Abstract
An outbreak of disease in a White Rhine laying goose flock was characterized by increased water uptake, increased mortality, production of eggs with abnormal shells, a 25% drop in egg production and 40% embryo mortality. Affected dead or sacrificed birds had sero-fibrinogranulocytic peritonitis and salpingitis, infiltration of the lamina propria in the uterus and heterophil granulocytes in the isthmus and magnum of the oviduct. Mycoplasmas, mainly identified as Mycoplasma sp. strain 1220, were isolated from the airsac, liver, ovary, magnum and peritoneum of some affected geese. Strain 1220 was originally isolated from a Hungarian gander with phallus inflammation and, according to detailed biochemical and serological examinations, it is expected to represent a new avian species within the genus Mycoplasma.
Introduction
Mycoplasmas were first isolated from geese by Kosovac & Djurisic (Citation1970), and the occurrence of several Mycoplasma and Acholeplasma species in geese was later confirmed by Stipkovits et al. (Citation1975). Mycoplasmas have been isolated from geese in association with various conditions such as airsacculitis and peritonitis (Stipkovits et al., Citation1975; Buntz et al., Citation1986; Gaillard-Perrin et al., Citation1983), salpingitis (Kosovac & Djurisic, Citation1970; Stipkovits et al., Citation1984a; Pfützner et al., Citation1988), embryo mortality (Benčina et al., Citation1988; Stipkovits et al., Citation1987), and phallus inflammation (Stipkovits et al., Citation1986). Natural cases of salpingitis in chickens caused by Mycoplasma gallisepticum were reported in Japan (Nunoya et al., Citation1997).
Intratracheal infection of goslings or goose embryos with mycoplasma and acholeplasma strains resulted in airsacculitis (Kisary et al., Citation1976; Stipkovits et al., Citation1987), and challenge of laying geese with unidentified mycoplasma isolates (subsequently identified as Mycoplasma cloacale and Mycoplasma anseris; L Stipkovits, personal communication) induced a fall in egg production and increased embryo mortality (Stipkovits et al., Citation1984a). Flocks with inflammation of the cloaca and phallus were treated successfully with different antibiotics such as tylosin tartrate, lincomycin/spectinomycin and tiamulin hydrogen fumarate (Czifra et al., Citation1986). In other waterfowl, such as ducks, Mycoplasma anatis, M. gallisepticum, M. cloacale, Mycoplasma synoviae, and Acholeplasma laidlawii were isolated more often (Kempf, Citation1990; Tiong, Citation1990).
Mycoplasma sp. strain 1220 was originally isolated from the phallus lymph of a gander in a Hungarian goose flock with inflammation of the cloaca and phallus. The organism is widely distributed in Hungary and represents 60% of all goose Mycoplasma isolates. Detailed biochemical and serological examinations have been performed and the organism is expected to represent a new avian Mycoplasma species (Varga, Citation1997). The present paper describes a case of salpingitis in laying geese and the isolation of Mycoplasma sp. strain 1220 from some of the affected birds.
Materials and Methods
Goose flock, clinical and pathological examination
The goose flock consisted of 2200 White Rhine layers and 700 ganders of the same type and age in their first traditional laying period. White Rhine geese are a cross between the Emden goose and local stock of Upper Rhine geese, and are used both for the production of fat liver (foie gras) and for meat.
The birds were 8 months old at the beginning of egg production in early February. They were kept indoors in a semi-intensive system on deep litter but had access to a yard by day. During the laying period they were provided with food and drinking water ad libitum. The food was produced locally and another five flocks received this same diet concurrently.
Egg production and hatch results, including numbers of infertile eggs, early embryo deaths and later embryo mortality, were recorded. Eggs were candled on the 10th day of incubation and any without any signs of germination were classified as infertile. Dead embryos detected at this time were classified as early embryo deaths.
The mortality of birds was recorded and cases were regularly necropsied. Samples of internal organs were collected from five dead geese and five sacrificed geese that were showing signs of illness. They were examined pathologically and histologically. Tissue sections were stained with haematoxylin and eosin. Ganders were not affected by the disease and no samples were collected from them.
Microbiological examination
Samples of the liver, spleen, various parts of the oviduct, and exudates in the oviduct and peritoneum were inoculated onto blood agar supplemented with 5% sheep blood and Drigalski agar. Drigalski agar is a modified MacConkey agar consisting of 1% lactose, 1% saccharose, 1% sodium thiosulphate pentahydrate, 0.008% bromothymol blue, 0.0005% crystal violet, 1% Bacto peptone (Difco), 0.5% sodium chloride, 0.5% “Lab Lemco” powder (Oxoid) and 1.4% agar.
Heat-fixed touch preparations from exudates in the oviduct and peritoneum were stained with 20% Ziehl–Neelsen carbol-fuchsin solution, treated with 1% acetic acid, and stained again with 3% malachite green according to Stamp et al. (Citation1950) and also with Giemsa stain (Hansen & Koster, Citation1936). They were examined for the presence of Chlamydophila spp.
Samples from the airsacs, spleen, exudates in the peritoneum and various parts of oviduct were inoculated into mycoplasma broth supplemented with 1% w/v glucose or arginine (Ernø & Stipkovits, Citation1973) and incubated at 37°C for 2 weeks. Cultures were checked every day for pH change and were inoculated onto mycoplasma agar medium B (Ernø & Stipkovits, Citation1973) on days 3, 6 and 10. Agar plates were incubated at 37°C for 14 days and examined every 2 days for the appearance of mycoplasma colonies. Mycoplasma isolates were identified by indirect immunofluorescence (Bradbury, Citation1998) using rabbit antisera against M. anseris (Bradbury et al., Citation1988), M. cloacale (Bradbury & Forrest, Citation1984) and Mycoplasma sp. strain 1220 (Stipkovits et al., Citation1984b).
Samples from the oviduct, liver and spleen of examined birds were tested for egg drop syndrome (EDS) virus by injection into the allantoic cavity of 10-day-old embryonated goose eggs. After 5 days of incubation, the allantoic fluid was checked for the presence of EDS virus by the haemagglutination test. Negative samples were further passaged through goose eggs up to a total of three passages. Sera from 20 recovered layers were examined by haemagglutination inhibition tests at the Veterinary Institute in Debrecen, Hungary, for the presence of antibodies to avian paramyxovirus subtypes 1 to 4, 6 and 7, avian influenza virus A haemagglutinin subtypes 1, 2, 4, 5 and 6 and EDS virus.
Results
Clinical signs
Egg production in the affected goose flock started at the beginning of February, 2 weeks earlier than expected, and it increased gradually. Three weeks later the steady increase in egg production stopped, and after a further few days the number of normal eggs decreased. Approximately one-half of the layers had conjunctivitis with lacrimation and the birds showed signs of depression. Drinking water intake increased and many birds showed a “penguin-like” posture. Between one and three layers died each day for the approximate 8-week duration of the clinical disease, amounting to a total of 33 birds. There was no mortality in the ganders.
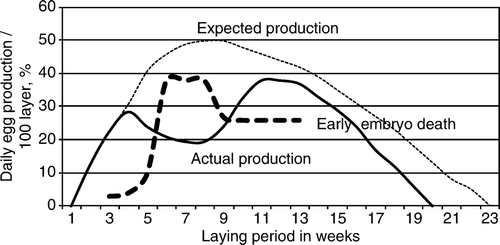
At the onset of disease 30% of layers laid daily (the maximum expected being 50%), but then production gradually decreased over 2 weeks to 20% and stayed at that level. After a further 2 weeks, egg production increased up to nearly 40% and for 4 weeks was maintained near to 40%, after which it dropped sharply. Egg production ceased 3 weeks earlier than was expected ().
Figure 1. Egg production curve of the affected goose flock (solid line) together with the expected production curve (dotted line) and the percentage of early embryo deaths (dashed line).
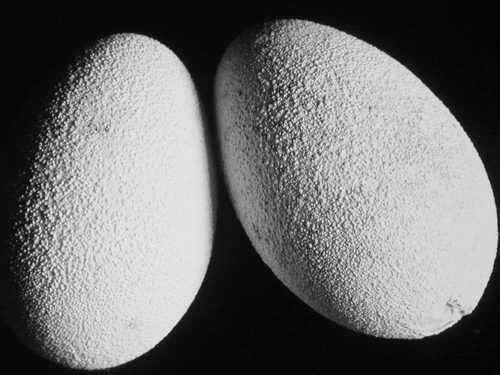
At the beginning of the disease the shell of many eggs had protuberances and a roughened granular surface due to deposition of calcium (). Later the shells became very thin and many eggs had either no shell or only a partially formed shell. Sometimes the shell membrane was absent, and in these cases the yolk was surrounded by turbid egg-white material. Some geese laid no eggs but only turbid egg-white material of various quantities, and in some cases this was covered with a grey fibrino-purulent exudate. The consistency of the egg-white in affected birds was fluid compared with normal material. Over the laying period, the number of eggs produced was 25% less than expected—and due to their very thin and abnormal shells, many eggs were unsuitable for incubation.
At the onset of disease 20% of incubated eggs were infertile, but later the number varied between 10% and 12%. At the beginning of egg production, the number of embryo deaths was 10% of incubated eggs; during the clinical disease it increased to 19%, and later it fell to 5%. At the beginning of production only 3% of embryos died in the early phase of incubation. During and after clinical disease and until the end of the laying period, between 30% and 40% early embryo deaths occurred.
At the end of the egg production period, 30% of layers were culled as being unsuitable for further breeding and the remainder were moved into another flock. None of the progeny of these flocks was used for breeding, but was sold for fat liver production.
Pathological examination
The cause of death of affected birds was not always established, although necrotic enteritis with fibrinous exudation was present in approximately 15% of the dead layers. Peritonitis and septicaemia was apparent in some dead birds, and in some cases could be attributed to cloacal injury during the passage of the egg.
Postmortem examination of five dead birds and five sacrificed birds that had been showing clinical signs of disease revealed sero-fibrinogranulocytic peritonitis and salpingitis, and histopathology confirmed lymphocytic and granulocytic infiltration of the lamina propria in the uterus and heterophil granulocytes in the isthmus and magnum of oviduct. In dead birds, the findings were dehydration, accumulation of sero-fibrinous exudate in the peritoneum and fibrinous membranes on the peritoneal walls. Development of ovarian follicles had ceased and there were one to two normal follicles alongside atretic follicles filled with turbid material, or only primary or secondary follicles. The oviduct was reduced in size and the wall was flaccid.
There were turbid exudates in the uterus of dead and sacrificed geese. Sometimes these exudates were fibrino-purulent. In some cases, fibrin masses formed compact shapes that could be as large as 15×20 mm2 (). Exudates were also seen in the magnum and infundibulum of the oviduct.
Figure 3. Oviduct of an affected goose showing a compact egg-shaped fibrinous mass in the uterus and ascending inflammation in the infundibulum that penetrated into the peritoneum (see arrows).
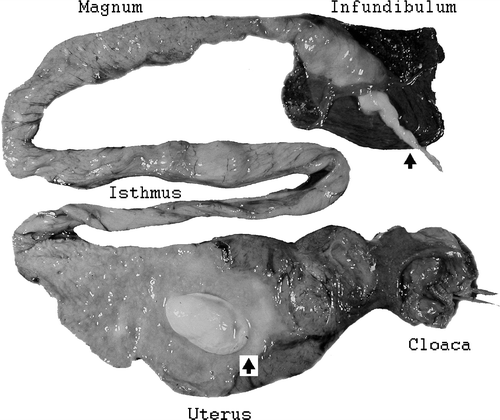
The mucous lining of the uterus and sometimes other regions of the oviduct was a pinkish-grey colour and oedematous, although in the magnum it was usually red. Exudates from the oviduct often appeared to extend through the infundibulum into the peritoneum. In such cases, exudates could be seen between the ovarian follicles. This apparent extension of exudates into the peritoneum could cause varying degrees of sero-fibrinogranulocytic peritonitis.
In the five sacrificed layers there was peritonitis in two and degeneration of follicles in three. Salpingitis was macroscopically visible in four or these birds and was confirmed histopathologically in all oviduct samples.
No macroscopic pathological lesions were recorded in other organs.
Histopathological examination revealed oedema and heterophil granulocyte infiltration of an apparently widened lamina propria in most parts of the oviduct both in dead and sacrificed birds (). The most frequent changes were seen in the uterus (shell gland). Heterophil granulocytes were detected mostly in the epithelial layer. Sometimes granulocyte infiltration of the mucous membrane was also observed in the isthmus and magnum. Purulent serous exudates and heterophil granulocytes were detected in folds of the afore-mentioned parts of the oviduct. General multifocal heterophil granulocyte and lymphocyte infiltration was noted around portal blood vessels in the liver. Similar lesions were recorded in sacrificed birds, but the severity of changes was less and less portal blood vessels were affected than in those geese that had died.
Figure 4. Oviduct of an affected goose with oedema in the uterus and infiltration of the lamina propria with heterophils (see arrow). Bar = 50 µm.
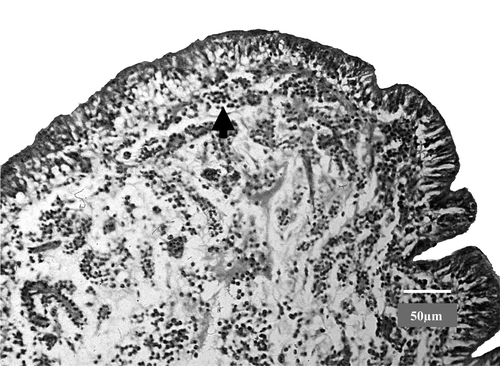
In two of the 10 layers a prolapsed cloaca had irregular erosions of the epithelial membrane of an everted mucosa covered by a fibrinous pseudomembrane. No microscopic lesions were seen in the spleen, heart or lung. Ganders were not affected by the disease.
Microbiological examination
No bacteria grew on blood agar or Drigalski agar from liver, spleen, oviduct and peritoneal exudates. There was no evidence of chlamydia in the stained touch preparations from exudates in the peritoneum and oviduct.
Mycoplasmas were isolated from two of the dead birds and three of the sacrificed birds that were examined. Mycoplasma sp. strain 1220 was isolated from the liver and oviduct of one dead bird, and M. anseris from peritoneum and oviduct of the other. Mycoplasma sp. strain 1220 was recovered from the peritoneum, airsac and oviduct of one sacrificed bird, from the peritoneum, oviduct and ovary of another, and from the peritoneum and ovary of a third.
EDS virus could not be detected in any of the samples examined and no antibodies to this virus were found by haemagglutination inhibition test in sera of slaughtered and recovered birds a few weeks after appearance of the disease. No antibodies were detected to avian paramyxoviruses subtypes 1 to 4 or subtypes 6 and 7 or to avian influenza virus A haemagglutinin subtypes 1 and 2 or 4 to 6.
Discussion
An acute sero-fibrinous and purulent fibrino-granulocytic salpingitis was detected in dead and sacrificed laying geese, and in the early stages of disease many eggs with rough calcium deposits developed in the inflamed oviduct. Later on, eggs with very thin or incomplete shells or with no shell at all were produced. Inflammation involved both the oviduct and peritoneum and there was acute sero-fibrinous peritonitis.
None of the histological features commonly associated with mycoplasma infection such as epithelial hyperplasia and lymphoplasmacytic infiltration of the affected organs were seen, but it is possible that the layers were still in the early acute phase of the infection and that it was too soon for them to have developed further histological findings. However mycoplasmas were isolated from exudates in the oviduct and peritoneum as well as from various inner organs (liver, oviduct and follicles).
Several species of Mollicutes have been detected in geese: M. anatis (Gaillard-Perrin et al., Citation1983), M. anseris (Bradbury et al., Citation1988), M. cloacale (Stipkovits et al., Citation1984b), Mycoplasma gallinarum (Stipkovits et al., Citation1975), M. gallisepticum (Buntz et al., Citation1986; Benčina et al., 1987; Levisohn & Kleven, Citation2000), M. synoviae (Benčina et al., 1987), Mycoplasma imitans (Bradbury et al., Citation1993) and A. laidlawii and Acholeplasma axanthum (Stipkovits et al., Citation1975). However, on the basis of serological and biochemical studies, most of our isolates were identified as Mycoplasma sp. strain 1220. The identification of this strain from geese with salpingitis together with negative results for other bacteria including chlamydia, for EDS virus and its antibodies, and for antibodies to paramyxoviruses and avian influenza viruses, suggests that the disease may have been associated with this mycoplasma, although further investigations are necessary to confirm this. Strain 1220 is commonly found in healthy Hungarian goose flocks, so reproduction of the condition by experimental challenge and/or the consistent isolation of this Mycoplasma from geese with similar disease problems would be useful evidence to back up the findings of the current study.
An earlier investigation of experimental infection of embryonated goose eggs with strain 1220 revealed increased embryo mortality with acute oedematous inflammation of the chorioallantoic membrane, diffuse heterophil granulocytic interstitial hepatitis and bronchopneumonia (Stipkovits et al., Citation1987). In the present study it would have been very useful to examine dead embryos from affected layers for mycoplasma infection, and this should be carried out in future investigations.
Salpingitis in chickens caused by M. gallisepticum has been reported in both natural and experimental cases (Nunoya et al. Citation1997; Pruthi & Kharole, Citation1981). In the natural infection, the condition was characterized by marked thickening of the oviduct mucosa due to epithelial hyperplasia and lymphoplasmacytic infiltration and there was a 23% drop in egg production. Keratoconjunctivitis due to M. gallisepticum infection has also been demonstrated in layer chickens (Nunoya et al., Citation1995).
In addition to inflammation of the oviduct, a mycoplasmaemia appears to have developed in our geese with general signs of increased thirst, conjunctivitis and a drop in egg production. The signs lasted for about 2 weeks, after which egg production increased but never reached the expected level. Furthermore, egg production decreased and then ceased approximately 3 weeks earlier than expected. Altogether 25% fewer eggs were produced over the laying season and there was a very high proportion (40%) of early embryo deaths. Embryo death was a typical feature of Mycoplasma infection in geese in our earlier studies (Stipkovits et al., Citation1984a). As a result of poor production during the first laying season, the flock was culled and replaced with new stock.
The laying season normally begins in the middle of February and continues until June, but the early onset of lay in this particular flock at the beginning of February may have had some bearing on the development of disease. It will be essential to investigate other goose flocks with similar signs of disease in order to establish the true economic impact of infection of geese with Mycoplasma sp. strain 1220.
References
- Benčina , D. , Tadina , T. and Dorrer , D. 1988 . Natural infection of geese with Mycoplasma gallisepticum and M. synoviae and egg transmission of the mycoplasmas . Avian Pathology , 25 : 528 – 533 .
- Bradbury , J.M. 1998 . “ Identification of mycoplasmas by immunofluorescence ” . In Methods in Molecular Biology; Mycoplasma Protocols , Edited by: Miles , R. and Nicholas , R. Vol. 104 , 119 – 125 . Totowa , NJ : Humana Press .
- Bradbury , J.M. and Forrest , M. 1984 . Mycoplasma cloacale, a new species isolated from a turkey . International Journal of Systematic Bacteriology , 34 : 389 – 392 .
- Bradbury , J.M. , Jordan , F.T.W. , Shimizu , T. , Stipkovits , L. & Varga Zs. 1988 . Mycoplasma anseris sp. nov. found in geese . International Journal of Systematic Bacteriology , 38 , 74 – 76 .
- Bradbury , J.M. , Saed Abdul-Wahab , O.M. , Yavary , C.A. , Dupiellet , J.-P. and Bové , J.M. 1993 . Mycoplasma imitans sp. nov. is related to Mycoplasma gallisepticum and found in birds . International Journal of Systematic Bacteriology , 43 : 721 – 728 .
- Buntz , B. , Bradbury , J.M. , Vuillaume , A. and Rousselot-Paillet , D. 1986 . Isolation of Mycoplasma gallisepticum from geese . Avian Pathology , 155 : 615 – 617 .
- Czifra , Gy. , Varga , Zs. , Dobos-Kovács , M. & Stipkovits , L. 1986 . Medication of inflammation of the phallus in geese . Acta Veterinaria Hungarica , 34 , 211 – 223 .
- Ernø , H. and Stipkovits , L. 1973 . Bovine mycoplasmas: Cultural and biochemical studies I . Acta Veterinaria Scandinavica , 14 : 439 – 449 .
- Gaillard-Perrin , D. , Nougayrede , P. and Vuillaume , A. 1983 . Caractérisation de quelques souches de mycoplasmes isolées chez l oie et le canard dans les Landes . Revue de Médecine Vétérinaire , 134 : 97 – 102 .
- Hansen , K. and Koster , H. 1936 . Nachweis von Brucella abortus (Bang) auf Grund der Alkalifestigkeit . Deutsche Tierarztiche Wochenschrift , 44 : 739 – 742 .
- Kempf , I. 1990 . The mycoplasmas of ducks . Recueil de Médecine Vétérinaire , 166 : 1111 – 1115 .
- Kisary , J. , El –Ebeedy , A.A. and Stipkovits , L. 1976 . II. Studies on pathogenicity of mycoplasmas in goslings and goose and chicken embryos . Avian Pathology , 5 : 15 – 20 .
- Kosovac A. & Djurisic , S. 1970 . Diseases of the female genital tract of geese and mycoplasmosis . In Proceedings of the IVth International Congress of the World Veterinary Poultry Association pp. 429 – 440 . Belgrade, Yugoslavia .
- Levisohn , S. and Kleven , S.H. 2000 . Avian mycoplasmosis (Mycoplasma gallisepticum) . Revue scientifique et technique—Office International des Epizooties , 19 : 425 – 442 .
- Nunoya , T. , Yagihashi , T. , Tajima , M. and Nagasawa , Y. 1995 . Occurrence of keratoconjunctivitis apparently caused by Mycoplasma gallisepticum in layer chickens . Veterinary Pathology , 32 : 11 – 18 .
- Nunoya , T. , Kanai , K. , Yagihashi , T. , Hoshi , S. , Shibuya , K. and Tajima , M. 1997 . Natural case of salpingitis apparently caused by Mycoplasma gallisepticum in chickens . Avian Pathology , 26 : 391 – 398 .
- Pfützner , H. , Brys , A. , Leirer , R. and Rott , M. 1988 . Mykoplasmeninfektionen bei Gansen . Monatshefte für Veterinarmedizin , 43 : 692 – 695 .
- Pruthi , A.K. and Kharole , M.U. 1981 . Sequential pathology of genital tract in chickens experimentally infected with Mycoplasma gallisepticum . Avian Diseases , 25 : 768 – 778 .
- Stamp , J. T. , McEwen , A. D. , Watt , J.A.A. and Nisbet , D. I. 1950 . Enzootic abortion of ewes. I. Transmission of the disease . Veterinary Record , 62 : 251 – 254 .
- Stipkovits , L. , El-Ebeedy , A.A. , Kisary , J. and Varga , L. 1975 . Mycoplasma infection of geese. I. Incidence of mycoplasmas and acholeplasmas in geese . Avian Pathology , 4 : 35 – 43 .
- Stipkovits , L. , Bové , J.M. , Rousselot , M. , Larrue , P. , Labat , M. and Vuillaume , A. 1984a . Studies on mycoplasma infection of laying geese . Avian Pathology , 14 : 57 – 68 .
- Stipkovits , L. , Varga , Zs. , Dobos-Kovács , M. & Sánta , M. 1984b . Biochemical and serological examination of some mycoplasma strains of goose origin . Acta Veterinaria Hungarica , 32 , 117 – 125 .
- Stipkovits , L. , Varga , Zs. , Czifra , Gy , & Dobos-Kovács , M. 1986 . Occurrence of mycoplasma in geese affected with inflammation of the cloaca and phallus . Avian Pathology , 15 , 289 – 299 .
- Stipkovits , L. , Varga , Zs. , Glávits , R. , Rátz , F. & Molnár , É. 1987 . Pathological and immunological studies on goose embryos and one-day-old goslings experimentally infected with a mycoplasma strain of goose origin . Avian Pathology , 16 , 453 – 468 .
- Tiong , S.K. 1990 . Mycoplasmas and acholeplasmas isolated from ducks and their possible association with pasteurellas . Veterinary Record , 127 : 64 – 66 .
- Varga , Zs. 1997 Study of goose mycoplasma strains. Candidate's dissertation of Hungarian Academy of Sciences , HAS 1051 Budapest, Hungary .