Abstract
Histomonosis or blackhead is a disease of gallinaceous birds, caused by the protozoan Histomonas meleagridis. As recent regulatory action has removed almost all drugs against this disease from the European market, the development of new prophylactics has become crucial. Identification of the protective immune mechanism would facilitate the choice and development of a vaccination strategy to prevent histomonosis. In this study, turkeys were either actively or passively immunized and were then challenged to assess the role of antibody-mediated immunity in the protection form this disease. Active immunization was performed either by experimental infection and treatment or by intramuscular injection with lysed H. meleagridis. Passive immunization was attempted by intraperitoneal administration of pooled, concentrated, neutralizing antisera from immunized donor animals to naïve turkeys. A significantly higher IgG response was observed after infection and treatment than after intramuscular injection, which in turn was higher than the responses of placebo and control birds. While active immunization of turkeys by intramuscular injection of dead H. meleagridis antigens appeared not to be protective against histomonosis, immunization by infection and treatment did induce protection. However, no significant level of protection could be observed in the passively immunized birds. These results suggest that serum antibodies to H. meleagridis may not be a key component in the protection against this parasite. It is, however, possible that the concentration of antibodies at the mucosal site is insufficient. Therefore, further investigation on mucosal immune responses is necessary.
Introduction
Histomonosis or blackhead—a disease of gallinaceous birds, especially of turkeys—is caused by the protozoan parasite Histomonas meleagridis. The disease is characterized by necrotic foci in the liver, thickening and ulceration of the caecal wall and sulphur-coloured droppings. Mortality and morbidity may reach 100% in turkeys, while the disease is much less severe in chickens (McDougald, Citation1991). Due to recent regulatory action, a registered product for the prevention or treatment of blackhead is no longer available in the European Union. This has encouraged the search for alternative products, such as a vaccine against H. meleagridis.
In the past, it has been established that the parasite causes higher mortality in young turkeys than in adults, demonstrating the possibility of immunity against H. meleagridis in the older birds (Tyzzer, Citation1934). However, immunization by intramuscular or intravenous injections with diseased tissue emulsions failed to induce protection against histomonosis (Higgins, Citation1915; Tyzzer et al., Citation1921). Recently, intramuscular vaccination with inactivated, cloned H. meleagridis was also unable to cause protection (Hess et al., Citation2008). In contrast, attempts to vaccinate turkeys with in vitro attenuated H. meleagridis have led to partial or even complete protection (Tyzzer, Citation1933, Citation1934, Citation1936; Lund et al., Citation1966; Hess et al., Citation2008), although the effector mechanisms of this protective immunity remain unclear. In 1963, Clarkson investigated the protective value of antibodies against this parasite. He passively immunized naïve birds with antisera collected from infected and treated birds. His results suggested that antibodies do not protect birds from histomonosis, although only a semi-quantitative analysis of the immune response was conducted and specific antibody titres were not measured (Clarkson, Citation1963). Hence, the objective of the present study was to determine the strength and protective value of an antibody response against H. meleagridis.
Materials and Methods
Turkeys and parasites
All animal experiments were approved by the Ethical Committee for Animal Experiments of the K.U.Leuven, according to the international regulations (project number P05098). Commercial turkeys (breed BIG6) were obtained as 1-day-old poults and housed on litter in a room with high effficiency particulate arresting (HEPA)-filtered air. They had free access to food and water.
To immunize and challenge the turkeys, H. meleagridis strain mdc (Bleyen et al., Citation2007) was cultured as described by McDougald & Galloway (Citation1973). Microscopic analysis and a diagnostic polymerase chain reaction (Bleyen et al., Citation2007) were performed to confirm the presence of H. meleagridis.
Experiment 1: analysis of protection after active immunization
Thirty-four turkeys were randomly divided into four groups. Two groups acted as control: an uninfected, non-immunized control (UNC, four birds) and an infected, non-immunized controls (INC, 10 birds). Two groups of 10 turkeys were immunized against H. meleagridis either by infection and treatment (IC) or by intramuscular immunization with H. meleagridis antigens (IM).
At 21 days of age (0 days post immunization [d.p.i.]), group IC was infected via the cloaca with 2×105 H. meleagridis protozoa, as described by McDougald & Fuller (Citation2005). Six days later, as clinical signs were showing, the birds were treated with ronidazole 10 mg/kg (Tricho Plus, Oropharma, Maastricht, The Netherlands). Also at 0 d.p.i., group IM was immunized intramuscularly with H. meleagridis antigens. The antigens were prepared by homogenizing infected caeca and foci of the liver in 0.9% NaCl solution (“physiological saline”) and lysing the cells (2×105 H. meleagridis/ml) by sonication. Protein concentrations were determined with a protein assay using bicinchoninic acid for the colourimetric detection and quantitation. (Pierce, Rockford, Illinois, USA). Each bird received 250 µg of protein, emulsified in Freund's incomplete adjuvant (Sigma, Steinheim, Germany). Three weeks later (20 d.p.i.), a booster immunization was administered to group IM in the same manner. Groups UNC and INC were not treated during this period of the experiment.
At 27 d.p.i., groups INC, IC and IM and were challenged via the cloaca with 105 H. meleagridis organisms. Ten days later (10 days post challenge), all animals were killed by decapitation for postmortem examination. Macroscopic liver lesions were scored as follows: 0 = normal; 1 = a few small foci (off-white and variable in appearance) visible on the surface of the liver; 2 = lesions covering one-half of the liver surface; 3 = necrotic lesions (often large) covering more than 50% of the liver surface; and 4 = death from histomonosis, with coalescing, necrotic lesions all over the liver surface. Lesions in the caeca were scored as follows: 0 = normal; 1 = a few scattered small lesions, but little or no thickening of the mucosal wall; 2 = yellow and foamy contents, lesions prominent but discreet and some bleeding and thickening of the mucosa visible; 3 = enlarged caeca, empty or filled with yellow caseous abnormal lumen contents, thickened walls and confluent lesions, entire caecum involved; and 4 = death from histomonosis, distended caeca with fragile walls and necrotic lesions.
Experiment 2: specific antibody response to active immunization
For the generation of immune serum, 49 turkeys were divided randomly into five groups. At 24 days of age, two groups—group HIC (10 birds) and —group HIM (10 birds)—received the same treatment as the IC and IM groups, respectively (see Experiment 1). A placebo group was inoculated via the cloaca with homogenized caeca and liver from an uninfected turkey (PIC, 10 turkeys), and another group was immunized intramuscularly (PIM, nine turkeys) with the same mixture. At 20 d.p.i., a booster immunization was administered to all groups in the same manner. The control group (CON, 10 turkeys) was neither vaccinated nor challenged. Blood samples were taken at 0, 6, 13, 20 and 27 d.p.i. (at necropsy). Serum was collected and stored at −20°C for indirect immunofluorescence assays (IFA). Seven days after the booster was given (27 dpi), the birds were killed by decapitation, all blood was collected and necropsy was performed. Antiserum collected from each group (HIC, HIM, PIC and PIM) was pooled and used for antibody transfer to naïve turkeys (Experiment 3, see below).
To determine serum IgG titres by IFA, 25 µl of a 2-day-old culture of H. meleagridis (5×105/ml) fixed in 4% paraformaldehyde were seeded onto the 6 mm wells of a Teflon-coated slide (Immuno-Cell, Mechelen, Belgium). They were air-dried and treated with blocking buffer (5% horse serum, 0.02% sodium azide in phosphate-buffered saline) for 30 min at 37°C. The slides were then incubated with two-fold dilutions of the test sera (in blocking buffer) for 30 min at 37°C. Finally, the slides were incubated with goat fluorescein isothyocyanate-labelled anti-turkey (IgG) antibodies (1/50 diluted in blocking buffer; KPL, Maryland, USA) for 30 min at 37°C. Fluorescence staining was examined microscopically at 500× magnification.
Experiment 3: analysis of protection after passive immunization
Antibodies from the pooled sera from Experiment 2 were precipitated in ammonium sulphate (40% wt./vol.) and the pellets were resuspended in 1/10 volume physiological saline. The antibody mixtures were subsequently dialysed extensively against physiological saline. Antibody titres were determined by IFA as described above.
Before antibody transfer, the pooled and concentrated antisera were evaluated for their ability to induce complement-mediated lysis of H. meleagridis. A 2-day-old culture was diluted in culture medium to a concentration of approximately 1.5×105 H. meleagridis/ml. Serum collected from a H. meleagridis-negative turkey (confirmed by IFA) was used as the source of complement. Pooled antisera from the HIC and the HIM groups were tested, while that from the CON group was used as a control. Prior to use, these test samples were inactivated at 56°C for 30 min. The assay was performed in a sterile 96-well Cellstar culture plate (Greiner Bio-One, Wemmel, Belgium).
Each test reaction contained 50 µl H. meleagridis culture, 50 µl tested antisera (undiluted, and 1/10, 1/100 and 1/1000 diluted) and 50 µl complement. Control reactions included parasites plus complement (PC) and parasites plus physiological saline (PP). The concentration of parasites in each reaction mixture was determined, using a Neubauer counting chamber, at the beginning of the experiment, after incubation at 37°C for 1 h, for 3 h and after overnight incubation. The percentage reduction in live parasites was calculated as follows:
For the passive immunization, 35 turkeys were divided in six groups (see later ). At 5 weeks of age, the birds were given an intraperitoneal injection of 4 ml concentrated antisera. The passively immunized groups, PASHIC and PASHIM, received antibodies from the HIC and HIM groups, respectively; while the placebo groups PASPIC and PASPIM received concentrated PIC and PIM antibodies, respectively. Groups POS and NEG were not immunized. Four hours after serum transfer, the birds in all groups except for the NEG group were challenged via the cloaca with 3×105 H. meleagridis. Ten days later (10 days post transfer), all of the turkeys were killed by decapitation for postmortem examination of livers and caeca as described above. To track the antibody titres before and after passive immunization, blood was taken 1 day before (−1 day post transfer), 1 day after serum transfer and at 10 days post transfer. Sera were collected and stored at −20°C until analysis by IFA as described above.
Statistical analysis
All statistical analyses were performed using the SAS 8.2 software (SAS Institute, Cary, North Carolina, USA). Lesion scores, antibody titres in the sera at each time point and mortality rates were compared using Kruskal–Wallis analysis to detect significant differences between multiple groups, and the Wilcoxon rank sum test was used to compare differences between each pair of groups. P≤0.05 was considered to be significant.
Results
Experiment 1: analysis of protection after active immunization
During immunization by infection and treatment, two birds of the IC group died of histomonosis (at 8 d.p.i. and 21 d.p.i.). After challenge, none of the immunized birds of the IC group and only one bird of the intramuscularly immunized IM group died (8 days post challenge). Six birds of the, Infected non-immunized control INC group died of histomonosis, three at 9 days post challenge and three at 10 days post challenge. There were no significant differences between the mortality rates of the uninfected UNC group and those of the immunized and challenged IC and IM groups, while the mortality of the challenged but non-immunized INC group differed significantly from all other groups
Table 1. Experiment 1: mortality and mean lesion scores of groups of turkeys after active immunization and challenge with H. meleagridis
In the liver, significant differences were seen between the mean lesion scores of the non-immunized INC group and the IC and UNC groups and between the IM group and the IC and UNC groups. No significant differences were observed between mean liver lesion scores of the INC and the IM group, or between the IC and the uninfected control UNC group. The mean caecal lesion score of the IC group was significantly lower than those of the INC and IM groups, but significantly higher than that of the UNC group. There were no statistical differences between the mean caecal lesion scores of the IM and INC groups, which were both significantly higher than those of the UNC and the IC groups. The mean caecal lesion scores of the surviving birds of the INC and the IM groups were however 1 and 2, respectively, while their mean liver lesions were 1.25 and 2, respectively .
Experiment 2: specific antibody response to active immunization
Analysis of the serum IgG response against H. meleagridis using IFA with fixed H. meleagridis as antigen showed that the parasites fluoresced intensely. Without primary incubation with antisera, no fluorescence could be detected, indicating the absence of autofluorescence or non-specific fluorescence.
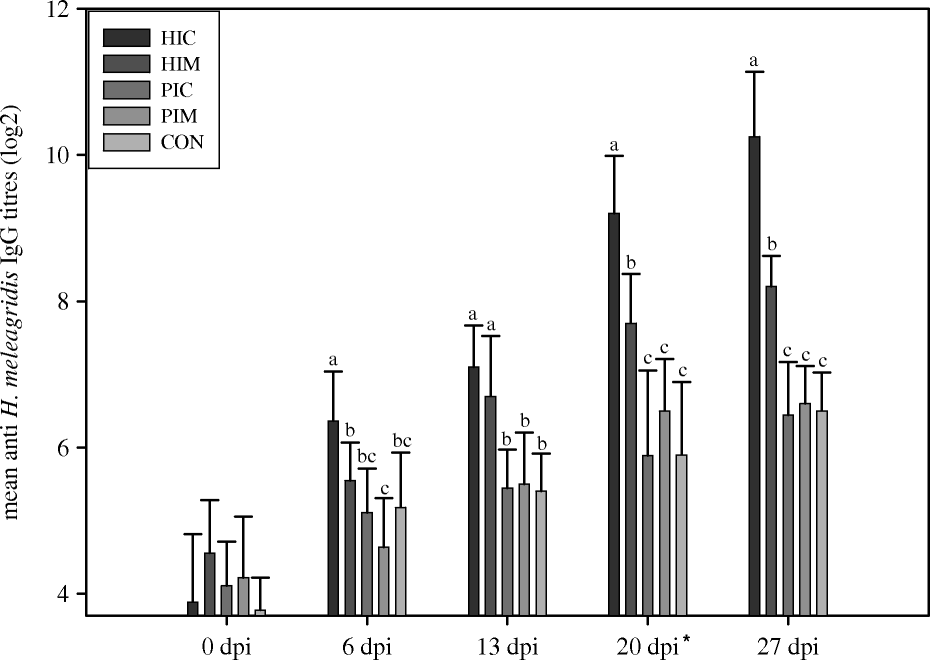
The mean anti-H. meleagridis antibody titres of the immunized groups (HIC and HIM) were significantly higher than those of the placebo (PIC and PIM) and control (CON) groups, from 6 d.p.i. (HIC) and from 13 d.p.i. (HIM) onwards (). From 6 d.p.i., significantly higher anti-H. meleagridis antibody titres were found in group HIC compared with group HIM—except for 13 d.p.i., when no significant differences could be detected between them. Throughout the entire experiment, no significant differences were observed between the placebo and the control groups.
Figure 1. Experiment 2: serum antibody responses of groups of turkeys after active immunization with H. meleagridis. Turkeys immunized at 0 d.p.i., with a booster at 20 d.p.i. (indicated by the asterisk). HIC, immunized by intracloacal infection with H. meleagridis; HIM, immunized intramuscularly with H. meleagridis antigens; PIC, immunized by intracloacal injection with H. meleagridis-negative liver and caecal homogenate; PIM, immunized as PIC but intramuscularly; CON, non-immunized control. Titres measured by indirect immunofluorescence. Different lowercase letters indicate significant differences between treatments at each time point.
Experiment 3: analysis of protection after passive immunization
Before antibody transfer, the pooled, concentrated antisera were evaluated for their bioactivity by examining their ability to induce complement-mediated lysis of H. meleagridis. The results of this test are presented in . Incubation of H. meleagridis with complement and the undiluted HIC or HIM concentrated serum pools resulted in a reduction in living parasites after 1 h. Moreover, after 3 h and after overnight incubation, growth of H. meleagridis was impeded by 100% in both samples. This reduction was detectable at serum dilutions of 1/10 and 1/100 but not at 1/1000. When incubating the parasites and the complement with the pooled CON sera or without test sera, no reduction and even growth (indicated in by the negative values) could be observed.
Table 2. Lytic activity of anti-H. meleagridis antibodies after different incubation times
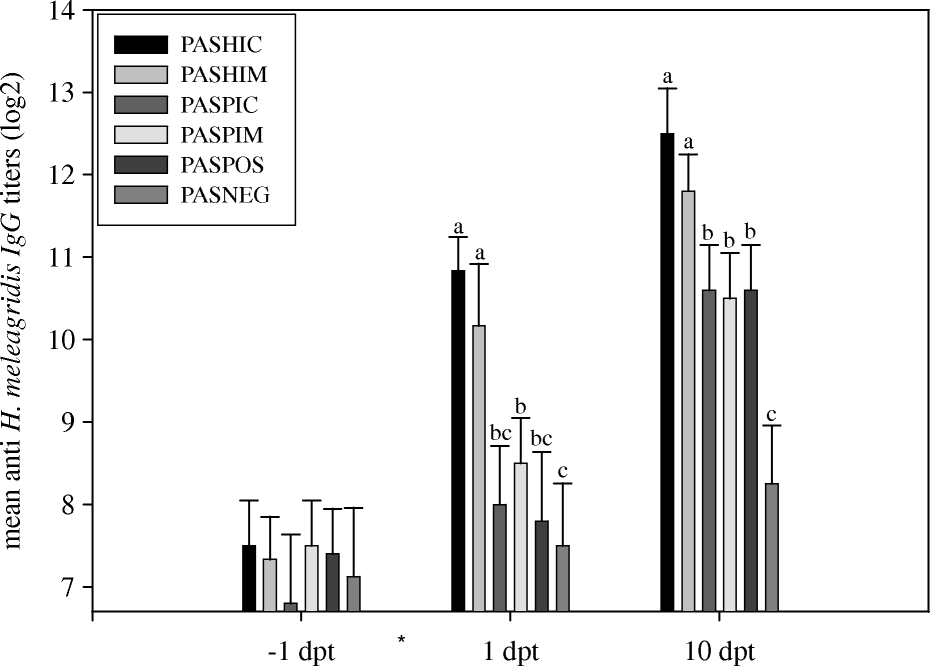
The anti-H. meleagridis antibody titres as determined by IFA are shown in . One day before passive serum transfer (−1 day post transfer), no statistical differences in antibody titres could be detected. One day after transfer (1 day post transfer), the titres of the passively immunized groups (PASHIC and PASHIM) increased respectively 9.8-fold and 7.5-fold compared with the titres at −1 day post transfer, and were significantly different from the titres of the other groups. The birds were challenged via the cloaca 4 h after passive serum transfer. On the day of necropsy (10 days post transfer), the titres of groups PASHIC and PASHIM were still significantly different from those from groups PASPIC, PASPIM and POS. The antibody titres from all challenged groups were significantly different from group NEG at 10 days post transfer.
Figure 2. Experiment 3: serum antibody responses of groups of turkeys after passive immunization and challenge with H. meleagridis. *Transfer of immune serum and challenge (4 h later) with H. meleagridis at 0 days post transfer (dpt). PASHIC, passively immunized with HIC antibodies; PASHIM, passively immunized with HIM antibodies; PASPIC, received antibodies from PIC; PASPIM, received antibodies from PIM; POS, non-immunized challenged group; NEG, non-immunized non-challenged group. Titres measured by indirect immunofluorescence. Different lowercase letters indicate significant differences between treatments at each time point.
All challenged groups showed lesions in the examined organs (), but no significant differences between the mean lesion scores of the challenged groups could be found. For the unchallenged NEG group, no lesions could be detected in the liver or caeca, resulting in a significant difference in mean lesion scores between this group and the challenged groups.
Table 3. Experiment 3: passive immunization and challenge against H. meleagridis
Discussion
In the present study, an anti-H. meleagridis serum antibody response was detected after immunization by infection and treatment and after intramuscular immunization with H. meleagridis antigens. Immunity induced by intramuscular injection of dead H. meleagridis antigens appeared not to be protective against histomonosis. In contrast, turkeys actively immunized by infection followed by treatment were protected against a H. meleagridis challenge. However, transfer of anti-H. meleagridis antibodies to naïve turkeys did not protect them against challenge via the cloaca with 3×105 H. meleagridis organisms.
Several earlier studies encouraged us to investigate the protective value of the serum antibodies against H. meleagridis. Tyzzer (Citation1934) suggested that protective immunity could be age-related, as older birds are more likely to be immune to this disease. Furthermore, a degree of protection against intracloacal challenge with H. meleagridis and against an oral challenge with H. meleagridis-contaminated Heterakis gallinarum eggs was detected after young turkeys were vaccinated with attenuated cultures of H. meleagridis (Tyzzer, Citation1933, Citation1934, Citation1936; Lund et al., Citation1966). Recently, cloned attenuated H. meleagridis has been shown to induce protection against an intracloacal challenge (Hess et al., Citation2008). However, examination of the immune response was not included in these studies so it was not possible to link this protection to a systemic or mucosal immune response.
In 1963 Clarkson performed a passive immunization experiment to determine whether antibodies could protect turkeys against H. meleagridis, and suggested that they could not or could not completely induce protection. However, he used only a semi-quantitative analysis of the immune response (Clarkson, Citation1963), whereas in the present study actively and passively immunized and challenged turkeys were examined on a quantitative basis, to determine the protective value of antibodies against this parasite.
In Experiment 1, the degree of protection was analysed after actively immunizing turkeys. Following challenge of turkeys immunized by infection and treatment (IC group), the caeca of the birds showed signs of infection, although the parasites did not appear to migrate to the liver or cause death. This clearly showed that turkeys are protected against H. meleagridis after infection and treatment. A second group of birds (group IM) was immunized by intramuscular injection with dead H. meleagridis antigens derived from infected livers and caeca. This choice of immunizing material should generate antibodies against all in vivo stages as the parasite appears in different stages within its host (Lee et al., Citation1969; Honigberg & Bennett, Citation1971) but it resulted in significantly higher mean lesion scores of both the liver and caeca than those of the IC and the negative control group—although, as far as we could judge the mortality at 10 days post challenge, only one of 10 birds died of histomonosis. Furthermore, the mean lesion scores of the surviving birds were higher in the IM group than in the INC (infected non-immunized control) group, suggesting that immunity induced by dead antigens is not protective against histomonosis. These findings are supported by the results of Hess et al. (Citation2008).
In a second active immunization experiment (Experiment 2) a strong anti-H. meleagridis serum antibody response was produced after experimental infection and recovery, while intramuscular immunization resulted in a slightly lower, but still significant, antibody response. The booster immunization resulted in both groups in a subsequent significant increase in parasite-specific serum antibodies.
In the passive immunization Experiment 3, the sera from the second experiment were concentrated and transferred to naïve animals. After transfer, the titres of the anti-H. meleagridis immunized groups (PASHIC and PASHIM) increased respectively 9.8-fold and 7.5-fold, resulting in higher mean antibody titres than those of the originating groups (HIC and HIM). However, despite the successful transfer of anti-H. meleagridis antibodies, no protective effect was observed, at least not against an intracloacal challenge with 3×105 parasites.
It is possible that the antibody titres of the concentrated pooled sera for passive immunization were not high enough to result in protection. This is considered unlikely because group IC of Experiment 1 was protected after being immunized in the same manner as the HIC group (Experiment 2), although these birds had lower antibody titres at the day of challenge than the titres after passive immunization. In vitro analysis of the bioactivity of the specific antisera showed that the antibodies transferred to the naïve animals could still activate the lysis of H. meleagridis but this activity decreased at a dilution of 1/100 and ceased at 1/1000, which could indicate that the anti-H. meleagridis activity of the antibodies was not strong enough to induce protection.
Another consideration is that the serum antibody titres of passively immunized groups might no longer have been high enough to be protective when the infection reached its systemic phase. Indeed, the transferred antibodies peaked in the serum approximately 1 day after injection (preliminary experiment; data not shown) and declined thereafter. However, the titres of the passively immunized groups remained readily detectable and significantly higher than those of the placebo groups. Thus, a certain level of protection should have been seen.
Lastly, and most importantly, the challenge dose might have been too high to simulate a natural challenge. A lower challenge dose might have generated differences in mean lesion scores, thus revealing the protective value of the transferred antibodies.
Despite these explanations, the most logical and direct conclusion of our study is that systemic immunity by serum antibodies against H. meleagridis is not, or is not primarily, responsible for protection against histomonosis. Instead, mucosal immune responses might be more important in the prevention of this disease and deserve further investigation.
Acknowledgements
The present study was supported by the Belgian Federal Public Service of ‘Health, Food Chain Safety and Environment’ and Alpharma Animal Health Inc. (Antwerp, Belgium). The authors would like to thank Petra Sintubin, Nathalie Wellens, Marisa Geens, Anh Dao Nguyen Pham, Nani Van Gerven, Tom Luyten, Marcel Samain and Karolien Loots for their skilled technical assistance. Joris De Gussem and Jeroen De Gussem are acknowledged for providing infected animals.
References
- Bleyen , N. , De Gussem , K. , De Gussem , J. and Goddeeris , B.M. 2007 . Specific detection of Histomonas meleagridis in turkeys by a PCR assay with an internal amplification control . Veterinary Parasitology , 143 : 206 – 213 .
- Clarkson , M.J. 1963 . Immunological responses to Histomonas meleagridis in the turkey and fowl . Immunology , 6 : 156 – 168 .
- Hess , M. , Liebhart , D. , Grabensteiner , E. and Singh , A. 2008 . Cloned Histomonas meleagridis passaged in vitro resulted in reduced pathogenicity and is capable of protecting turkeys from histomonosis . Vaccine , 26 : 4187 – 4193 .
- Higgins , C.H. 1915 . Entero-hepatitis or blackhead in turkeys . Bulletin 17 (pp. 1 – 11 ). Ottawa : Dominion of Canada, Department of Agricultural, Health of Animals Branch .
- Honigberg , B.M. and Bennett , C.J. 1971 . Light microscopic observations on structure and division of Histomonas meleagridis (Smith) . Journal of Protozoology , 18 : 687 – 697 .
- Lee , D.L. , Long , P.L. , Millard , B.J. and Bradley , J. 1969 . The fine structure and method of feeding of the tissue parasitizing stages of Histomonas meleagridis . Parasitology , 59 : 171 – 184 .
- Lund , E.E. , Augustine , P.C. and Ellis , D.J. 1966 . Immunizing action of in vitro-attenuated Histomonas meleagridis in chickens and turkeys . Experimental Parasitology , 18 : 403 – 407 .
- McDougald , L.R. 1991 . “ Other protozoan diseases of the intestinal tract ” . In Diseases of Poultry , 9th edn , Edited by: Calnek , B.W. , Barnes , H.J. , Beard , C.W. , Reid , W.M. and Yoder , H.W. Jr . 804 – 811 . Ames : Iowa State University Press .
- McDougald , L.R. and Fuller , L. 2005 . Blackhead disease in turkeys: direct transmission of Histomonas meleagridis from bird to bird in a laboratory model . Avian Diseases , 49 : 328 – 331 .
- McDougald , L.R. and Galloway , R.B. 1973 . Blackhead disease: in vitro isolation of Histomonas meleagridis as a potentially useful diagnostic aid . Avian Diseases , 17 : 847 – 850 .
- Tyzzer , E.E. 1933 . The immunizing properties of an attenuated strain of Histomonas meleagridis . Journal of Parasitology , 19 ( Suppl .), 9 .
- Tyzzer , E.E. 1934 . Studies on histomoniasis or blackhead infection, in the chicken and the turkey . Proceedings of the American Academy of Arts and Sciences , 69 : 189 – 264 .
- Tyzzer , E.E. 1936 . A study of immunity produced by infection with attenuated culture-strains of Histomonas meleagridis . Journal of Comparative Pathology , 49 : 285 – 303 .
- Tyzzer , E.E. , Fabyan , M. and Foot , N.C. 1921 . Further observations on “blackhead” in turkeys . Journal of Infectious Diseases , 29 : 268 – 286 .