Abstract
In this experiment we evaluated the transmission characteristics of a chicken-adapted low-pathogenicity avian influenza virus (LPAIV) of subtype H6N2, from infected chickens to Japanese quail and Pekin ducks, which are commonly sold in live bird markets located in Southern California. The layout of the cages and bird-handling practices were similar to those found in Southern California live bird markets. Five out of 20 chickens were inoculated with LPAIV H6N2, and placed in direct contact with five chickens and in indirect contact with 10 chickens, 10 Japanese quail and 10 Pekin ducks. Transmission of LPAIV was followed in each bird daily for 15 days post inoculation by real-time reverse transcriptase-polymerase chain reaction testing of oropharyngeal and cloacal swab samples. This strain of H6N2 LPAIV, isolated from commercial poultry in California, was transmitted to chickens, quail, and ducks from chickens. An antibody response was detected in ducks by haemagglutination inhibition tests, but avian influenza virus was only detected by reverse transcriptase-polymerase chain reaction in one duck. Avian influenza virus was detected in quail (5 and 7 days post inoculation) before chickens (8 and 9 days post inoculation), all of which were in indirect contact with infected chickens; however, this difference was not statistically significant.
Introduction
Avian influenza viruses (AIVs) are enveloped, single-stranded negative-sense RNA viruses that belong to the Orthomyxoviridae family and can cause disease affecting humans, poultry, and wild birds (Swayne & Halvorson, Citation2003). Live bird markets (LBMs) have been involved in many AIV epidemics worldwide; however, little is known about AIV transmission in the market setting (Senne et al., Citation1992; Swayne & Halvorson, Citation2003; Webster, Citation2004). Increased knowledge of low-pathogenicity avian influenza virus (LPAIV) transmission will facilitate our understanding of the epidemiology of AIV, with the goal of planning better control and eradication programmes. One study compared the transmission rate of LPAIVs and highly pathogenic avian influenza viruses (HPAIVs) among chickens (van der Goot et al., Citation2003). It consisted of eight experiments, each conducted with 10 specific pathogen free chickens co-housed in a single cage, five inoculated with LPAIV or HPAIV H5N2. Although the study illustrated the speed at which AIV can spread among chickens in direct contact with many infectious chickens, it does not show how AIV might spread among cages of chickens housed the way they are in LBMs or in some commercial poultry settings. Transmission rates from chickens to other bird species that are also commonly housed together in LBMs, such as ducks and quail, would also be valuable in order to plan better AIV control and eradication programmes.
The transmission and replication of AIV have been studied in Japanese quail (Coturnix coturnix japonica), a bird commonly sold in LBMs worldwide (Perez et al., Citation2003). Japanese quail (hereafter referred to as quail) have been identified as highly susceptible frequent hosts of many AIV isolates in LBMs (Liu et al., Citation2003). However, in another study of AIV transmission in quail, three quail and three chickens were exposed via aerosol and faecal contact to three AIV-inoculated quail without detectable transmission over 12 days (Perez et al., Citation2003). Markarova et al. ( Citation2003) inoculated 4-week-old Japanese quail with AIVs representative of subtypes H1 to H15, originally isolated from wild waterfowl and shorebirds, but were unable to detect transmission to quail placed in the same cage as inoculated quail. These studies help to document both the variability in host adaptation and transmissibility among AIV, but also point to the importance of quail in the complex transmission cycles in LBMs.
Birds of the Anseriformes and Charadriiformes orders generally do not show signs of AIV infection and are known reservoir hosts of LPAIV since all AIV subtypes have been isolated from these birds (Swayne & Halvorson, Citation2003). However, it is clear that ducks and shorebirds are not always susceptible to infections with AIV, particularly AIVs that are adapted to chickens. One exception has been in the recent HPAIV H5N1 outbreak in Asia, where transmission of H5N1 from chickens to domestic and wild migratory birds was reported (Chen et al., Citation2004; Hulse-Post et al., Citation2005; Songserm et al., Citation2006). In a study conducted by Alexander et al. (Citation1986), four out of eight H5-subtype AIVs studied were re-isolated from inoculated and in-contact ducks. Additionally in autumn 2005, a chicken-adapted LPAIV (H6N2) was detected in five out of the 10 samples collected from ducks sold at a LBM located in Southern California (Yee et al., 2007). In other research, when Pekin ducks (Anas platyrhynchos) were inoculated with a chicken-adapted HPAIV, A/Chicken/Pennsylvania/1370/83 (H5N2), virus was only detected in one out of 12 ducks (Wood et al., Citation1985). Indirect contact transmission of a chicken-adapted LPAIV to domestic ducks has not been studied previously. Since ducks are commonly sold and kept in close proximity to chickens and quail in LBMs worldwide, information on the effective transmission of a chicken-adapted LPAIV from chickens to other avian species would fill a gap in a critical AIV transmission pathway.
In the present study, we simulated an LBM setting with three avian species commonly sold in LBMs located in Southern California in order to characterize the direct contact transmission between chickens and indirect contact transmission of AIV from chickens to quail and ducks.
Methods and Materials
Animals
All birds were acquired through poultry suppliers that provided birds for LBMs in Southern California. The suppliers participated in active and passive surveillance performed by the California Custom Slaughter LPAIV Control Programme (Yee et al., Citation2008). There were no AIV infections in any of the suppliers’ flocks immediately prior to or during the experimental period based on the results of state and federal monitoring (C.J. Cardona, personal communication). All of the chicken strains used in this experiment are of the types and strains sold in California LBMs (Yee et al., 2008) and were market age at the time of placement. All chickens used in this experiment were between 6 and 24 weeks of age; broiler pullets (n=4) were 6 to 8 weeks of age, and brown pullets (n=26) and roosters (n=10) were 8 to 24 weeks old. Pekin ducks (n=20) and Japanese quail (n=20) were 8 to 12 weeks and 7 weeks of age, respectively.
Virus
The virus used to inoculate birds in this study, A/chicken/CA/1772/02 (H6N2) (hereafter referred to as H6N2 AIV), was isolated from chickens in Southern California and was provided for use in these studies by Dr Peter Woolcock..
Experimental procedures
Five chickens were inoculated intranasally with 107 EID50 median embryo infective dose H6N2 AIV on day 1 and were placed in a cage with five chickens (Cage 1). Cage 1 contained five roosters, two broilers, and three brown pullets and was located above a cage containing 10 ducks (Cage 4) with a tray separating the cages to catch faeces. Ten quail (Cage 3) were located above a cage of 10 brown pullets (Cage 2) and across from Cages 1 and 4. Cages 1 and 4 were 12 inches from Cages 2 and 3 (). Food and water were provided ad libitum in pans similar to those found in LBMs located in Southern California.
Figure 1. Sideview diagram of the experimental layout. The birds were sampled and handled in numerical order of Cage 1, Cage 2, Cage 3 and Cage 4, every day for 16 days.
A technician wore a hycar apron, rubber gloves, and rubber boots, which were stored in the room where the birds were kept and were only cleaned and disinfected after day 16. The purpose of the apron, gloves, and boots was to serve as a means of mechanically transferring AIV between the cages and individual birds to simulate handling conditions that occur in LBMs. The technicians did not know the identity and location of the inoculated birds. Oropharyngeal and cloacal samples were collected from each bird, and each bird was handled daily for 16 days by the technician. Blood samples were collected on days 0 (pre-inoculated), 7, 14, and 16 of the experiment. Samples were first collected from Cage 1, which contained the inoculated birds, followed by the second cage of chickens, quail and then ducks. Sample collection and bird handling was conducted daily in the same order. Gross necropsy was performed for every bird at the end of 16 days as described (Cardona & Cutler, Citation2002). These procedures were replicated in a second trial.
Laboratory tests
Virus was detected in all samples by real-time reverse transcriptase-polymerase chain reaction (rRT-PCR) following published methods (Spackman et al., Citation2003). The same rRT-PCR test was run on known dilutions of the challenge virus to establish a standard curve that could be used estimates of the number of virus particles. The haemagglutinin inhibition (HI) tests on sera were performed using standard methods (World Organisation for Animal Health, Citation2005). Haemagglutination inhibition titres of 1:8 or greater were considered positive.
Statistical analyses
The time to initial detection was the first day AIV was detected by rRT-PCR in a bird. Mann–Whitney non-parametric two-sample tests were conducted to compare the two study replicates and the median day to AIV detection of the index versus the direct contact chickens in Cage 1. The Kruskal–Wallis test, a non-parametric analysis of variance rank test, was used to test differences in time to detection of AIV between cage groups (Kruskal & Wallis, Citation1952). The mean rank time to infection for each cage was compared to determine whether there was a difference in AIV spread among the birds, based on their species. Times to detection for index cases were not included in the time to detection analysis between cages. A non-parametric Bonferroni–Dunn procedure was used to perform multiple pairwise comparisons between group mean ranks following each statistically significant Kruskal–Wallis test. The overall error rate was set at 5% (Daniel, Citation1990).
Results
No significant lesions were evident during necropsy of the ducks or quail in Trial 1. The lesions in chickens in Cage 1 included moderate splenomegaly (three inoculated, three direct contact chickens), mild to moderate egg yolk peritonitis and airsacculitis (two inoculated chickens), pale kidneys (two inoculated chickens) and moderate, bilateral lung consolidation (one direct contact chicken) in Trial 1. Chickens in Cage 2 did not present with any grossly evident lesions in Trial 1.
In total 10 chickens in Trial 2 had moderate splenomegaly (Cage 1, four inoculated, three direct contact; Cage 2, three indirect contact), four chickens (Cage 1, two inoculated, two direct contact) and two ducks had mild abdominal airsacculitis with fibrin tags, two chickens (Cage 1, one inoculated, one direct contact) and one duck had extensive egg yolk peritonitis, one duck had mild hepatomegaly, and one chicken (Cage 2) had dilated, thin-walled intestines. Two chickens died in Trial 2; an inoculated chicken (Cage 1) on day 7 and an indirect contact chicken (Cage 2) on day 14. AIV was not detected in the chicken that died on day 14. One duck died on day 14 and was the only duck in which AIV was detected. The duck had an enlarged liver and airsacculitis.
AIV was detected in the inoculated chickens for 4 to 7 days and 1 to 9 days consecutively for Trials 1 and 2, respectively. In the direct contact chickens in Cage 1, AIV was detected for 4 to 6 consecutive days in Trial 1 and for 6 to 13 consecutive days in Trial 2. The cycle threshold values detected in swab samples from inoculated chickens were similar for both trials (26.13 to 40.25 for Trial 1 and 27.32 to 39.46 for Trial 2). At the peak in inoculated birds, 103.3 virus particles were detected from an oropharyngeal sample in Trial 1 and 101.7 virus particles were detected from a cloacal sample in trial 2. For the indirect contact chickens in Cage 2, AIV was detected for 1 to 4 consecutive days in Trial 1 and for 1 to 3 consecutive days in Trial 2. The cycle threshold values for the rRT-PCR-tested samples collected from the chickens in Cage 2 ranged from 23.78 to 44.53 in Trial 1 and from 26.32 to 41.65 in trial 2. The highest numbers of virus particles in indirect contact chickens were 105.6 and 103 from oropharyngeal samples in Trials 1 and 2, respectively. AIV was detected in quail for 1 to 6 consecutive days in Trials 1 and 2. The cycle threshold values for the rRT-PCR-tested samples collected from the quail in the room ranged from 25.08 to 31.85 in Trial 1 and from 23.22 to 43.71 in Trial 2. Virus particle numbers in quail peaked in oropharyngeal samples at 105.1 and 103.5 in Trials 1 and 2, respectively. All birds were HI test-negative prior to inoculation. All birds where AIV was detected by rRT-PCR on or prior to day 10 had a greater than 5 log2 HI titre response on day 14. The day 14 log2 HI titre range for Cage 1 was 7 to 11 for Trial 1 and was 9 to 10 for Trial 2. The day 14 log2 HI titre ranges for all chickens in Cage 2 where AIV was detected by RT-PCR prior to day 10 was 7 to 11 for Trial 1 and was 3 to 11 for Trial 2. Positive HI titres were detected in five quail in Trial 1 and two quail in Trial 2. The day 14 log2 HI titre range for the five quail was 5 to 11 in Trial 1. The day 14 log2 HI titre for the two quail was 6. Positive HI titres were detected in eight out of 10 ducks in each trial. The day 14 log2 HI titre range for positive ducks was 5 to 11 in both trials.
Since AIV was detected by RT-PCR in only one duck for both trials, the median time to detection for ducks was not included in the statistical analysis. It should be noted that this single duck infection was verified by isolation of the virus and sequencing of the haemagglutinin gene, confirming its identity as the inoculating strain (data not shown). The cumulative incidence of AIV detection for both trials is illustrated in . AIV was first detected outside Cage 1 in quail for both trials on days 6 and 8, respectively. The median time to detection in inoculated chickens for both trials was 1.5 days (range: 1 to 9 days). There was no statistically significant difference in the median time to detection in direct contact birds and inoculated chickens between Trial 1 (median = 3 days) and Trial 2 (median = 2 days; P=0.603). The time to detection in the inoculated chickens (median = 1.5 days) was significantly earlier than the other chickens in Cage 1 (median = 3 days; P=0.011). A Kuskal–Wallis non-parametric analysis of variance test of the time to detection between the cages was statistically significant (P < 0.001). The time to detection for chickens that had direct contact with infectious chickens (Cage 1) was significantly earlier than that for chickens with indirect contact (Cage 2, median = 13 days) and quail (median = 12 days) (). There was no significant difference in the median time to detection between chickens in Cage 2 and quail (P=0.655).
Figure 2. Cumulative incidence of H6N2 detection by rRT-PCR for each interspecies AIV-transmission trial.
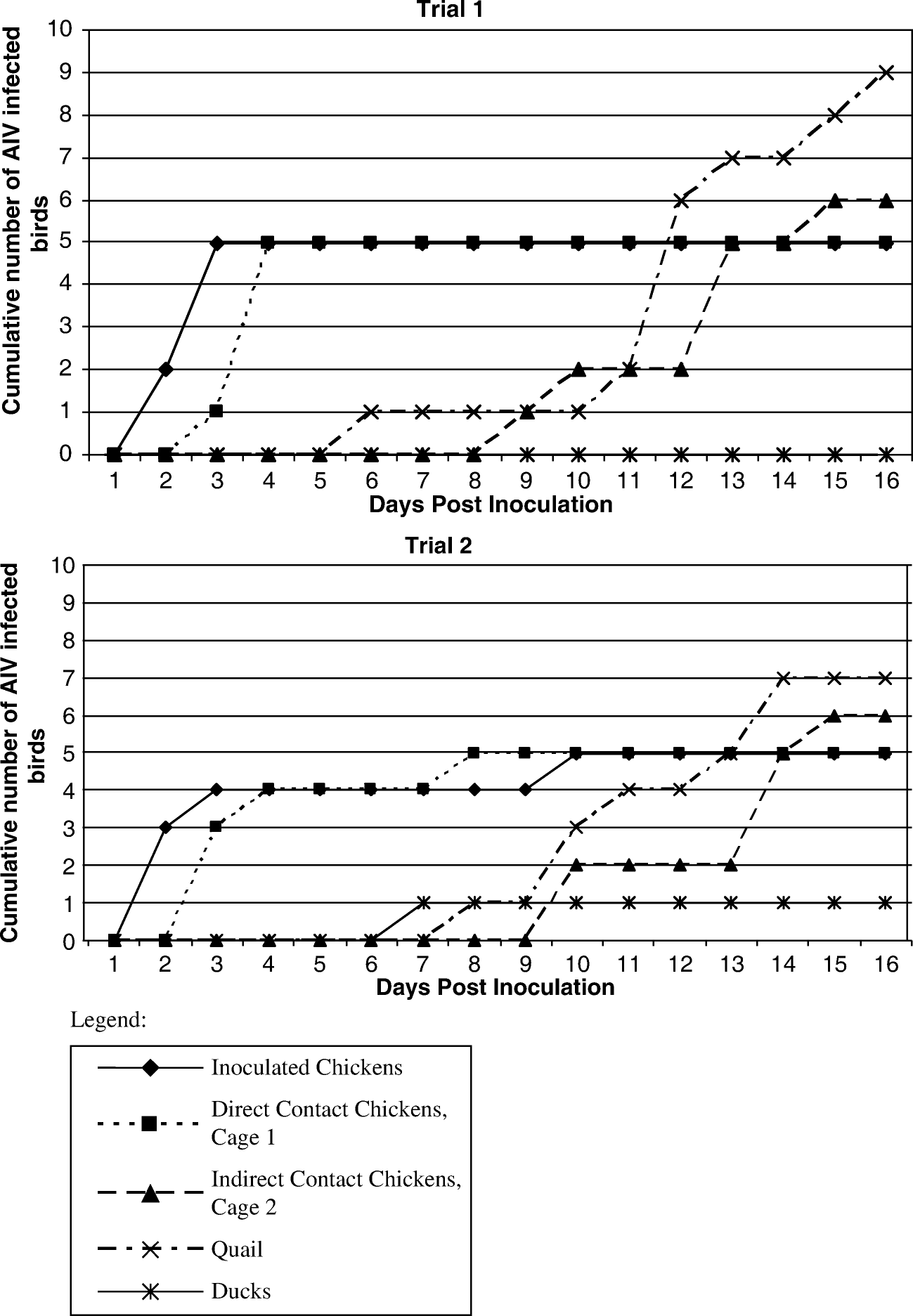
Table 1. Median time to AIV detection by RT-PCR
Discussion
In the present study we have demonstrated the transmission of LPAIV from chickens to chickens, quail and, in one case, ducks. Although this initial LBM layout was designed to replicate Southern California LBM conditions, there are many other market types and more studies are needed to further evaluate the effects of layout on AIV transmission characteristics. AIV was not detected until day 9 in one inoculated chicken in Trial 2. It is possible that virus was present at a titre not detectable by RT-PCR, or the bird was shedding intermittently or was not initially infected by the inoculum and only later infected by contact with infected birds. Although AIV was not detected in this bird until day 9, there was no significant effect on the experiment as there was no statistical difference observed between the two trials.
Although the difference in time to detection in quail compared with the second cage of chickens was not statistically significant, AIV was detected in quail 3 days earlier in Trial 1 and 2 days earlier in Trial 2 than it was detected in the first chicken in the second cage of chickens. Birds in the second cage of chickens were also handled directly after the inoculated cage of chickens. In our previous studies of H6N2 transmission, handling infectious birds prior to indirect contact chickens was not observed as an effective transmission mode of transmission (K.S. Yee, manuscript submitted). Previous AIV transmission studies in quail have had mixed results. Perez et al. (Citation2003), in their studies of Asian duck H9 AIV, did not observe transmission from inoculated to susceptible quail through either direct or indirect contact 5 to 7 days post inoculation, but did observe transmission of an Asian quail H9 AIV to quail and chickens with faecal contact. It is possible that transmission of the AIV subtypes studied by Perez et al. would have been observed if the experiment were extended beyond 7 days post inoculation. None of the chickens was infected with the Asian quail H9 AIV via aerosol contact. Detection of AIV transmitted to quail in the current study is significant since previous experiments using Japanese quail have not resulted in efficient transmission of AIV to direct contact birds (Liu et al., Citation2003). In our experiment, H6N2 AIV had the genetic characteristics of adaptation to chickens and was detected in quail with aerosol contact only within 5 and 7 days of exposure to inoculated birds. The effect of the quail in LBMs on the dynamics of AIV transmission and detection should be further studied. In a surveillance study of LBMs located in Hong Kong, there was a strong association between presence of quail and detection of AIV in those LBMs (Lau et al., Citation2007).
Indirect (non-faecal) contact transmission rate of AIV to chickens was similar to that observed in another study with H6N2 AIV, where the virus was detected between 7 and 13 days post inoculation in index birds (K.S. Yee, manuscript submitted). Our results contrast with those from previous transmission studies using a different AIV strain. In van der Goot et al.'s (2003) LPAIV H5N2 study, no transmission was observed in two of the four replicates between chickens with direct contact. But AIV was transmitted to two and three out of five chickens in two of the replicates (van der Goot et al., Citation2003). Our results did not show any statistical differences between the replicate studies.
Transmission of AIV from birds of the order Galliformes to Anseriformes species is reported rarely; the most recently observed is HPAIV H5N1 detected in wild waterfowl as well as in domestic ducks and chickens in Asia, Europe, and Africa (Anonymous, Citation2005, Citation2006; Eurosurveillance Editorial Office, Citation2006). One explanation for the results of this study is that cellular receptors bound by AIV in the respiratory tracts of ducks are different from those of chickens, resulting in less efficient establishment of infection. In our experiment, AIV was detected in only one duck by rRT-PCR, which supports the theory that transmission of chicken-adapted AIV to ducks is rare, requiring additional molecular changes to the virus (Nguyen et al., Citation2005). However, our HI test results show that not only can AIV move between cages indirectly, but that indirect contact with infectious chickens is adequate to transmit H6N2 to ducks. Our results suggest that ducks may not amplify and shed H6N2, but our study results do contradict a prevalence study of LPAIV in multiple-species LBMs located in Southern California where LPAIV H6N2 was detected by rRT-PCR in five out of 10 ducks sampled (Yee et al., Citation2007). Ducks may shed H6N2 at low level that cannot be detected by rRT-PCR or at an interval that could not be detected by the sampling schedule. Further transmission studies would be useful including faecal exposure of ducks by infectious chickens as faecal exposure was not evaluated in this study.
We have demonstrated AIV transmission among different bird species commonly found in LBMs; however, further studies using different subtypes of LPAIV should be performed for better understanding of inter-species transmission. Results from this experiment are limited to the characteristics of this AIV; however, the methods may be used to quantify transmission parameters and then used to simulate the impact of an LPAIV H6N2 and generalize an outbreak of any chicken-adapted LPAIV in a LBM among different species of birds.
Acknowledgements
The authors thank Dr Jinling Li and Dr Zeng-Qi Yang for their laboratory guidance and performing the HI tests. They also thank Nicole L Anchell, Nugget Dao, Phuong Dao, and Sara Leisgang for providing technical and laboratory support for this study. The work was supported by the Center for Animal Disease Modeling and Surveillance at the University of California Davis, and by the Avian Influenza Coordinated Agricultural Project, USDA/CSREES grant 2005-35605-15388, “The Prevention and Control of Avian Influenza in the United States”.
References
- Alexander , D.J. , Parsons , G. and Manvell , R.J. 1986 . Experimental assessment of the pathogenicity of eight avian influenza A viruses of H5 subtype for chickens, turkeys, ducks, and quail . Avian Pathology , 15 : 647 – 666 .
- Anonymous . 2005 . Recent avian influenza outbreaks in Asia . Center for Disease Control and Prevention. Available online at http://www.cdc.gov/flu/avian/outbreaks/asia.htm (accessed 18 April 2005) .
- Anonymous . 2006 . Avian influenza—situation in Egypt—update 3 , 6 April. Available online at http://www.who.int/csr/don/2006_04_06a/en/index.html (accessed 2 January 2007) .
- Cardona , C.J. & Cutler , G. 2002 . Chapter 30, Section F. Necropsy techniques . In D.C. Bell & W.D. Weaver ( Eds. ), Commercial Chickens: Meat and Egg Production ( pp. 572 – 576 ). Springer Science and Business Media .
- Chen , H. , Deng , G. , Li , Z. , Tian , G. , Li , Y. , Jiao , P. , Zhang , L. , Liu , Z. , Webster , R.G. and Yu , K. 2004 . The evolution of H5N1 influenza viruses in ducks in southern China . Proceedings of the National Academy of Sciences U S A , 101 : 10452 – 10457 .
- Daniel , W. 1990 . Multiple comparisons . In Applied Nonparametric Statistics (p. 241). PWS-KENT .
- Eurosurveillance Editorial Office 2006 . More detections of avian influenza in wild birds in Europe: Commission approves limited poultry vaccination . Available online at http://www.eurosurveillance.org/ew/2006/060223.asp (accessed 23 February 2006) .
- Hulse-Post , D.J. , Sturm-Ramirez , K.M. , Humberd , J. , Seiler , P. , Govorkova , E.A. , Krauss , S. , Scholtissek , C. , Puthavathana , P. , Buranathai , C. , Nguyen , T.D. , Long , H.T. , Naipospos , T.S. , Chen , H. , Ellis , T.M. , Guan , Y. , Peiris , J.S. and Webster , R.G. 2005 . Role of domestic ducks in the propagation and biological evolution of highly pathogenic H5N1 influenza viruses in Asia . Proceedings of the National Academy of Sciences USA , 102 : 10682 – 10687 .
- Kruskal , W. and Wallis , W. 1952 . Use of ranks in one-criterion variance analysis . Journal of the American Statistical Association , 47 : 583 – 621 .
- Lau , E.H. , Leung , Y.H. , Zhang , L.J. , Cowling , B.J. , Mak , S.P. , Guan , Y. , Leung , G.M. and Peiris , J.S. 2007 . Effect of interventions on influenza A (H9N2) isolation in Hong Kong's live poultry markets, 1999–2005 . Emerging Infectious Diseases , 13 : 1340 – 1347 .
- Liu , M. , He , S. , Walker , D. , Zhou , N. , Perez , D.R. , Mo , B. , Li , F. , Huang , X. , Webster , R.G. and Webby , R.J. 2003 . The influenza virus gene pool in a poultry market in South central china . Virology , 305 : 267 – 275 .
- Makarova , N.V. , Ozaki , H. , Kida , H. , Webster , R.G. and Perez , D.R. 2003 . Replication and transmission of influenza viruses in Japanese quail . Virology , 310 : 8 – 15 .
- Nguyen , D.C. , Uyeki , T.M. , Jadhao , S. , Maines , T. , Shaw , M. , Matsuoka , Y. , Smith , C. , Rowe , T. , Lu , X. , Hall , H. , Xu , X. , Balish , A. , Klimov , A. , Tumpey , T.M. , Swayne , D.E. , Huynh , L.P. , Nghiem , H.K. , Nguyen , H.H. , Hoang , L.T. , Cox , N.J. and Katz , J.M. 2005 . Isolation and characterization of avian influenza viruses, including highly pathogenic H5N1, from poultry in live bird markets in Hanoi, Vietnam, in 2001 . Journal of Virology , 79 : 4201 – 4212 .
- Perez , D.R. , Lim , W. , Seiler , J.P. , Yi , G. , Peiris , M. , Shortridge , K.F. and Webster , R.G. 2003 . Role of quail in the interspecies transmission of H9 influenza A viruses: molecular changes on HA that correspond to adaptation from ducks to chickens . Journal of Virology , 77 : 3148 – 3156 .
- Senne , D. , Pearson , J.E. and Panigrahy , B. 1992 . “ Live poultry markets: a missing link in the epidemiology of avian influenza ” . In Proceedings of the 3rd International Symposium on Avian Influenza , 50 – 58 . Madison, WI, USA .
- Songserm , T. , Jun-on , R. , Sae-Heng , N. , Meemak , N. , Hulse-Post , D. , Sturm-Ramirez , K. and Webster , R. 2006 . Domestic ducks and H5N1 influenza epidemic, Thailand . Emerging Infectious Diseases , 12 : 575 – 581 .
- Spackman , E. , Senne , D.A. , Bulaga , L.L. , Myers , T.J. , Perdue , M.L. , Garber , L.P. , Lohman , K. , Daum , L.T. and Suarez , D.L. 2003 . Development of real-time RT-PCR for the detection of avian influenza virus . Avian Diseases , 47 : 1079 – 1082 .
- Swayne , D. and Halvorson , D.A. 2003 . “ Influenza ” . In Diseases of Poultry , 11th edn , Edited by: Saif , Y. , Barnes , H. , Glisson , J. , Fadly , A. , McDougald , L. and Swayne , D. 135 – 160 . Ames : Iowa State Press .
- van der Goot , J.A. , de Jong , M.C. , Koch , G. and Van Boven , M. 2003 . Comparison of the transmission characteristics of low and high pathogenicity avian influenza A virus (H5N2) . Epidemiology and Infection , 131 : 1003 – 1013 .
- Webster , R.G. 2004 . Wet markets—a continuing source of severe acute respiratory syndrome and influenza? . Lancet , 363 : 234 – 236 .
- Wood , J.M. , Webster , R.G. and Nettles , V.F. 1985 . Host range of A/Chicken/Pennsylvania/83 (H5N2) influenza virus . Avian Diseases , 29 : 198 – 207 .
- World Organisation for Animal Health . 2005 . Chapter 2.7.12: avian influenza . In Manual of Diagnostic Tests and Vaccines for Terrestrial Animals . OIE. Available online at http://www.oie.int/Eng/Normes/Mmanuel/A__0037.htm (accessed 24 October 2008).
- Yee , K. , Carpenter , T.E. , Cardona , C.J. and Dao , P. 2007 . “ Prevalence of low pathogenic avian influenza in southern California live bird markets ” . In Proceedings of the 56th Western Poultry Disease Conference , 154 Las Vegas, NV, USA .
- Yee , K.S. , Carpenter , T.E. , Mize , S. and Cardona , C.J. 2008 . The live bird market system and low-pathogenic avian influenza prevention in Southern California . Avian Diseases , 52 : 348 – 352 .