Abstract
A 30-year-old Salvin's Amazon parrot (Amazona autumnalis salvini) with a history of a lifelong poor diet and inappropriate housing was presented in lateral recumbency to a veterinary teaching hospital for further evaluation. Radiological and ultrasonographic examination revealed a mild proventricular dilatation, mild hepatomegaly, signs of enteritis and airsacculitis. The main laboratory findings included a mild macrocytic hyperchromic anaemia, hypoglobulinaemia, decreased bile acids and increased alkaline phosphatase. In this bird a liver pathology was suspected because of the clinical, laboratory and ultrasonographic findings. The bird was treated with supportive care and metabolic aids. After initial improvement of the clinical signs, the bird's condition deteriorated and it died. Pathological findings revealed an endocarditis and myocarditis due to Lactobacillus jensenii and a bacteraemia. Endocarditis due to Lactobacillus sp. is a rare phenomenon in humans not yet described in animals. It is associated with severe underlying illnesses leading to translocation of otherwise non-pathogenic bacteria in the bloodstream. A similar pattern might be assumed in animals with compromised immunity.
Introduction
Lactobacilli are Gram-positive rods belonging to the lactic acid bacteria group (Bernardeau et al., 2007). They are found in the mucosal membranes of mammals (oral cavity, intestines and urogenital tract) and birds (crop and intestines) (Bernardeau et al., Citation2007; Gong et al., Citation2007). Lactobacilli are ubiquitous in the diet, especially in fermented products (Bernardeau et al., Citation2007). They are assumed to have a low pathogenicity (Moudden et al., Citation2007). However, cases of infection due to lactobacilli have been reported in humans (Atkins et al., Citation1990; Antony et al., Citation1996; Cannon et al., Citation2005; Land et al., Citation2005; Salvana & Frank, Citation2006; Chanet et al., Citation2007; Moudden et al., Citation2007). Lactobacillus casei and Lactobacillus rhamnosus are the most commonly found species, but there are also reports of Lactobacillus jensenii causing endocarditis and septicaemia (Atkins et al., Citation1990; Cannon et al., Citation2005; Salminen et al., Citation2006; Moudden et al., Citation2007). To our knowledge, this is the first case report of a natural endocarditis and bacteraemia due to a Lactobacillus sp. in an animal.
Case Report
A 30-year-old Salvin's Amazon parrot (Amazona autumnalis salvini) was presented in lateral recumbency. The bird was kept in the kitchen on a poor diet with the owner as a heavy smoker. It showed weakness, apathy, a head tilt to the left and bilateral clenched claws, with deterioration of the signs within a few hours. Physical examination revealed the bird to be in a hypovolaemic shock with severe dehydration, opisthotonus, and tremors. Both feet showed mild plantar reddening and clenched claws. The bird was in a good nutritional status. Lung and heart auscultation revealed no abnormal findings. After initial examination, a survey radiograph in the ventrodorsal projection was obtained to exclude heavy metal intoxication. Additionally, lead levels in the blood were tested and were found not to be suspicious of lead intoxication. The bird was rehydrated with intravenous fluids (5% glucose 5 ml/kg and 0.9% saline 5 ml/kg) and treated with antibiotics (enrofloxacin 15 mg/kg intramuscularly twice daily (Baytril 2.5%; Provet AG, Switzerland], and metronidazol 30 mg/kg per orally twice daily [Metronidazol-Tropfen; Streuli Pharma AG, Switzerland]) in case of a suspected septicaemia in this highly depressed bird.
A day after initial presentation, the bird was in an upright position with a mild head tilt to the left side. Both feet were still held clenched, but the bird moved around and was eating by itself. In the blood evaluation several values were altered, including a mild heterophilia (79%, reference range 33% to 73%) and lymphopaenia (18.5%, reference range 22% to 66%) without changes of the leukocyte count (Fudge, Citation2000). A slight macrocytic hyperchromic anaemia was noted (haematocrit 40%, reference range 42% to 55%; red blood cells 1.96×106/µl, reference range 2.45 to 3.18×103/µl; mean corpuscular hemoglobin 66 pg, reference range 47.2 to 56.8 pg; mean corpuscular volume 204 fl, reference range 160 to 175 fl). Plasma chemistry revealed a mildly increased alkaline phosphatase (155 U/l, reference range 15 to 150 U/l) and decreasing of the bile acids (4.8 µmol/l, reference range 24 to 120 µmol/l). Globulins were decreased (16 g/l, reference range 19 to 23 g/l), with a decreased albumin:globulin ratio (0.63, reference range 0.71 to 1.44).
Based on the laboratory changes, clinical signs and anamnesis, the primary differentials at this point were hepatic disease and hepatoencephalopathy. Other possibilities were neuromuscular disease (Sarcocystis sp. infection), idiopathic vestibular syndrome, and neoplasia. A cloacal swab was taken for Chlamydophila psittaci antigen test (Antigen ELISA IDEIA™ Chlamydia, K 6002; DAKO, Cambridgeshire, UK), which was negative. Ventrodorsal radiographs and right lateral radiographs were obtained and a mild proventricular dilatation, signs of enteritis, and mild airsacculitis were found. Gram staining of the faeces revealed 70% Gram-positive cocci, 20% Gram-positive rods and 10% Gram-negative rods. The faecal examination confirmed the radiological diagnose of enteritis because of the relative high degree of Gram-negative bacteria in the faeces, which are not considered to be normal commensales in healthy psittacines (Harrison & McDonald, Citation2006).
To further evaluate the liver, an ultrasonographic examination of the coelom was performed using a 5 to 8 MHz microconvex transducer (ATL HDI 5000; Philips AG Medical Systems, Zurich, Switzerland) with a ventromedian approach. The ultrasonographic findings lead to the diagnosis of mild enteritis, minimal coelomic effusion and mild hepatomegaly. Treatment was adjusted for suspected hepatopathy with milk thistle extract (5 mg/kg per orally twice daily [Milk Thistle; Holland & Barret, USA]), lactulose (0.3 ml/kg per orally twice daily [Duphalac; Solvay Pharma AG, Switzerland]), and vitamin K1 (0.2 mg/kg intramuscularly once daily [Konakion MM; Roche Pharma, Switzerland]). Vitamin E and selenium (1 mg/kg selenium and 25 mg/kg vitamin E intramuscularly once [Selen-E Vetag; Intervet, Switzerland]) was given because of the neuromuscular signs. Fluid therapy was changed to non-lactated solutions given subcutaneously. Two days later the clinical state of the bird deteriorated and the bird was again recumbent. When informed by telephone, the owners wished no further efforts until they could visit the patient. The bird died before they arrived and was submitted for postmortem.
On gross postmortem examination, a mild cardiomegaly and a friable grey–brown mass measuring 0.2×0.1 cm2 was found in the right ventricle attached to the endocardium. Findings also included a mildly enlarged firm liver with rounded edges and a mildly distended duodenum. The spleen was of normal size. The kidneys, lungs, airsacs and gastrointestinal tract (except of the mild distension of the duodenum) showed no macroscopically visible changes. Samples of cardiac blood and liver tissue were collected aseptically and submitted for bacterial culture using aerobic and anaerobic incubation at 37°C for 24 h on 5% sheep blood agar plates. Further sections of all major organs were fixed in 10% buffered formalin, stained with haematoxylin and eosin, and evaluated microscopically. Histological examination of the heart, liver, kidneys, lungs, ventriculus and proventriculus were made.
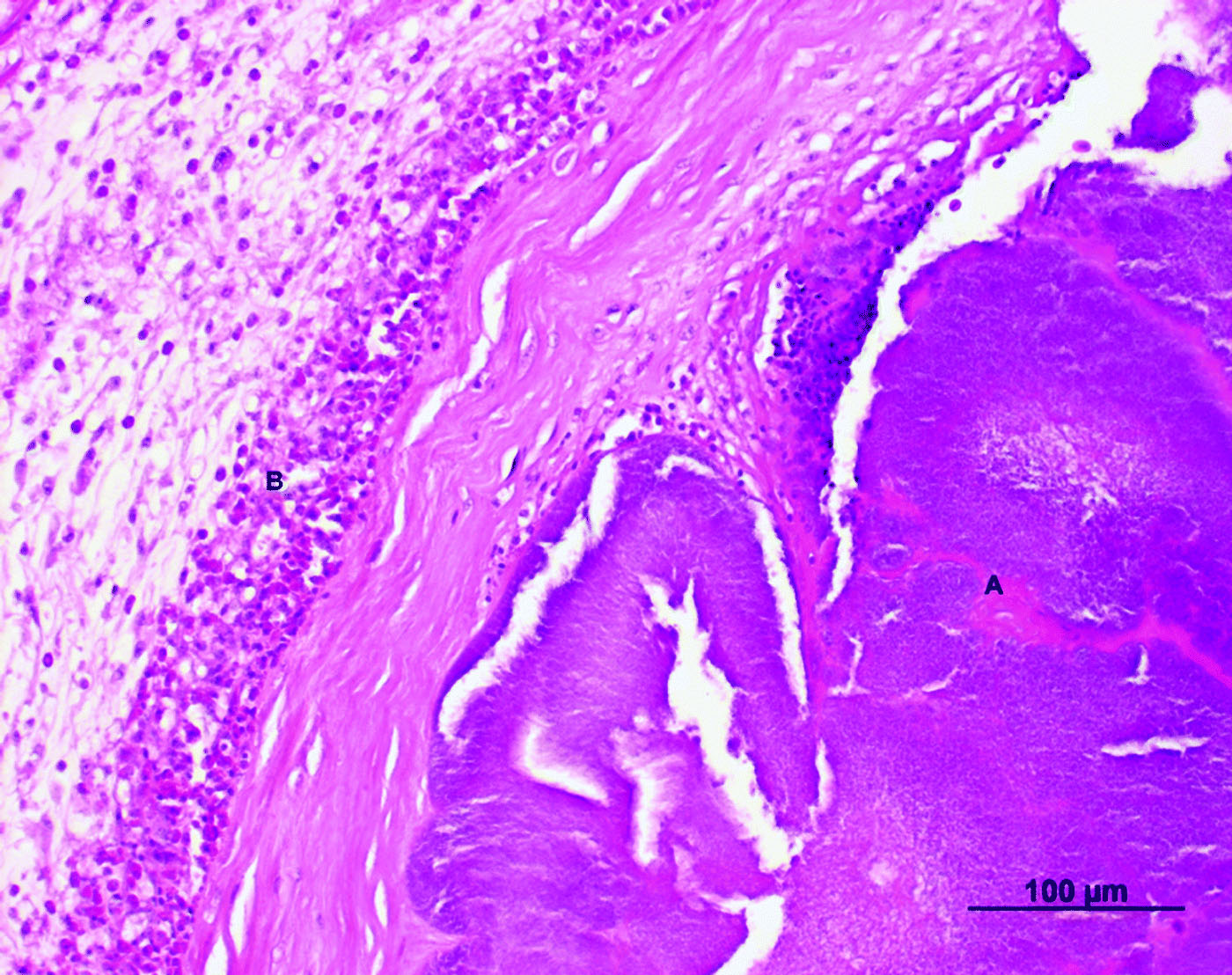
Throughout the heart lumen and in the endocardium (), bacterial colonizations (A) were noted. The thickened endocardium () showed a subendocardial inflammatory infiltrate consisting of granulocytic cells (B), primarily of eosinophils and heterophils. The myocard showed a disintegration of myofibrils with interstitial oedema and petechial bleedings. An inflammatory response consisting of granulocytic cells was also seen.
Figure 1. Cross-section of the valvular region of the right ventricle of an Amazon parrot (A. autumnalis salvini) (haematoxylin and eosin stain). Note heterophils (B) infiltrating the thickened encocardium and bacterial colonization (A) throughout the heart lumen.
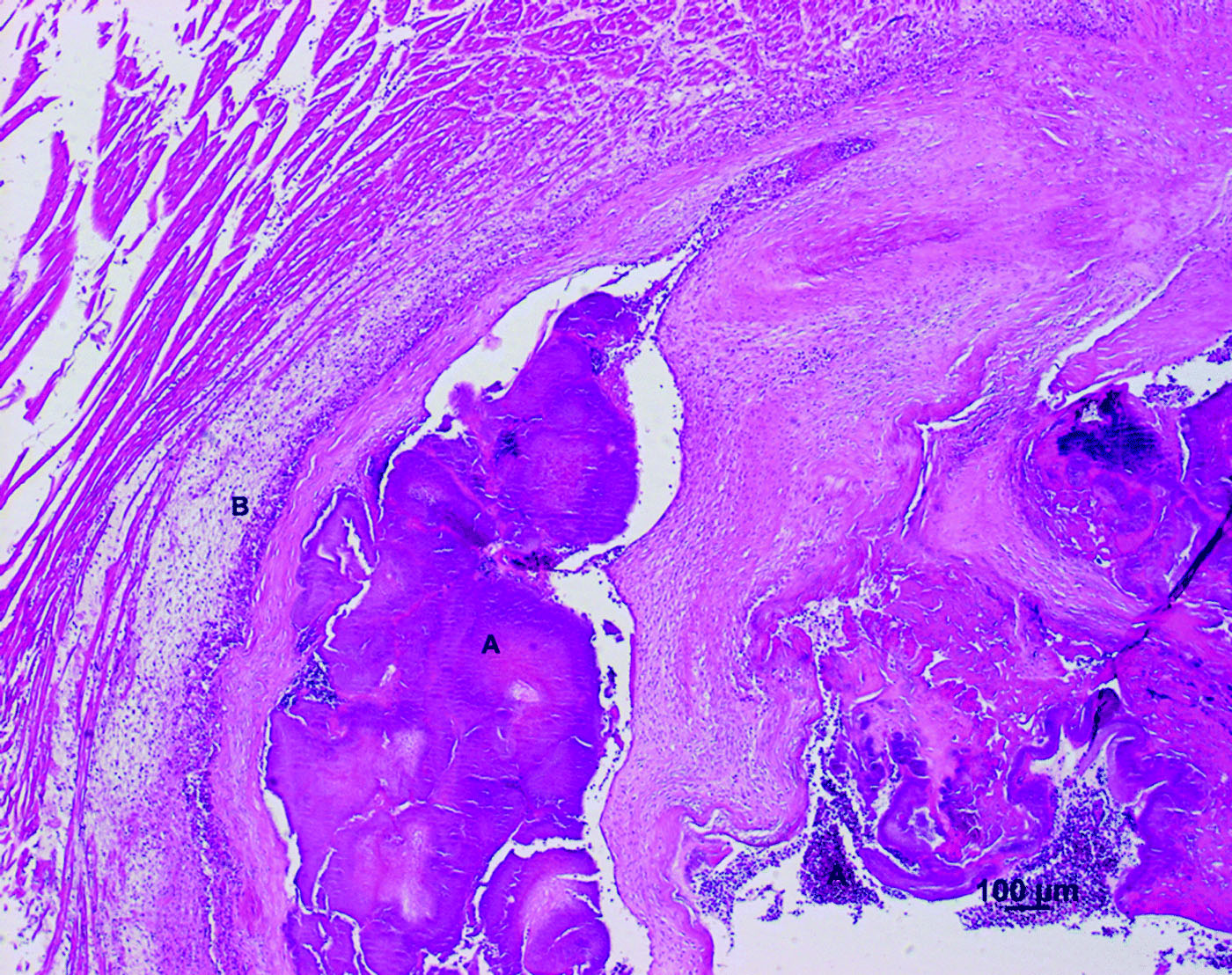
Figure 2. Cross-section of the valvular region of the right ventricle of an Amazon parrot (A. autumnalis salvini) (haematoxylin and eosin stain). The thickened encocardium showed an inflammatory infiltrate consisting of granulocytic cells (B) and bacterial nests (A)
The serosa of the ventriculus and the proventriculus showed an inflammatory infiltration with heterophilic cells and a few bacterial rods. The liver exhibited a disseminated necrosis and an inflammatory reaction comprised primarily of heterophils. The kidneys displayed a degenerative necrotizing glomerulonephritis with infiltrations of heterophils and eosinophils. An anthrasilicosis of the lung was noted. The lung tissue showed an interstitial pneumonia with hyperaemia, perivascular oedema formation and an inflammatory response primarily consisting of eosinophils. Cultures of the heart, blood and liver tissue collected postmortem yielded pure cultures on blood agar after incubation at 37°C for 24 to 48 h. For further specification of the bacteria grown on blood agar, the 16S rDNA was amplified using universal primers and were sequenced (Lane, Citation1991). The acquired sequences were compared with data-bank entries and the best hit was obtained with L. jensenii. A suspension of the isolate was prepared and transferred according to the manufacturer's instruction into the culture medium of an ATB™ VET strip (bioMérieux, Lyon, France). After 18 to 24 h of incubation, the strip was read automatically by the ATB instrument and revealed resistance to oxacillin, kanamycin, lincomycin, colistin, cotrimoxazole, sulfamethizole, flumequine, oxoline acid, enrofloxacin, and metronidazole. The bacteria showed sensitivity to penicillin, amoxicillin, cephalothin, cefaperazon, streptomycin, spectinomycin, gentamicin, apramycin, chloramphenicol, tetracycline, doxycycline, erythromycin, pristinomycin, tylosin, nitrofurantoin, fusidin acid, rifampicin, and cefquinome.
Discussion
Pathological findings in the represented case revealed an endocarditis and myocarditis due to L. jensenii. Bacterial infection of the heart has been described in various avian species (De Wit & Schoemaker, Citation2005). It can be a result of bacteraemia from chronic infections as salpingitis, hepatitis and pododermatitis, and can lead to endocarditis, myocarditis or epicarditis (Schmidt et al., Citation2003a; De Wit & Schoemaker, Citation2005). The prognosis for small animals with bacterial endocarditis is poor to grave (Kittleson & Kienle, Citation1998). All cases of birds with endocarditis described in literature are based on postmortem examinations (Isaza et al., Citation1992; Greenwood et al., Citation1996; Harari & Miller, Citation2007), so one can assume that in birds with endocarditis the prognosis is grave as well. Endocarditis in birds may cause valvular insufficiency with or without associated murmur. No signs of heart disease such as a heart murmur were noted during physical examination of the patient. An echocardiography, which could have revealed the endocarditis, was not performed. Other signs of heart diseases in birds are lethargy, dyspnea, weakness and collapse or syncope (Strunk & Heather Wilson, Citation2003). Diagnosis of endocarditis in animals is based on anamnesis (sudden onset of signs), physical examination (heart murmur), positive blood cultures and leukocytosis and signs of vegetative lesions of the endocardium in echocardiography (Isaza et al., Citation1992; Kittleson & Kienle, Citation1998; Strunk & Heather Wilson, Citation2003). Treatment of endocarditis includes antibiotic therapy, ideally based on culture results, and supportive care. Associated signs such as renal or congestive heart failure as well as fluid and electrolyte imbalances should be treated likewise (Kittleson & Kienle, Citation1998; Peddle, Citation2007). Management and control of the underlying disease is crucial (Peddle, Citation2007).
Different bacterial pathogens have been described for endocarditis in birds, such as Streptococcus sp., Pasteurella sp., Enterococcus sp., Enterobacter sp., Staphylococcus sp., and Erysipelothrix rhusiopathiae (Isaza et al., Citation1992; Gerlach, Citation1994; Greenwood et al., Citation1996; Harari & Miller, Citation2007). In this case, pure cultures of L. jensenii were isolated from heart and liver tissues. To date, no case of endocarditis and bacteraemia due to Lactobacillus sp. in an animal can be found in the literature. Cases of infection due to Lactobacilli have been reported in humans as a rare phenomenon, associated with compromised immunity, structural heart disease, recent surgery, prolonged antibiotic therapy and severe comorbid conditions (Atkins et al., Citation1990; Antony et al., Citation1996; Cannon et al., Citation2005; Land et al., Citation2005; Hammermann et al., Citation2006; Salvana & Frank, Citation2006; Chanet et al., Citation2007; Moudden et al., Citation2007). L. casei and L. rhamnosus are the most commonly found species, but there are also reports of L. jensenii causing endocarditis and septicaemia (Atkins et al., Citation1990; Cannon et al., Citation2005; Salminen et al., Citation2006; Moudden et al., Citation2007). It is assumed that the mucosa of the gastrointestinal and urogenital tract in humans with severe underlying illnesses is friable and allows translocation of otherwise non-pathogenic bacteria (Hammermann et al., Citation2006). A translocation of mucosa-associated bacteria after mucosal lesion (e.g. due to enteritis) appears possible in birds as well. Histological examination in this case revealed not only an endocarditis and myocarditis, but also a high presence of eosinophils in the heart, kidneys and lungs. The exact function of the avian eosinophil is still unclear (Campbell, Citation1995). It is supposed that eosinophils have different functions such as participation in inflammatory responses, phagocytosis and bactericidal and parasiticidal activity. Eosinophilia has been seen in gastrointestinal parasitism (Campbell, Citation1995; Latimer & Rakich, Citation2007). Studies also suggest that avian eosinophils may participate in the modulation of inflammation in delayed hypersensitivity reactions (Latimer & Rakich, Citation2007). Eosinophilic myocarditis can be the result of some parasitic infections, such as sarcocystosis, as it is described in birds (Strunk & Heather Wilson, Citation2003). However, in this case no protozoal parasites were seen on histological examination.
A delayed hypersensitivity reaction to staphylococcal dermatitis known as “Amazon foot necrosis” is reported in Amazon parrots (Burgmann, Citation1995; Schmidt et al., Citation2003b). Owners of the affected birds are often heavy smokers, as was the case in the described bird. It is assumed that some element in the tobacco smoke may initiate this disease (Schmidt et al., Citation2003b). First, the bird's feet and legs become erythematous, then the bird starts chewing at the feet, which leads to an ulcerative dermatitis with possible secondary bacterial infection (Burgmann, Citation1995). Because relapses are very common, other contributing factors to this condition—like immunosuppression, underlying infectious diseases and topical irritations of the skin—are assumed (Burgmann, Citation1995).
In the present case, a combination of different factors (poor husbandry and nutrition, age of the bird, exposure to tobacco smoke) might have led to a delayed hypersensitivity reaction plus immunosuppression, which allowed a translocation of usually non-pathogenic intestinal microorganisms in the bloodstream followed by a fatal endocarditis and bacteraemia. The chosen antibiotic regime could not succeed because L. jensenii, responsible for the bacteraemia in this bird, was resistant against enrofloxacine and metronidazole. Lactobacilli have a high natural resistance to bacitracine, cefoxitine, ciprofloxacine, fusidic acid, kanamycine, gentamicine, metronidazole, nitrofurantoine, norfloxacine, streptomycin, sulphadiazine, trimethoprim/sulphamethoxazole, and vancomycin (Bernardeau et al., Citation2007). However, the antimicrobial susceptibility of the large genus Lactobacillus seems to be species dependent (Salminen et al., Citation2006). Antibiotic treatment with cephalosporins and quinolones is not advisable because of the low effectiveness of these antibiotics against the isolates (Antony et al., Citation1996). Susceptibility testing may be important for definitive and successful treatment.
In the present report the underlying disease was not diagnosed with the applied procedures. An echocardiography would have given further information but was not performed because of absence of a heart murmur and other clinical signs suggestive of heart disease. In such cases of a highly depressed patient, septicaemia should be suspected and a blood culture may lead to the right diagnosis and causal therapy after sensitivity testing.
Acknowledgements
The authors thank Marcus Clauss for comments on the manuscript, and thank Helene Pendl and Ludwig Hoelze for their support.
References
- Antony , S. , Stratton , C. and Dummer , J. 1996 . Lactobacillus bacteraemia: description of the clinical course in adult patients without endocarditis . Clinical Infectious Diseases , 23 : 773 – 778 .
- Atkins , M. , Nicolson , L. , Harrison , G. , Paull , A. , Malnick , H. and Morrison , D. 1990 . Lactobacillus jensenii prosthetic valve endocarditis . Journal of Infection , 21 : 322 – 324 .
- Bernardeau , M. , Vernoux , J. , Henri-Dubernet , S. and Guéguen , M. 2007 . Safety assessment of dairy microorganisms: the Lactobacillus genus . International Journal of Food Microbiology , 126 ( 3 ) : 278 – 285 .
- Burgmann , P. 1995 . Common psittacine dermatological diseases . Seminars in Avian and Exotic Pet Medicine , 4 : 169 – 183 .
- Campbell , T. 1995 . “ Avian hematology ” . In Avian Hematology and Cytology , Edited by: Campbell , T. 3 – 19 . Ames : Iowa State Press .
- Cannon , J. , Lee , T. , Bolanos , J. and Danzinger , L. 2005 . Pathogenic relevance of Lactobacillus: a retrospective review of over 200 cases . European Journal of Clinical Microbiological Infectious Diseases , 24 : 31 – 40 .
- Chanet , V. , Brazille , P. , Honore , S. , Michel , M. , Schaeffer , A. and Zarrouk , V. 2007 . Lactobacillus septic arthritis . Southern Medical Journal , 100 : 531 – 532 .
- De Wit , M. and Schoemaker , N. 2005 . Clinical approach to avian cardiac disease . Seminars in Avian and Exotic Pet Medicine , 14 : 6 – 13 .
- Fudge , A. 2000 . “ Laboratory reference ranges for selected avian, mammalian and reptilian species ” . In Laboratory Medicine , Edited by: Fudge , A. 375 – 400 . Philadelphia , PA : WB Saunders .
- Gerlach , H. 1994 . “ Bacteria ” . In Avian Medicine: Principles and Application , Edited by: Ritchie , B. , Harrison , G. and Harrison , L. 975 – 983 . Lake Worth , FL : Wingers .
- Gong , J. , Si , W. , Forster , R. , Huang , R. , Yu , H. , Yin , Y. , Yang , C. and Han , Y. 2007 . 16S rRNA gene-based analysis of mucosa-associated bacterial community and phylogeny in the chicken gastrointestinal tracts: from crop to ceca . FEMS Microbiology Ecology , 59 : 147 – 157 .
- Greenwood , A. , Marshall , J. and Tinsley , E. 1996 . Vegetative endocarditis in a Waldrapp ibis . Avian Pathology , 25 : 387 – 391 .
- Hammermann , C. , Bin-Nun , A. and Kaplan , M. 2006 . Safety of probiotics: comparison of two popular strains . British Medical Journal , 333 : 1006 – 1008 .
- Harari , J. and Miller , D. 2007 . Ventricular septal defect and bacterial endocarditis in a whistling swan . Journal of the American Veterinary Medical Association , 183 : 1296 – 1297 .
- Harrison , G. and McDonald , D. 2006 . “ Nutritional considerations Section II ” . In Clinical Avian Medicine , Edited by: Harrison , G. and Lightfoot , T. 108 – 140 . Palm Beach , FL : Spix Publishing .
- Isaza , R. , Buergelt , C. and Kollias , G. 1992 . Bacteraemia and vegetative endocarditis associated with a heart murmur in a Blue-and-Gold macaw . Avian Diseases , 36 : 1112 – 1116 .
- Kittleson , M. and Kienle , R. 1998 . “ Infective endocarditis ” . In Small Animal Cardiovascular Medicine , Edited by: Kittleson , M. and Kienle , R. 402 – 412 . St Louis , MO : Mosby .
- Land , M. , Rouster-Stevens , K. , Woods , C. , Cannon , M. , Cnota , J. and Shetty , A. 2005 . Lactobacillus sepsis associated with probiotic therapy . Pediatrics , 115 : 178 – 181 .
- Lane , D. 1991 . “ rRNA sequencing ” . In Nucleic Acids Techniques in Bacterial Systematics , Edited by: Stackebrandt , E. and Goodfellow , M. 115 – 147 . Chichester : John Wiley & Sons .
- Latimer , K. and Rakich , P. 2007 . Avian cytology . Veterinary Clinics of North America—Exotic Animal Practice , 10 : 131 – 154 .
- Moudden , M. , Boukhira , A. , Sarret , D. , Cazajous , G. , Labaye , J. , Herody , M. , Soler , C. and Didelot , F. 2007 . Septicemia due to Lactobacillus jensenii: bacteriological diagnostic orientation . Annales de Biologie Clinique , 65 : 299 – 302 .
- Peddle , G. 2007 . Canine bacterial endocarditis: a review . Journal of the American Animal Hospital Association , 43 : 258 – 263 .
- Salminen , M. , Rautelin , H. , Tynkkynen , S. , Poussa , T. , Saxelin , M. , Valtonen , V. and Jaervinen , A. 2006 . Lactobacillus bacteraemia, species identification, and antimicrobial susceptibility of 85 blood isolates . Clinical Infectious Diseases , 42 : 35 – 44 .
- Salvana , E. and Frank , M. 2006 . Lactobacillus endocarditis: case report and review of cases reported since 1992 . Journal of Infection , 53 : e5 – e10 .
- Schmidt , R. , Reavill , D. and Phalen , D. 2003a . “ Cardiovascular system ” . In Pathology of Pet and Aviary Birds , Edited by: Schmidt , R. , Reavill , D. and Phalen , D. 3 – 17 . Ames : Iowa State Press .
- Schmidt , R. , Reavill , D. and Phalen , D. 2003b . “ Integument ” . In Pathology of Pet and Aviary Birds , Edited by: Schmidt , R. , Reavill , D. and Phalen , D. 177 – 197 . Ames : Iowa State Press .
- Strunk , A. and Heather Wilson , G. 2003 . Avian cardiology . Veterinary Clinics of North America—Exotic Animal Practice , 6 : 1 – 28 .