Abstract
An outbreak of neurological disease occurred in pheasant chicks on a game farm in 2007. The disease was first seen in the 10th hatching of chicks on the farm. Affected chicks showed trembling and incoordination from the time of hatching, and subsequently blindness and cataract formation was seen in some of the affected chicks at 3 weeks of age. The peak mortality and culling figure was 21.0% in the worst affected hatch, compared with a maximum of 11.7% in the first nine hatches. No further cases were evident by 7.5 weeks of age. Histopathological examination showed a moderate acute encephalomyelitis in some, but not all, of the chicks with neurological signs. The clinical presentation and histopathological findings were typical of vertically transmitted avian encephalomyelitis as seen in chickens, although avian encephalomyelitis virus could not be detected in inoculated embryonated chicken eggs. However, serological testing by enzyme-linked immunosorbent assay for antibodies to the virus was positive in four of five affected 3-week-old birds and in 23 out of 29 adult breeding birds, and reverse transcriptase-polymerase chain reaction testing of RNA extracted from brain and pancreas tissue of affected chicks yielded nucleotide sequences aligned 82% and 83% with three avian encephalomyelitis sequences in a sequence database. The evidence suggested that the neurological disease was attributable to infection with a strain of avian encephalomyelitis virus that appeared to have entered the flock at the start of the breeding season, and was possibly introduced by carrier pheasants brought on to the farm early in the season.
Introduction
Avian encephalomyelitis (AE) is an infectious viral disease that has a virtually worldwide distribution in domestic fowl. It affects young chicks, in which it causes incoordination (ataxia) and tremors—giving rise to the former name of “epidemic tremor” (Tannock & Shafren, Citation1994; Calnek, Citation2008). The causative agent is avian encephalomyelitis virus (AEV), which is currently classed as a member of the family Picornaviridae (Marvil et al., Citation1999; Todd et al., Citation1999). AE is essentially an enteric infection and is transmitted between birds by oral ingestion, but it can also be transmitted vertically from infected breeding females through the egg to the chick, resulting in clinical signs at hatching (Calnek, Citation2008). The disease is controlled in poultry by vaccination of breeding flocks during the growing period to prevent this vertical transmission. AE has also been described in some other species of birds, including an outbreak of presumptive AE in pheasants (Phasianus colchicus) in which high mortality was reported in Mongolian and ring-necked pheasant chicks and clinical signs of ataxia, paralysis and tremor were described. The clinical signs were reproduced experimentally by inoculation of domestic fowl chicks with material from the affected pheasant chicks (Mathey, Citation1955).
Experimental infection in pheasants, red-legged partridges (Alectoris rufa) and grey partridges (Perdix perdix) was described by Bodin et al. (Citation1981). Of these three species of game birds, pheasants were found to be the least susceptible to infection with a strain of AEV derived from domestic fowls. Infection of adult pheasants resulted in no clinical signs but lesions were evident histologically. Inoculation of embryonated pheasant eggs produced no lesions although the virus was maintained in the embryos, resulting in pathogenic effects when material was re-inoculated into domestic fowl eggs. The authors concluded that there was little risk of pheasants suffering from infection with the virus although they were nonetheless considered potential vectors of infection. In a serological survey in Britain, neutralizing antibody against AEV was detected in pheasants, turkeys and red-legged partridges but not in starlings (Sturnus vulgaris), woodpigeons (Columba palumbus), collared doves (Streptopelia decaocto) or a limited range of finches, corvids or ducks (Steenis, Citation1971). However, detection of the virus has recently been reported in domestic pigeons in Turkey (Toplu & Alcigir, Citation2004).
The present paper describes the investigation of an outbreak of neurological disease in pheasant chicks on a game farm in Britain in 2007 and the demonstration of AEV in affected chicks.
Materials and Methods
Case history
The outbreak occurred on a game farm producing chicks from its own breeding birds. The females (hens) used for breeding had been run as far as possible as a closed flock. The males (cocks) ran with the hens through the winter and were transferred as a ratio of one cock to 10 hens into laying pens in early March. In February 2007 additional cocks (approximately 11% of the total number) were brought in to supplement the breeding flock. They came from a customer and were derived from the farm's own stock. They were quarantined for approximately 3 weeks before being moved to the laying pens. Two types of laying pen were used, mostly open wire mesh-covered block pens containing 30 hens and three cocks but also aviaries containing 10 hens and one cock. The hens remained in the same pens throughout the breeding season but some of the cocks were moved between pens. Eggs were collected by hand and set within a few days in the farm's own hatchery. Average egg production in April was 17.4 eggs/hen (compared with 16.7 eggs/hen in April 2006) and in May was 23.0 eggs/hen (25.2 eggs/hen in May 2006). The eggs were set to produce a sequence of two hatches a week starting in early May and continuing for 21 hatches. Chicks from the first nine hatches were not noticeably affected, but from Hatch 10 (early June) onwards chicks started to hatch showing trembling, most markedly of the head, and incoordination. The rate of mortality and culling from 1-day-old to 7.5 weeks varied between 6.6% and 11.7% in Hatches 1 to 9 and rose to 15.4% in Hatch 10, 21.0% in Hatch 11, and in subsequent hatches varied between 11.5% and 19.7%, including losses due to flooding. Many of the affected chicks were culled in the first few days of life but some of the surviving chicks from Hatch 11 continued to show incoordination and a proportion were noted to be blind, but no cases of incoordination were evident at 7.5 weeks of age.
Laboratory investigation
Three laboratory submissions of samples relating to the outbreak were examined. The first submission comprised 11 live affected 3-day-old chicks from Hatch 10, the second comprised 30 live affected and dead 1-day-old chicks from Hatch 13 and, following a visit to the farm, the third comprised 29 bloods collected at random from adult breeding birds (both cocks and hens) for serology, and five live 3-week-old birds (poults) from Hatch 11, which were clinically affected with incoordination and/or blindness. Bloods were taken from these five poults. Following submission to the laboratory the live birds were examined clinically and humanely euthanized.
Gross pathology and histopathology
Representative numbers of the 1-day-old and 3-day-old chicks underwent diagnostic postmortem examination, and tissues were collected for bacteriology, histopathology and virology. Examination of the 3-week-old poults was confined to sampling for histopathology and virology only. Samples of the brain, yolk sac and liver were cultured on 5% sheep blood agar and MacConkey agar (Oxoid, Basingstoke, UK) and were incubated aerobically for 18 to 36 h. Samples taken from the birds for histopathology were fixed in buffered formol saline, and sections of the tissues were prepared using conventional methods.
Virus isolation and detection
Samples of pooled brain from 1-day-old and 3-day-old pheasant chicks, and pooled brain and pancreas from 3-week-old poults, were processed for attempted virus isolation as follows; bacteria-free suspensions were prepared and inoculated into the yolk sac of 5-day-old to 6-day-old embryonated specific pathogen-free chickens eggs. The eggs were candled daily for embryo inertia, and after 7 days one-half of the inoculated eggs were chilled and the embryos examined for gross lesions such as muscle atrophy. Samples of brain from the inoculated embryos were pooled and passaged in a further batch of 5-day-old to 6-day-old embryonated specific pathogen-free chickens eggs. The eggs were candled daily and, prior to hatching, all the embryos were examined for gross lesions of AE. Samples were also inoculated and passaged onto confluent monolayers of chicken embryo liver cell cultures.
Serology
Five serum samples from affected 3-week-old poults and 29 sera from the breeding flock were tested using a commercial AEV enzyme-linked immunosorbent assay (ELISA) (IDEXX Laboratories Ltd, Chalfont St Peter, UK). Briefly, each serum sample was diluted 1:500 in sample diluent and tested by ELISA according to the manufacturer's instructions. The presence of AEV antibody was determined by relating the value of the test sample to the positive control mean (S/P ratio). Serum samples with S/P ratios > 0.2 were considered positive.
Analysis by reverse transcription-polymerase chain reaction (RT-PCR)
Sample preparation
Samples of brain from 3-day-old chicks and of brain and pancreas from 3-week-old poults were suspended in equal volumes of phosphate-buffered saline. Tissues were disrupted by briefly vortexing with glass beads, and then passing them through a series of successively smaller-gauged needles. They were treated with sodium dodecyl sulphate to a final concentration of 0.5%. Suspensions were clarified by centrifugation at 700×g for 20 min at 17°C. Supernatants were used for RNA extraction and were stored at −20°C.
RNA extraction
RNA was extracted from 200 µl viral preparation from the pheasant brains and pancreas using the RNeasy minikit (Qiagen, Crawley, UK) according to the manufacturer's instructions.
Reverse transcription-polymerase chain reaction
The RT-PCR was carried out using the SuperScript™ III One-Step RT-PCR System with Platinum® Taq DNA Polymerase kit (Invitrogen Ltd, Paisley, UK). The primers MK AE 1 and MK AE 2, which flank a 619 base pair (bp) DNA sequence containing the VP2 gene, were previously described by Xie et al. (Citation2005). The reaction mixture contained 1 x buffer, 1 µl SuperScript™ III RT/Platinum® Taq Mix, 25 pmol each MK AE 1 and MK AE 2 primer, 2.5 µl RNA and sterile diethylpyrocarbonate-treated distilled water to 25 µl. The cycling conditions comprised an initial RT step of 45°C for 30 min, followed by denaturation at 94°C for 5 min, then 35 cycles of 94°C for 1 min, annealing at 62°C for 1 min and extension at 72°C for 1 min, with a final extension step at 72°C for 7 min and a 4°C hold.
Detection and purification of amplified PCR products
The RT-PCR products were visualized on 1% agarose gel (Mast Group Ltd, Merseyside, UK) stained with ethidium bromide (0.8 µg/ml) and were exposed to ultraviolet light. PCR products of 619 bp were excised from the gel and purified using the Qiaquick® PCR purification kit (Qiagen).
Nucleotide sequence determination and analysis
Purified PCR products were sequenced using the MK AE 1 and MK AE 2 primers and the Big Dye® Terminator v3.1 cycle sequencing kit (Applied Biosystems, Warrington, UK) according to the manufacturer's recommendations. Nucleotide sequences were analysed using the Vector NTI software suite (Invitrogen Ltd) and similarity searches were performed against the non-redundant nucleic acid sequence database using the BLASTN programme at the NCBI, USA. The 575 bp DNA sequence of the AEV amplicon (minus primer sequences) from the brain of the 3-week-old poults was submitted to GenBank and given accession number EU327593.
Results
Clinical examination
Clinical examination of the submitted 1-day-old and 3-day-old chicks showed a variable tremor—in some cases very marked and evident especially when the chicks were upright. The chicks were incoordinated, falling on their backs and unable to right themselves easily, and they also had a tendency to sit on their hocks, and to sit or lie with their legs at unusual angles. The chicks tended to move about with splayed legs and wings, often falling over. Many of the chicks showed curling of the toes. The 1-day-old chicks were mostly bright and alert but the more severely affected 3-day-old chicks were dull. Postmortem examination of the 1-day-old chicks confirmed they had not yet fed but no gross abnormalities were noted. Some of the 3-day-old chicks had fed but most showed depletion of the coronary fat deposits, and distension of the ureters indicative of dehydration. Clinical examination of the blind poults on the farm at 3 weeks of age showed a pale grey opacity of one or both eyes () but there was no evidence of conjunctivitis, increased lacrimation or photophobia.
Figure 1. A 3-week-old pheasant poult showing a cataract in the eye. The poult had recently been fitted with a plastic “bit” to prevent feather pecking.

Following postmortem examination, bacterial culturing from the chicks yielded light growths of coliforms, enterococci and staphylococci but no organisms predominated and there were no consistent bacterial isolates from the brain of any of the chicks.
Histopathological analysis
Histopathological examination of the brain of the clinically affected 1-day-old and 3-day-old chicks showed a mild to moderate acute encephalomyelitis mostly limited to the brainstem, but not all chicks were affected. The changes comprised one or more degenerating vacuolated neurons present in the brainstem nuclei, mostly with no obvious accompanying inflammatory cell reaction. Similar changes were visible in the spinal cord in some samples. No abnormalities were noted in the proventriculus, gizzard, pancreas/duodenum or myocardium. In the 3-week-old poults, all five samples showed evidence of an acute to sub-acute encephalitis with gliosis evident principally in the brainstem and to a lesser extent in the Purkinje and molecular layers and in the central white matter of the cerebellum. Light perivascular lymphocytic cuffing of a few blood vessels in the cerebrum and degenerating vacuolated neurons were present in the brainstem or spinal cord of some samples. In two out of the five, there was a single glial focus (“glial flare”) in the molecular layer of the cerebellum. Three eyes showed liquefaction of the lens fibres in the lateral and medial subcapsular zones and dysplasia of the lens epithelium consistent with cataract.
No abnormalities were noted in the proventriculus, gizzard, pancreas/duodenum, myocardium or peripheral nerve tissues.
Virological, serological and RT-PCR analysis
During two passages, no gross abnormalities were recorded in the inoculated embryos and no cytopathic effect was recorded in the chicken embryo liver cultures. The ELISA results were positive for AEV antibodies in four (80%) of the sera from the 3-week-old poults (S/P ratios = 0.37 to 1.29) and 23 (79%) of the sera from the adult breeding birds (S/P ratios = 0.21 to 2.21).
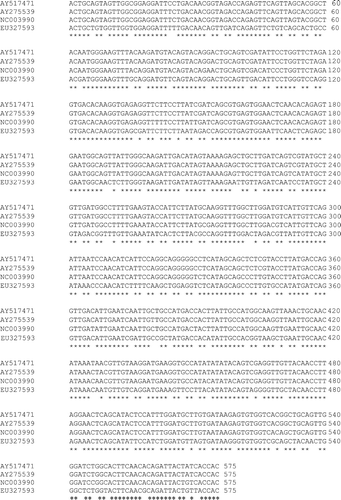
Gel electrophoresis analysis revealed that DNA amplicons of approximately 619 bp were produced when the RNA extracted from infected pheasant brains and pancreas were subjected to RT-PCR. The nucleotide sequences of the fragments amplified from both pheasant brain samples were determined to be identical, and this sequence was compared by multiple alignment with three other AEV sequences available in the sequence databases. These were NC003990 (Calnek vaccine strain; Marvil et al., Citation1999), AY275539 (L2Z strain) and AY517471 (van Roekel vaccine strain; Wei et al., Citation2004). The nucleotide sequence of the pheasant AEV sequence (EU327593) aligned 83% with NC003990 and 82% with both AY517471 and AY275539 (). The amino acid sequence of EU327593 aligned 100% with NC003990, 99% with AY517471 and 97% with AY275539 (data not shown).
Figure 2. Nucleotide sequence multiple alignment of the 575 bp amplicons within the VP2 gene of AEV. *Sequence of complete identity. The multiple alignment was generated using the ClustalW2 program available from the European Bioinformatics Institute (http://www.ebi.ac.uk/Tools/clustalw2/index.html; Larkin et al., Citation2007).
Discussion
The clinical signs of tremor and incoordination in the affected 1-day-old and 3-day-old pheasant chicks were typical of epidemic tremor and resembled those reported in cases of AE in domestic fowl chicks. In the latter, naturally occurring outbreaks of the disease do not usually appear until 1 to 2 weeks of age reflecting horizontal transmission of AEV at or shortly after hatching (Calnek et al., Citation1960; Calnek, Citation2008). However, vertical transmission of AEV from parent birds can result in chicks being affected at the time of hatching, as in this outbreak, although there was no concurrent marked drop in egg production and hatchability as can occur in chickens (Calnek et al., Citation1960). The outbreak resulted in a marked increase in mortality and culling in Hatches 10 and 11. The cataracts seen in some of the Hatch 11 poults at 3 weeks of age matched those reported as a sequel to neurological signs associated with AE in chickens, from approximately 10 weeks of age onwards (Peckham, Citation1957; Bridges & Flowers, Citation1958). The development of cataracts in pheasant chicks following AE, and apparently much sooner after infection than in chickens, has not been previously reported. A field outbreak of presumptive AE has been reported previously in pheasant chicks, causing 50% mortality and culling, although the age of the chicks was not given (Mathey, Citation1955). However, pheasants were considered less susceptible to AE than grey or red-legged partridges when inoculated with the egg-adapted Van Roekel strain of AEV of domestic fowl origin (Bodin et al., Citation1981) and less susceptible than domestic fowl or turkeys. Nevertheless the clinical signs seen in this outbreak in the pheasant chicks at hatching implicated the vertical transmission of AEV from infected breeding hens; experimental infection of domestic fowl was reported to result in egg transmission of the virus between 6 and 13 days post inoculation (Calnek et al., Citation1960), after which it appears to be prevented by the host immune response. No other recognized causes of encephalitis were demonstrated in the affected pheasant chicks, and in particular there was no virological evidence of Newcastle disease, which is a differential diagnosis of neurological signs at this age (Calnek, Citation2008).
The histopathological findings in the brain and spinal cord of the 1-day-old and 3-day-old pheasant chicks were noteworthy for the paucity of lesions, and in some chicks no abnormalities were detected despite the presence of marked clinical signs during life. A similar lack of histopathological changes in chicks under 7 days of age has been reported in AE domestic fowl (Calnek et al., Citation1960). In the samples where lesions were observed, they mainly comprised neuronal vacuolar degeneration, mild gliosis, and perivascular lymphocytic cuffing of occasional blood vessels consistent with a mild non-suppurative encephalomyelitis. Lesions were more apparent in the 3-week-old poults where all five brains examined were affected, although the lesions were in all cases still relatively mild compared with those commonly seen in chicks of the domestic fowl or turkey with AE. Focal gliosis of the Purkinje and molecular layers was noted in some of the 3-week-old poults, but no evidence was seen of the Purkinje cell degeneration described by Hishida et al. (Citation1986) as the most characteristic change in domestic fowl chicks horizontally infected with AEV. However, in experimental infection this was a reversible change and the cells had returned to normal at 15 to 17 days post inoculation. Central chromatolysis of large neurons in brainstem nuclei was not found, and “glial flares” in the molecular layer of cerebellum, which are often prominent in chickens and turkeys, were only a minor feature in two of the five brains examined. Similarly, no lymphoid proliferations with follicle formation such as occur in naturally infected chickens (Peckham, Citation1957) were detected in the muscle layers of the proventriculus, gizzard or duodenum, or in the pancreas or myocardium.
Histopathological examination of the eyes of the 3-week-old poults with ocular opacity confirmed the presence of cataract, a lesion previously recognized in natural AE (Peckham, Citation1957; Bridges & Flowers, Citation1958). The relative scarcity of microscopic changes even in the 3-week-old clinically affected pheasant chicks may be explained by the very limited susceptibility of this species to the effects of AEV (Bodin et al., Citation1981).
Attempts to detect AEV in the brain and pancreas of the affected pheasants by embryo inoculation and cell culture were unsuccessful. Detecting field and non-egg-adapted strains of AEV is more difficult than detecting egg-adapted strains (Tannock & Shafren, Citation1994), and other possible reasons could include a low titre of virus present, neutralization of the virus by a maternally-derived immune response, a different host response to the virus in pheasants compared with that in chickens or different characteristics of the agent in pheasants. However, evidence of AEV was detected in the pheasant chicks following RT-PCR amplification; over the 575 bp region compared, the translated amino acid sequences from the pheasant AEV and three chicken AEVs were very similar with amino acid identities being in the range 97% to 100%. In contrast, whereas comparisons of the three chicken AEV sequences showed nucleotide identities of between 95% and 99%, the pheasant AEV displayed 82% to 83% nucleotide identity with the three chicken AEV strains over the same region. Based on the nucleotide sequence results, it is evident that the AEV infecting these pheasants was distinct from those from chickens. This demonstrated the value of RT-PCR amplification in the detection of AEV in a non-standard host species with minimal central nervous system lesions and where virus isolation has proved unsuccessful. Similarly, immunohistochemistry was used by Toplu & Alcigir (Citation2004) to demonstrate AEV in pigeons, and would have been a useful diagnostic aid in this case also.
Antibodies to AEV were detected in both the adult pheasants and the poults by a commercial ELISA test. Previous experience with the ELISA test kit used indicated that it is able to detect antibody to AEV in pheasant serum (R.E. Gough, unpublished observations), although the test kit is not validated for use in this species. It is known that there are common antigenic sites on chicken, turkey and pheasant immunoglobulins (Narat et al., Citation2004), so it is not unreasonable to expect that the polyclonal anti-chicken immunoglobulin conjugate used in this ELISA kit will cross-react to a considerable extent with both turkey and pheasant immunoglobulins. Although not validated in pheasants, the positive results concur with previous observations that seroconversion occurs in this species (Steenis, Citation1971).
The source of AEV in the pheasants was not confirmed although the high seroprevalence in the adult breeding stock at the end of the breeding season indicated there had been widespread exposure to infection. There was no known history of contact with chickens or other gallinaceous species, and the differences in nucleotide sequences between the pheasant and known chicken strains suggest a chicken source was less likely. There would have been opportunity for contact with non-gallinaceous wild birds through the winter, including pigeons (in which AE has been reported by Toplu & Alcigir, Citation2004), but the timing of the outbreak suggests these were unlikely to have been a source of infection. The circumstantial evidence suggests that AEV entered the flock via the cock pheasants introduced to the farm in February (or conceivably as a result of contact with wild pheasants, perhaps in the wintering pens). If the cocks were the source, it appears they carried the virus through their 3-week period of quarantine and infected the hens from March onwards. Viral contamination of the environment of the breeding pens may also have contributed to the dissemination of infection. Some aspects of the epidemiology of AEV remain undefined, including the role of a carrier status, but the virus can remain infectious in the environment for long periods of time (Calnek, Citation2008). In addition it may have been carried between the breeding pens by the keepers and by the moving of cock birds during the season. A combination of factors such as these presumably accounted for the appearance of clinical AE in chicks in Hatches 10 and 11 as a result of a gradual build up of infection in the breeding pens.
The outbreak of AE represented a significant economic loss in the flock, prompting the owner to consider means of preventing the disease recurring in the future. In domestic fowl, AE is self-limiting and a simple humoral response provides lifelong protection. Vaccination is widely used to control AE in breeding flocks in order to prevent vertical transmission (reviewed by Calnek, Citation1998), and might be suitable as a means of enhancing immunity in pheasants although no licensed vaccines are available in this species in the UK. Nevertheless, if pheasants are relatively resistant to the effects of clinical AE (Bodin et al., Citation1981) and antibodies are present in the population (Steenis, Citation1971), then vaccination may not be considered necessary in many flocks. Further investigation is required to establish the prevalence of AEV antibodies in the wider pheasant population.
AE was demonstrated as the cause of the outbreak of neurological disease in this pheasant flock. It is probable that disease arose as a result of the entry of AEV via infected carrier birds into a naïve breeding flock at a critical time at the start of the breeding season, despite the introduced birds undergoing a period of quarantine. In hindsight, this episode has shown the inadvisability of introducing new stock close to the breeding season when there is risk of disease agents including AEV being vertically transmitted through the egg. AE should be considered a differential in the diagnosis of neurological signs and blindness in young pheasants.
Acknowledgements
The authors are grateful to the owner of the pheasants for much help during the investigation of this outbreak and for furnishing the production records, and to Dr G. MacKenzie for carrying out some of the histopathology.
References
- Bodin , G. , Pellerin , J.L. , Milon , A. , Geral , M.F. , Berthelot , X. and Lautie , R. 1981 . Étude de la contamination expérimentale du gibier à plumes (faisans, perdrix rouges, perdrix grises) par le virus de l'encéphalomyélite infectieuse aviaire . Revue Médicine Véterinaire , 132 : 805 – 816 .
- Bridges , C.H. and Flowers , A.I. 1958 . Iridocyclitis and cataracts associated with an encephalomyelitis in chickens . Journal of the American Veterinary Medical Association , 132 : 79 – 84 .
- Calnek , B.W. 1998 . Control of avian encephalomyelitis: a historical account . Avian Diseases , 42 : 632 – 647 .
- Calnek , B.W. 2008 . Avian encephalomyelitis . In Y.M. Saif , A.M. Fadly , J.R. Glisson , L.R. McDougald , L.K. Nolan & D.E. Swayne Diseases of Poultry 12th ed pp. 430 – 431 . Ames , IA : Blackwell Publishing .
- Calnek , B.W. , Taylor , P.J. and Sevoian , M. 1960 . Studies on avian encephalomyelitis. IV Epizootology . Avian Diseases , 4 : 325 – 347 .
- Hishida , N. , Odagiri , Y. , Kotani , T. and Horiuchi , T. 1986 . Morphological changes of neurons in experimental avian encephalomyelitis . Japanese Journal of Veterinary Science , 48 : 169 – 172 .
- Larkin , M.A. , Blackshields , G. , Brown , N.P. , Chenna , R. , McGettigan , P.A. , McWilliam , H. , Valentin , F. , Wallace , I.M. , Wilm , A. , Lopez , R. , Thompson , J.D. , Gibson , T.J. and Higgins , D.G. 2007 . Clustal W and Clustal X version 2.0 . Bioinformatics , 23 : 2947 – 2948 .
- Marvil , P. , Knowles , N.J. , Mockett , A.P.A. , Britton , P. , Brown , T.D.K. and Cavanagh , D. 1999 . Avian encephalomyelitis virus is a picornavirus and is most closely related to hepatitis A virus . Journal of General Virology , 80 : 653 – 662 .
- Mathey , W.J. 1955 . Avian encephalomyelitis in pheasants . Cornell Veterinarian , 45 : 89 – 93 .
- Narat , M. , Biček , A. , Vadnjal , R. and Benčina , D. 2004 . Production, characterisation and use of monoclonal antibodies recognising IgY epitopes shared by chicken, turkey, pheasant, peafowl and sparrow . Food Technology and Biotechnology , 42 : 175 – 182 .
- Peckham , M.C. 1957 . Case report—lens opacities in fowls possibly associated with epidemic tremors . Avian Diseases , 1 : 247 – 255 .
- Steenis , G. van . 1971 . Survey of various avian species for neutralising antibody and susceptibility to avian encephalomyelitis virus . Research in Veterinary Science , 12 : 308 – 311 .
- Tannock , G.A. and Shafren , D.R. 1994 . Avian encephalomyelitis: a review . Avian Pathology , 23 : 603 – 620 .
- Todd , D. , Weston , J.H. , Mawhinney , K.A. and Laird , C. 1999 . Characterisation of the genome of avian encephalomyelitis virus with cloned cDNA fragments . Avian Diseases , 43 : 219 – 226 .
- Toplu , N. and Alcigir , G. 2004 . Avian encephalomyelitis in naturally infected pigeons in Turkey . Avian Pathology , 33 : 381 – 386 .
- Wei , L. , Liu , J. , Yao , W. , Zhang , F. and Zhou , J. 2004 . Determination of the whole genome of an avian encephalomyelitis virus isolate from China . Chinese Journal of Virology , 20 : 230 – 236 .
- Xie , Z. , Khan , M.I. , Girshick , T. and Xie , Z. 2005 . Reverse transcriptase-polymerase chain reaction to detect avian encephalomyelitis virus . Avian Diseases , 49 : 227 – 230 .