Abstract
In a recent study, several US infectious laryngotracheitis virus (ILTV) strains and field isolates were genotyped by polymerase chain reaction and restriction fragment length polymorphism (PCR-RFLP) into nine different genotypes. All of the commercial poultry isolates were identified within genotypes IV, V, and VI. Based on the PCR-RFLP, Group IV isolates were characterized as genetically identical to the chicken embryo origin (CEO) vaccines, Group V as genetically closely related to the CEO vaccines, and Group VI as genetically different to the vaccine strains. The objective of this study was to determine the pathogenicity and growth characteristics of six ILTV commercial poultry isolates as compared with the CEO vaccine. Two isolates representative of PCR-RFLP Groups IV, V, and VI were selected. Differences in disease severity, viral tissue distribution in chickens, and plaque formation ability in cell culture were observed among viral genotypes IV, V, and VI, and between V-A and V-B isolates. Mild respiratory clinical signs were produced by IV-A, IV-B and the CEO vaccine, while VI-A and VI-B isolates produced severe respiratory signs and severe depression, and during the peak of clinical signs both isolates were re-isolated from the conjunctiva, sinus, trachea and thymus. Similarly to Group VI isolates, V-A and V-B produced severe respiratory signs, depression, and were re-isolated from conjunctiva, sinus, and trachea; on cell culture, both isolates produced significant larger plaques than any of the other isolates analysed. Overall, differences in pathogenicity and growth characteristics were observed among genetically closely related US ILTV isolates; however, complete genomes will be necessary to identify molecular determinants linked to the pathogenic viral phenotypes.
Introduction
Infectious laryngotracheitis is a highly contagious disease of chickens with a worldwide distribution that causes severe production losses due to increased morbidity, moderate mortality, decreased egg production, weight losses, and/or predisposition to other respiratory avian pathogens (Guy & Bagust, Citation2003). Infectious laryngotracheitis virus (ILTV) is a member of the genus Iltovirus, family Herpesviridae, subfamily Alphaherpesvirinae (Davison et al., Citation2005). The disease is characterized by acute respiratory signs, and is common in areas of intense poultry production. In the United States, two types of live-attenuated vaccines have been traditionally utilized to control infectious laryngotracheitis; vaccines attenuated by multiple passages in embryonated eggs (chicken embryo origin [CEO]) (Samberg et al., Citation1971); and the vaccine generated by multiple passages in tissue culture (tissue culture origin) (Gelenczei & Marty, Citation1965). In a recent study, several US field isolates were genotyped using polymerase chain reaction and restriction fragment length polymorphism (PCR-RFLP) (Oldoni & Garcia Citation2007). As previously found (Guy et al., Citation1989; Keller et al., Citation1992; Keeler et al., Citation1993), multiple PCR-RFLP analyses showed that most of the US isolates from commercial poultry were closely related to the CEO vaccines (Groups IV and V), while a third group of isolates (Group VI), also from commercial poultry, showed PCR-RFLP patterns different to the vaccine strains. Although only four out of the 73 ILTV-predicted open reading frames (Thureen & Keeler, Citation2006) were utilized to separate viral genotypes, this analysis provided the framework to compare the pathogenicity and growth characteristics of different ILTV genotypes. The pathogenicity of ILTV isolates has been evaluated measuring the trachea microscopic pathology induced by different ILTV isolates after intra-tracheal inoculation (Guy et al., Citation1990; Han et al., Citation2002). Most recently the pathogenicity of vaccines and field strains was evaluated considering clinical signs, body weight gain, and the presence of viral DNA in the trachea (Kirkpatrick et al., Citation2006). It was found that measurement of clinical signs and body weight were better indicators of ILTV strain pathogenicity than the trachea pathology index (Kirkpatrick et al., Citation2006). In addition, although not linked to pathogenicity, in vitro viral growth characteristics, such as plaque size formation, have been utilized to phenotypically distinguish among ILTV isolates (Russell & Turner, Citation1983).
The aim of the present study was to evaluate the pathogenicity and viral replication of ILTV isolates representative of PCR-RFLP genotype Groups IV, V, and VI as compared with the CEO vaccine, and to establish associations between viral genotypes and specific biological characteristics of current US isolates. The biological characterization of ILTV isolates was based on clinical signs, examination of viral replication in different tissues during the full course of the infection, and the plaque-forming ability of the different viral isolates in cell culture.
Materials and Methods
Viruses and cells
The commercial ILTV vaccine strain Trachivax® (Schering Plough, Millsboro, Delaware, USA) and six ILTV isolates were used in this study. Strains and isolates were selected based on their different PCR-RFLP characterization as previously described (Oldoni & Garcia, Citation2007). Field isolates from PCR-RFLP Groups IV, V, and VI were identified as follows: IV-A for isolate 301/K/06/BR, IV-B for isolate 10/C/97/BR, V-A for isolate 19/F/05/BR, V-B for isolate 402/A/06/BR, VI-A for isolate 417/A/06/BR, and VI-B for 2/A/04/BR. These six field isolates were recovered from the upper respiratory tract of chickens during outbreaks of the disease characterized by severe morbidity and moderate to high mortality. All field isolates were pre-incubated with adenovirus and reovirus antisera (NVSL, Ames, Iowa, USA), and were then propagated on the chorioallantoic membrane of 10-day-old chicken embryos using standard techniques (Tripathy, Citation1998). To confirm that viral stocks were free of adenovirus, reovirus, and chicken anaemia virus, the viral stocks were tested for adenovirus (Meulemans et al., Citation2001), chicken anaemia virus (Todd et al., Citation1992), and reovirus (Kapczynski et al., Citation2002) by PCR. The third chorioallantoic membrane passage for the six field isolates was titrated in chicken kidney (CK) cells. CK cells were also utilized to expand the USDA reference strain as previously described by Hughes & Jones (Citation1988). The viral plaque assay was performed in the chicken liver tumour cell line LMH (Kawaguchi et al., Citation1987), as previously described by Devlin et al. (Citation2006).
Virus titration
The CEO vaccine, the USDA reference strain, and field isolates were titrated in 96-well plates using a CK cell concentration of 8 x 105 cells/ml. Viral inoculums were diluted from 10−1 to 10−9, and five replicates of each dilution were inoculated onto CK cells. After 5 days of incubation at 37°C, plates were evaluated for the presence of the ITLV cytopathic effect (CPE). The Reed and Muench method was utilized to estimate the 50% tissue culture infective dose (TCID50).
Experimental design
One hundred and ninety-two White Leghorn specific pathogen free chickens were divided into eight groups of 24 birds each. Birds were housed in individual stainless steel isolation units with filtered air and positive pressure at the Poultry Diagnostic and Research Center (Athens, Georgia, USA), and were fed a standard diet and water ad libitum. At 4 weeks of age, seven groups of birds were inoculated with the six selected field isolates and the CEO vaccine. Each of the six field isolates was applied at a final dose of 102.5 TCID50 per chicken. The CEO vaccine was applied at the dose recommended by the manufacturer (Trachivax®; Schering Plough) of approximately 104.3 TCID50 per chicken. Four aliquots of 50 µl were administered per chicken in each eye and nostril. In the same room a group of 24 birds was inoculated with phosphate-buffered saline solution (PBSS) in a similar fashion and kept as the negative control in a separate unit.
Scoring of clinical signs
Video recording, 5 min per group of chickens, was performed daily from day 1 to day 14 post inoculation. Videos were analysed daily and clinical signs were scored on the basis of breathing patterns, conjunctivitis, the level of depression, and mortality observed per unit per day. A daily total clinical sign score was calculated for each unit. Dyspnea was scored on a scale of 0 (normal breathing), 1 (mild dyspnea and open mouth breathing) and 2 (gasping with an extended neck). The condition of the conjunctiva was scored on a scale of 0 (normal), 1 (swollen and/or partial closure of the eyes) and 2 (complete closure of the eyes). The level of depression was scored on a scale of 0 (normal, or not depressed), 1 (depressed) and 2 (severely depressed). For mortality, the score was 0 (no mortality) and 3 (mortality). Statistical analysis was performed to compare clinical sign scores at different days post inoculation (d.p.i.) within each group. Multiple comparisons with baseline values were made using a Bonferroni correction to limit the overall type-I error rate to 5%.
Sample collection and processing
Samples were collected from two chickens at 2, 4, 5, 7, 9, 11 and 14 d.p.i. from chickens inoculated with the IV-A, IV-B, V-A, V-B, VI-A, VI-B isolates, CEO vaccine, and sterile PBSS. Two birds from each group were sacrificed by carbon dioxide gas inhalation at 2, 4, 5, 7, 9, 11 and 14 d.p.i. The remaining 10 birds were sacrificed at 15 d.p.i. Sinuses and conjunctiva swabs were collected and placed in 1 ml sterile PBSS containing a 2% antibiotic–antimycotic 100X (Gibco, Grand Island, New York, USA) and 2% newborn calf serum (Gibco). The trachea was dissected from the larynx to the bronchial bifurcation, and was cut longitudinally; one-half was collected in buffered formalin, and the epithelium of the other half was scraped and re-suspended in 1 ml PBSS. The head was removed and the trigeminal ganglia extracted. After extraction, the trigeminal ganglia was collected and minced in 1 ml PBSS. The caecal tonsils were dissected and cut longitudinally, washed, minced, and re-suspended in 1 ml PBSS. One lung was extracted and a lung section was minced, and re-suspended in 1 ml PBSS. At 5, 7 and 9 d.p.i., the bursa, thymus and spleen were extracted and sections of these tissues were collected in 1 ml PBSS as described for other tissues. All samples collected in PBSS were stored at −80°C until processing for virus isolation and DNA extraction. Samples were frozen and thawed three times, thawed at 37°C, frozen at −80°C, and subjected to centrifugation for 2 min at 7500×g. before tissue culture inoculation.
Transverse segments of the larynx/trachea, eyelid and thymus were fixed in buffered formalin for 48 h, embedded in paraffin and stained with haematoxylin and eosin for histopathological examinations.
Virus isolation
Virus isolation was performed in adult CK cells as previously described by Rodríguez-Avila et al. (Citation2007). The bursa, thymus, spleen, lung, trigeminal ganglia, trachea and caecal tonsil tissue suspensions, and the conjunctiva, sinuses, and cloacal swab suspensions were inoculated directly into CK cells and incubated at 37°C, 5% CO2 for 5 days. Each sample was inoculated in duplicate, and all samples were passed blindly three times in CK cells. Samples were considered positive by virus isolation when the CPE characteristic of ILTV was observed, and were considered negative after three passages without detecting ILTV CPE.
DNA extraction and real-time PCR Taqman® assay
DNA extraction from tissues and swab suspensions was performed using the MegaZorb DNA Mini-prep 96-weel kit (CORTEX BIOCHEM™; San Leandro, California, USA) according to the manufacturer's recommendations. The real-time PCR Taqman® assay (ReTi-PCR) was executed as previously described (Callison et al., 2007). The primers and probe are located in the viral glycoprotein C gene were synthesized by IDT (Coralville, Iowa, USA) and BioSearch Technologies (Novato, California, USA), respectively. The final amplification reaction volume was 25 µl, including 12.5 µl of 2x master mix (Quantitect Probe PCR kit; Qiagen, Valencia, California, USA), primers to a final concentration of 0.5 µM, probe to a final concentration of 0.1 µM, 1 µl HK-UNG (Epicentre, Madison, Wisconsin, USA), 2 µl water, and 5 µl DNA template. The reaction was amplified with the Smart Cycler (Cepheid, Sunnyvale, California, USA) using a programme of 50°C for 2 min, 95°C for 15 min, and 40 cycles of 94°C for 15 sec, 60°C for 60 sec with optics on. For each ReTi ILTV assay reaction, the threshold cycle number was determined to be the PCR cycle number at which the fluorescence of the reaction exceeded 30 units of fluorescence. The genome copy number (GCN) per amplification reaction was expressed as the log10 value and was estimated using the standard curve equation (y= − 0.289x+12.487) generated from a plasmid containing the glycoprotein C gene. GCN values per reaction were estimated for samples with threshold cycle number ≤38.00. The GCN log10 value reported per sample was either the average of two samples when viral DNA was detected in both, or the value obtained for one sample in order to illustrate the virus replication dynamics during the full course of the infection.
Plaque formation ability
Aliquots of isolates IV-A, IV-B, V-A, V-B, VI-A, and VI-B, CEO vaccine, and the USDA reference strain were re-suspended in 0.5 ml modified Eagle's medium with 5% calf serum to a titre between 102.5 and 103.0 TCID50/ml and inoculated in triplicate onto 50% to 60% confluent LMH cell monolayers in six-well plates. After inoculation, the virus was adsorbed for a period of 2 h at room temperature, the supernatant was removed and replaced with 2.5 ml methylcellulose overlay medium containing 5% calf serum, and the plates were incubated for 96 h post inoculation. After incubation, cells were fixed with 2% formaldehyde for 1 h at room temperature. Plates were washed with running water and stained with 1% crystal violet in 50% ethanol for 15 min at room temperature. After staining, plates were washed in running water and dried. The diameters (µm) for 100 plaques induced by each virus were measured using the ImageJ software (http://rsb.info.nih.gov/ij). The percentage of plaques within different size ranges were calculated and plotted for each viral isolate, the CEO vaccine and the USDA strain. The mean plaque diameter for each virus was calculated and compared using the statistical analyses of variance (ANOVA-Tukey).
Results
Clinical signs
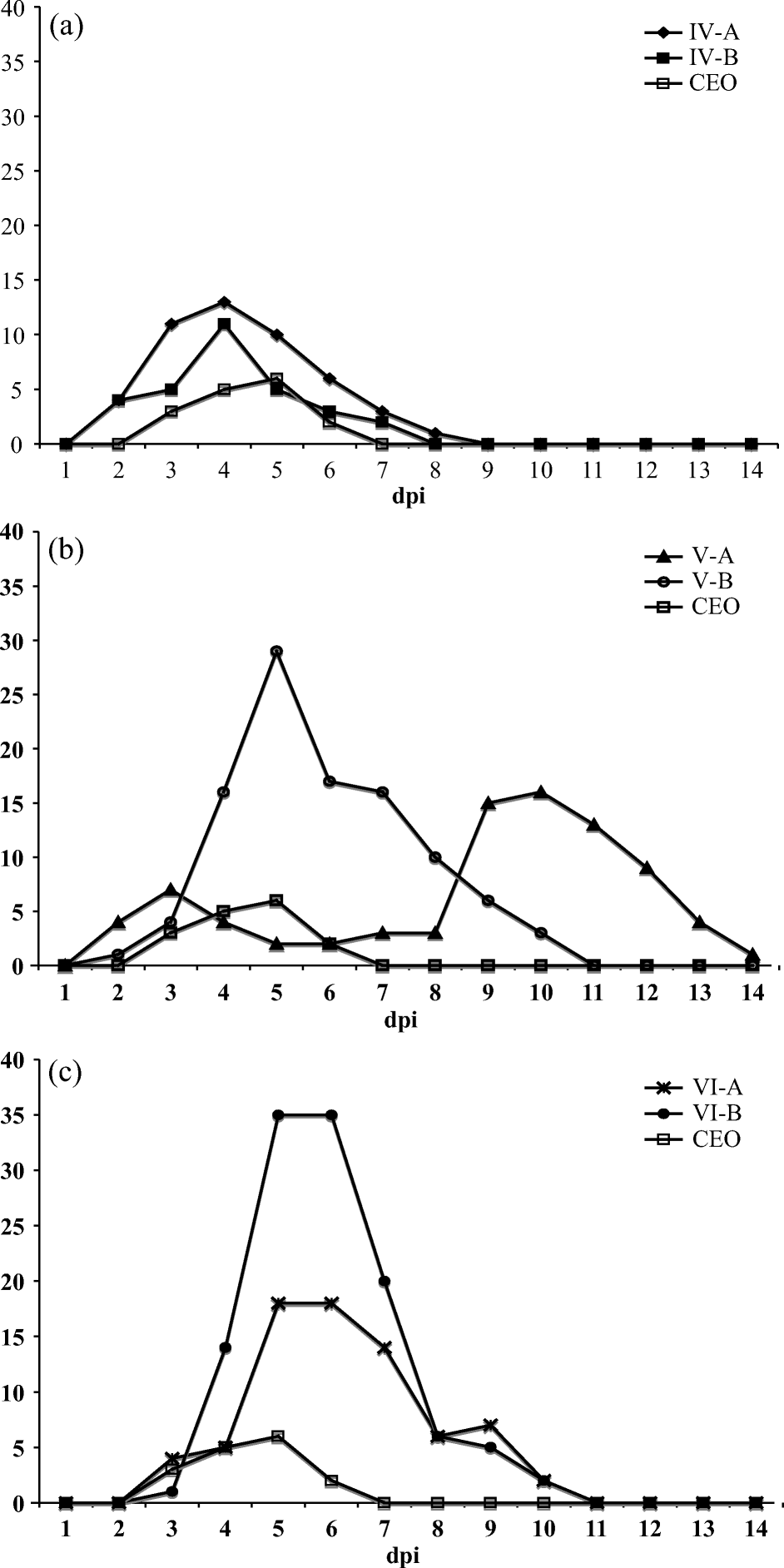
Total clinical sign scores were recorded daily per group and are summarized in a for isolates IV-A and IV-B, in b for isolates V-A and V-B, and in c for isolates VI-A and V1-B as compared to the CEO vaccine. Groups of chickens inoculated with IV-A and IV-B isolates showed mild to moderate dyspnea with open mouth breathing, and low to moderate depression from 3 to 5 d.p.i. (a). Groups of chickens inoculated with isolates V-B, VI-A and VI-B showed severe dyspnea, mild to severe conjunctivitis, and severe depression between 4 and 7 d.p.i. (b and 1c). Chickens inoculated with the V-A isolate showed severe dyspnea, mild conjunctivitis, and severe depression between 9 and 12 d.p.i. (b). The group of chickens inoculated with the CEO vaccine showed only mild conjunctivitis and mild depression, and clinical signs cleared by day 7 d.p.i. In chickens infected with IV-A and IV-B, clinical signs cleared by 8 d.p.i. (a); in chickens infected with V-B (b), VI-A and VI-B (c), clinical signs cleared by 11 d.p.i.; while chickens infected with V-A virus showed clinical signs, particularly depression, up to 14 d.p.i. (b). The peak scores of total clinical signs ranged from 18 to 35 for chickens infected with V-B, VI-A and VI-B isolates (b and 1c), while groups infected with IV-A and IV-B isolates reached total clinical signs scores of 11 to 13, respectively (a). A single mortality was recorded for the V-A-infected group of chickens at 12 d.p.i. The mean rank clinical signs scores averaged for all viral groups, from 3 to 9 d.p.i., differed significantly from the mean rank clinical signs scores of all groups averaged on 1 d.p.i. (Friedman test, P<0.001). This is indicative that the appearance and disappearance of clinical signs for each individual isolate was significantly different. Statistical analysis of clinical signs scores between isolates was precluded because scores were recorded as a group-level measurement, rather than measurement on individual birds per group, and consequently did not provide information on within-group variation.
Real-time PCR Taqman® assay
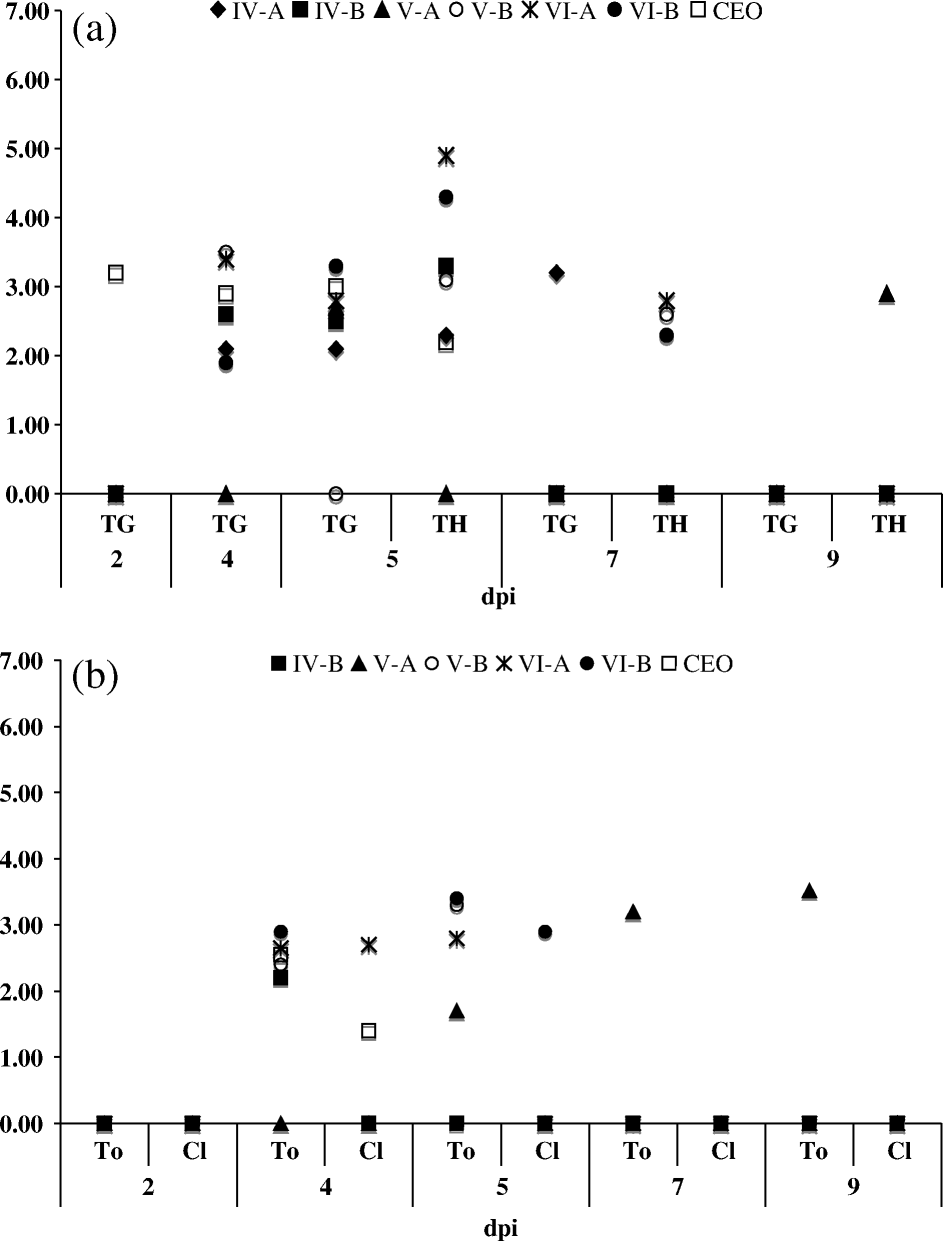
In all infected groups, viral DNA was detected in the conjunctiva, sinuses, trachea, trigeminal ganglia, caecal tonsils, thymus and cloaca. No viral DNA was detected from the bursa, spleen and lung. Viral DNA was detected most consistently, between 2 and 9 d.p.i., in the conjunctiva to sinuses, followed by the trachea. shows the average amount of viral DNA, expressed in GCN log10 per reaction, detected in samples of conjunctiva (a), sinus (b), and trachea (c) for all groups of infected chickens at 2, 4, 5, 7 and 9 d.p.i. The peak of viral replication for isolates IV-A, IV-B, V-B, VI-A and VI-B, and the CEO vaccine was determined between 4 to 5 d.p.i. During this period, the samples tested had an average of 4 to 6 GCN log10 viral DNA per reaction in the conjunctiva (a) and the sinuses (b), while an average of 2 to 4 GCN log10 viral DNA was detected in the trachea (c). Different to other isolates, the peak of viral replication in the conjunctiva, sinuses, and trachea for isolate V-A was at 9 d.p.i. with an average of 6 GCN log10 viral DNA detected in the trachea (c).
Figure 2. Average GCN log10 value detected per sample by ReTi-PCR (quantitative PCR). (2a) Conjunctiva, (2b) sinuses and (2c) trachea of chickens inoculated with isolates IV-A, IV-B, V-A, V-B, VI-A and VI-B and CEO vaccine.
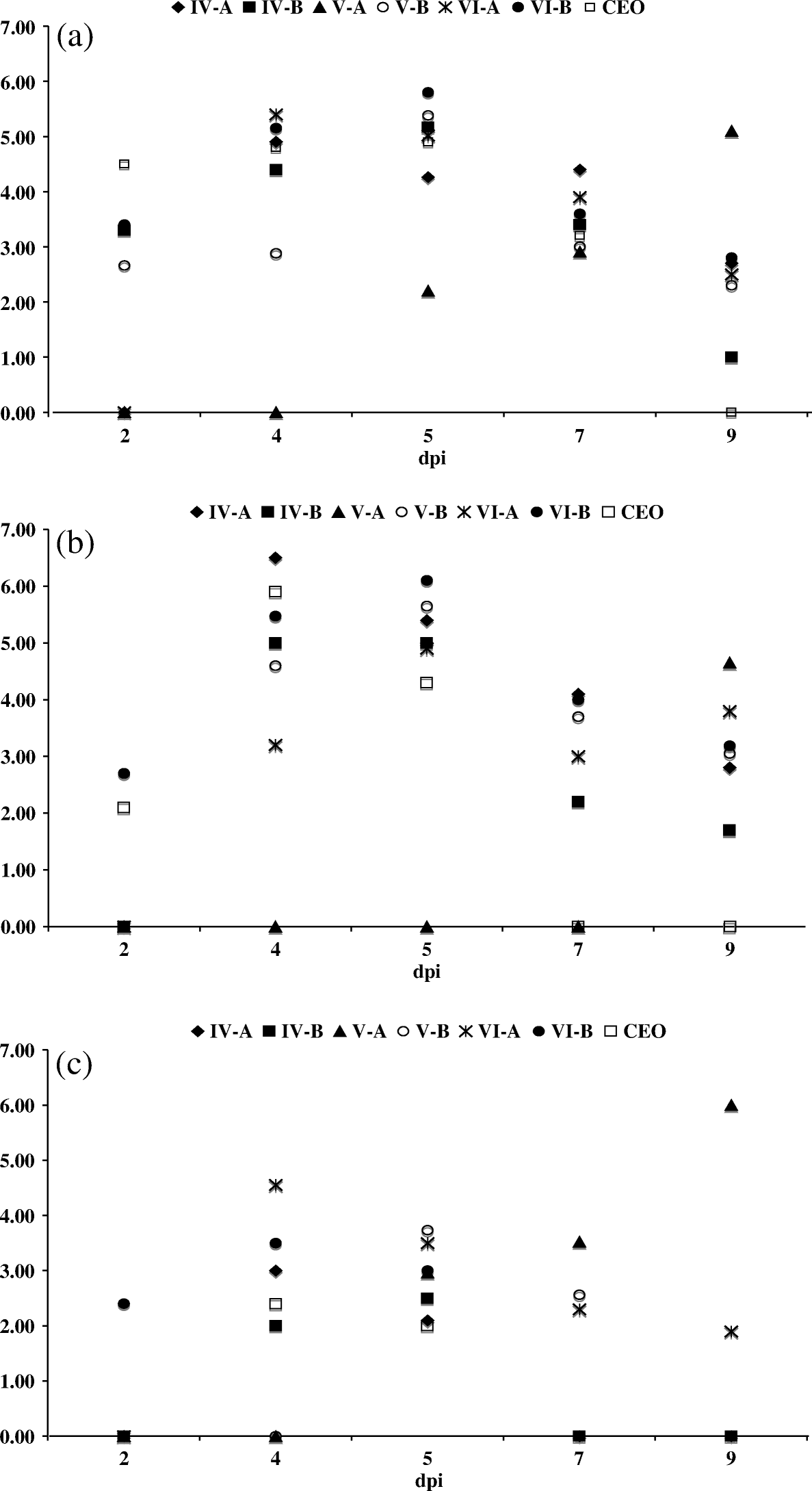
Average viral DNA ranging from 2 to 3.5 GCN log10 per reaction was detected in the trigeminal ganglia during 4 and 5 d.p.i. for all isolates and the CEO vaccine-infected chickens (a). In the thymus, viral DNA was detected at 5 d.p.i. for all groups of infected chickens, with the exception of V-A-infected chickens where viral DNA was detected in the thymus at 9 d.p.i. In the caecal tonsils, with the exception of IV-A-infected chickens, viral DNA ranging from 1.5 to 3.0 GCN log10 per reaction were detected for all isolates and the CEO vaccine-infected chickens between 4 and 5 d.p.i. (b). Sporadic detection of viral DNA in the cloaca was observed but it was not consistent for all of the infected groups (b). Parallel samples collected at similar times post vaccination from PBSS-inoculated birds were all negative by Re-Ti PCR (data not shown).
Virus isolation
Virus was isolated from the conjunctiva and sinus of all infected chickens, from the trachea of chickens infected with V-A, V-B, VI-A and VI-B, and from the thymus of chickens infected with VI-A and VI-B. No virus was isolated from the lung, spleen, bursa, caecal tonsils, cloaca, and the trigeminal ganglia. presents the total number of virus isolations obtained from the conjunctiva, sinus, trachea and thymus for all infected groups. Seventy per cent of the positive virus isolations showed viral CPE at the first passage, 28% at the second passage and 2% at the third passage in CEK cells. All virus isolation-positive samples were collected between 2 and 9 d.p.i.; no virus isolations were recorded for samples collected at 11 and 14 d.p.i. All samples collected from chickens inoculated with PBSS were negative by virus isolation after three passages in CK cells.
Table 1. Virus isolation results on adult CK cells
Histopathology examination
Minimal to mild lesions on the mucus glands and epithelium, as well as lymphocytic infiltrates, were observed in the trachea for all ILTV-infected groups. Minimal to mild lesions in the trachea were observed between 4 and 9 d.p.i. for chickens inoculated with IV-A, IV-B, V-B, VI-A and VI-B isolates, and the CEO vaccine. Heterophilic infiltrates in the lamina propria with oedema, haemorrhage and the presence of syncytial cells with intranuclear inclusion bodies were observed exclusively, at 9 d.p.i., in the trachea of chickens inoculated with the V-A isolate. Syncytial cells with intranuclear inclusion bodies and fibrinoheterophilic infiltrate were also observed in the larynx of chickens inoculated with IV-B, V-A, V-B, VI-A, and VI-B isolates. In the conjunctiva, all infected groups presented syncytial cells with intranuclear inclusion bodies and lymphoheterophilic blepharoconjunctivitis indicating active viral replication. In addition, chickens infected with the V-A isolate presented catarrhal conjunctivitis. Analysis of the thymus and the trigeminal ganglia from chickens infected with the six isolates and the CEO vaccine did not present significant lesions indicative of viral replication. No lesions indicative of infection were observed in the tissues of chickens inoculated with PBSS.
Plaque formation ability
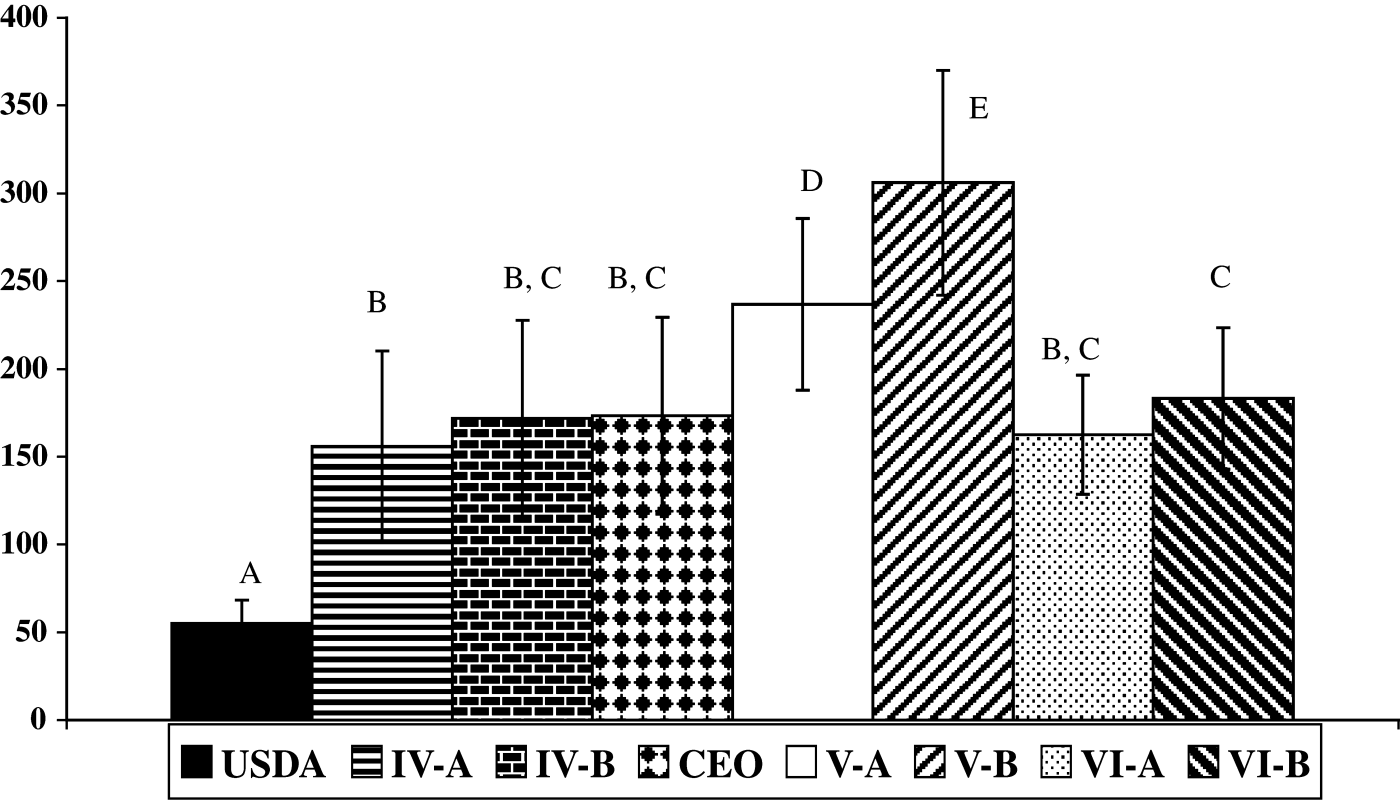
The average plaque size for each isolate, the CEO vaccine and the USDA strain are shown in . No significant differences were observed among the mean plaque diameter calculated for IV-A isolate (156±54 µm), IV-B isolate (172±56 µm), CEO vaccine (173±564 µm), VI-A isolate (163±34 µm), and VI-B isolate (183±40 µm). In contrast, significant differences were observed between the average plaque diameters calculated for isolates V-A (237±49 µm) and V-B (306±64 µm). Overall the V-A and V-B isolates produced significant larger plaques than any of the viruses analysed (, P<0.05). The USDA reference strain produced a significantly smaller mean plaque diameter (55±13 µm) than the other field and vaccine viruses.
Discussion
This study presents the biological characterization of US ILTV isolates from commercial poultry representative of PCR-RFLP Groups IV, V, and VI, as compared with the CEO vaccine. All field isolates used in this study were able to produce mild to severe clinical signs of the disease when inoculated at a dose of 102.5 TCID50 per chicken. Analysis of total clinical signs scores reflected different levels of disease severity. Isolates IV-A, IV-B and the CEO vaccine produced milder disease than V-A, V-B, VI-A, and VI-B. The duration of clinical signs varied among isolates, with V-A isolate presenting the longest duration of clinical signs among all viruses evaluated. In contrast to results from a previous pathogenicity study, where the mortality rate reached 100% (Kirkpatrick et al., Citation2006), in this study mortality was not significant, severe clinical signs cleared 11 d.p.i., and the rate of viral isolations from the conjunctiva and sinus was higher than from the trachea. The lack of mortality and reduced trachea replication observed for isolates IV-A and IV-B, the CEO vaccine can be attributed to the combined eye–nostril route of inoculation and the lower dose (102.5 TCID50 per chicken) administered. In an earlier study the main sites of virus replication (after eye-drop inoculation) were the conjunctiva, the sinuses, and, to a lesser degree, the trachea (Robertson & Egerton, Citation1981). However, in a more recent study with the CEO vaccine, active viral replication in the trachea was detected 2 to 5 days post vaccination by eye-drop application (Rodríguez-Avila et al., Citation2007). In agreement with previous reports (Robertson & Egerton, Citation1981; Kirkpatrick et al., Citation2006), the severity of clinical signs observed did not correlated with trachea pathology since no microscopic lesions were observed in the tracheas, and the typical syncytia and inclusion body formation characteristic of ILTV were restricted to the conjunctiva and the larynx of chickens inoculated with V-B, VI-A, VI-B isolates. Only chickens inoculated with the V-A isolate presented syncytia and inclusion bodies in the trachea, which may have contributed to the longer duration and slower clearance of clinical signs for this group.
In addition to the conjunctiva, sinus, and trachea, viral DNA was detected for all ILTV-infected groups in the thymus at day 5 d.p.i., but only the VI-A and VI-B isolates were recovered from the thymus. However, histopathological analysis indicated no active viral replication in the thymus. The occasional detection of viral DNA and isolation of virus from the thymus suggest that this site may play a role on the pathogenicity of ILTV infection. However, further studies need to be conducted to determine whether the thymus is a site where the virus persists during sub-acute stages of infection.
As previously reported by Williams et al. (Citation1992) ), in the present study viral DNA of all field isolates and the CEO vaccine was detected in the trigeminal ganglia, indicating the migration of the virus to the nervous system and the establishment of a latent infection. The CEO vaccine was detected in the trigeminal ganglia as early as 2 d.p.i. (a). In a recent study (Rodríguez-Avila et al., Citation2007) viral DNA was detected in the trigeminal ganglia of birds 4 days after contact exposure to CEO origin-vaccinated pen mates. Together these results further confirm the involvement of the trigeminal ganglia during the early pathogenesis of ILTV infection (Bagust, Citation1986). With the exception of chickens infected with the IV-A isolate, viral DNA was detected in the caecal tonsils of all infected groups. The detection of viral DNA in the caecal tonsils coincided with the peak of viral replication in the conjunctiva, sinuses and trachea. One possibility is that macrophages and/or other cells of the immune system transported viral particles to the caecal tonsils. It has been reported that different strains of ILTV have the ability to infect macrophages at different rates in vitro (Calnek et al., Citation1986). Plaque formation ability in LMH cells for isolates V-A and V-B was characterized by large plaque formation, suggesting that this group of isolates is more efficient in cell-to-cell spread than the other commercial poultry isolates and the CEO vaccine. Therefore, isolates V-A and V-B not only differed on their clinical presentation (duration of clinical signs), but also in their tissue culture growth characteristics. These results suggest that, although categorized in the same PCR-RFLP group, these isolates are phenotypically different, emphasizing that an extensive biological variability may exist if we examined a larger pool of isolates. Sequence differences between V-A and V-B isolates have been detected in the glycoprotein B (data not shown); however, the association of gB gene genetic changes with in vitro and in vivo characteristics of Group V isolates remains to be studied. Based on PCR-RFLP analysis, the IV-A and IV-B isolates are genetically similar to the CEO vaccine. In this study it was evident that they share in vivo and in vitro characteristics with this vaccine strain. On the other hand, viral genotypes V and VI are genetically different; however, they share similar pathogenicity traits. An indication that the genomic differences identified for isolates within PCR-RFLP Groups V and VI are probably not associated with their pathogenicity, but may be associated with their different in vitro growth characteristics. We are aware that six isolates are not representative of the biological diversity that may exist among circulating ILTV isolates. As it was observed for V-A and V-B isolates, biological differences are present among isolates within the same genotype. Therefore, it is relevant to obtain complete genome sequences of multiple viral isolates representative of the PCR-RFLP genotypes in order to clearly identify molecular determinants of pathogenicity.
Acknowledgements
This project was sponsored by University of Georgia Veterinary Medical Agricultural Research Funds.
References
- Bagust , T.J. , Calnek , B.W. and Fahey , K.J. 1986 . Gallid-1 herpesvirus infection in the chicken. 3. Reinvestigation of the pathogenesis of infectious laryngotracheitis in acute and early post-acute respiratory disease . Avian Diseases , 30 : 179 – 190 .
- Callison , S.A. , Riblet , S.M. , Oldoni , I. , Sun , S. , Zavala , G. , Williams , S. , Resurreccion , R.S. , Spackman , E. and García , A. 2006 . Development and validation of a real-time Taqman® PCR assay for the detection and quantitation of infectious laryngotracheitis virus in poultry . Journal of Virological Methods , 50 : 537 – 544 .
- Calnek , B.W. , Fahey , K.J. and Bagust , T.J. 1986 . In vitro infection studies with infectious laryngotracheitis virus . Avian Diseases , 30 : 327 – 336 .
- Davison , A.J. , Eberle , R. , Hayward , G.S. , Mcgeoch , D.J. , Minson , A.C. , Pellet , P.E. , Roizman , B. , Studdert , M.J. and Thiry , E. 2005 . “ Herpesviridae ” . In Virus Taxonomy: Eighth Report of the International Commitee on Taxonomy of Viruses , Edited by: Fauquet , C.M. , Mayo , M.A. , Maniloff , J. , Desselberg , U. and Ball , L.A. 193 – 212 . San Diego : Elsevier Academic Press .
- Devlin , J.M. , Browning , G.F. , Hartley , C.A. , Kirkpatrick , N.C. , Mahmoudian , A. , Noormohammadi , A.H. and Gilkerson , J.R. 2006 . Glycoprotein G is a virulence factor in infectious laryngotracheitis virus . Journal General Virology , 87 : 2839 – 2847 .
- Gelenczei , E.F. and Marty , E.W. 1965 . Strain stability and immunologic characteristics of a tissue-culture-modified infectious laryngotracheitis virus . Avian Diseases , 14 : 44 – 56 .
- Guy , J.S. and Bagust , T.J. 2003 . “ Laryngotracheitis ” . In Diseases of Poultry , Edited by: Saif , Y.M. , Barnes , H.J. , Glisson , J.R. , Fadly , A.M. , Mcdougald , L.R. and Swayne , D.E. 121 – 134 . Ames : Iowa State University Press .
- Guy , J.S. , Barnes , H.J. , Munger , L.L. and Rose , L. 1989 . Restriction endonuclease analysis of infectious laryngotracheitis viruses: comparison of modified-live vaccine viruses and North Carolina field isolates . Avian Diseases , 33 : 316 – 323 .
- Guy , J.S. , Barnes , H.J. and Morgan , L.M. 1990 . Virulence of infectious laryngotracheitis viruses: comparison of modified-live vaccine viruses and North Carolina field isolates . Avian Diseases , 34 : 106 – 113 .
- Han , M.G. , Kweon , C.H. , Mo , I.P. and Kim , S.J. 2002 . Pathogenicity and vaccine efficacy of a thymidine kinase gene deleted infectious laryngotracheitis virus expressing the green fluorescent protein gene . Archives of Virology , 147 : 1017 – 1031 .
- Hughes , C.S. and Jones , R.C. 1988 . Comparison of cultural methods for primary isolation of infectious laryngotracheitis virus from field materials . Avian Pathology , 17 : 295 – 303 .
- Kapczynski , D.R. , Sellers , H.S. , Simmons , V. and Schultz-Cherry , S. 2002 . Sequence analysis of the S3 gene from a turkey reovirus . Virus Genes , 25 : 95 – 100 .
- Kawaguchi , T. , Nomura , K. , Hirayama , Y. and Kitagawa , T. 1987 . Establishment and characterization of a chicken hepatocellular carcinoma cell line, LMH . Cancer Research , 47 : 4460 – 4464 .
- Keeler , C.L. Jr , Hazel , J.W. , Hastings , J.E. and Rosenberger , J.K. 1993 . Restriction endonuclease analysis of Delmarva field isolates of infectious laryngotracheitis virus . Avian Diseases , 37 : 418 – 426 .
- Keller , L.H. , Benson , C.E. , Davison , S. and Eckroade , R.J. 1992 . Differences among restriction endonuclease DNA fingerprints of Pennsylvania field isolates, vaccine strains, and challenge strains of infectious laryngotracheitis virus . Avian Diseases , 36 : 575 – 581 .
- Kirkpatrick , N.C. , Mahmoudian , A. , Colson , C.A. , Devlin , J.M. and Noormohammadi , A.H. 2006 . Relationship between mortality, clinical signs and tracheal pathology in infectious laryngotracheitis . Avian Pathology , 35 : 449 – 453 .
- Meulemans , G. , Boschmans , M. , Van Den Berg , T.P. and Decaesstecker , M. 2001 . Polymerase chain reaction combined with restriction enzyme analysis for detection and differentiation of fowl adenoviruses . Avian Pathology , 30 : 655 – 660 .
- Oldoni , I. and Garcia , M. 2007 . Characterization of infectious laryngotracheitis virus isolates from the US by polymerase chain reaction and restriction fragment length polymorphism of multiple genome regions . Avian Pathology , 36 : 167 – 176 .
- Robertson , G.M. and Egerton , J.R. 1981 . Replication of infectious laryngotracheitis virus in chickens following vaccination . Australian Veterinary Journal , 57 : 119 – 123 .
- Rodríguez-Avila , A. , Oldoni , I. , Riblet , S.M. and García , M. 2007 . Replication and transmission of live-attenuated Infectious laryngotracheitis virus (ILTV) vaccines . Avian Diseases , 51 : 905 – 911 .
- Russell , R.G. and Turner , A.J. 1983 . Characterization of infectious laryngotracheitis viruses, antigenic comparison by kinetics of neutralization and immunization studies . Canadian Journal of Compendium Medicine , 47 : 163 – 171 .
- Samberg , Y. , Cuperstein , E. , Bendheim , U. and Aronovici , I. 1971 . The development of a vaccine against avian infectious laryngotracheitis. IV. Immunization of chickens with a modified laryngotracheitis vaccine in the drinking water . Avian Diseases , 15 : 413 – 417 .
- Thureen , D.R. and Keeler , C.L. Jr . 2006 . Psittacid herpesvirus 1 and infectious laryngotracheitis virus: Comparative genome sequence analysis of two avian alphaherpesviruses . Journal of Virology , 80 : 7863 – 7872 .
- Todd , D. , Mawhinney , K.A. and Mcnulty , M.S. 1992 . Detection and differentiation of chicken anemia virus isolates by using the polymerase chain reaction . Journal of Clinical Microbiology , 30 : 1661 – 1666 .
- Tripathy , D.N. 1998 . “ Infectious laryngotracheitis ” . In A Laboratory Manual for the Isolation and Identification of Avian Pathogens , Edited by: Swayne , D.E. , Glisson , J.R. , Jackwood , M.W. , Pearson , J.E. and Reed , W.M. 11 – 115 . Kennett Square , PA : American Association of Avian Pathologists .
- Williams , R.A. , Bennett , M. , Bradbury , J.M. , Gaskell , R.M. , Jones , R.C. and Jordan , F.T. 1992 . Demonstration of sites of latency of infectious laryngotracheitis virus using the polymerase chain reaction . Journal of General Virology , 73 : 2415 – 2420 .