Abstract
To understand the basis of the resistance of pigeons to Asian lineage highly pathogenic avian influenza virus (HPAIV) subtype H5N1, we examined the presence of influenza virus receptors, sialic acids linked to galactose by an α-2,3 linkage (SAα2,3Gal) or by an α-2,6 linkage (SAα2,6Gal), in respiratory and intestinal tracts of pigeons and compared the distributions of N-acetylneuraminic and N-glycolylneuraminic acids in the trachea and intestines of pigeons and chickens. Results suggested that the epithelial surfaces of the larynx, trachea, bronchus, and bronchiole of pigeons contained abundant SAα2,6Gal with little or no SAα2,3Gal. In contrast, the epithelial surfaces of the pharynx, trachea, bronchus, and bronchiole of chickens contained mainly SAα2,3Gal, a well-recognized receptor for avian influenza viruses including H5N1 HPAIV. A similar distribution pattern of N-acetylneuraminic and N-glycolylneuraminic acids in the trachea and intestines of pigeons and chickens was observed. Overall, the results suggest that SAα2,6Gal is the major receptor in the pigeon airway, which may partly contribute to the resistance of pigeons to Asian lineage HPAIV subtype H5N1.
Introduction
The emergence of Asian lineage highly pathogenic (HP) avian influenza virus (AIV) subtype H5N1 in 1996 and the parallel cases of human infections by AIV H5N1 raise a major concern for a potential pandemic outbreak of human influenza A virus (Xu et al., Citation1999; Li et al., Citation2004; Shinya et al., Citation2006). In addition to humans, a number of wild and migratory birds, especially waterfowl, and wild mammals including tigers are susceptible to Asian lineage HPAIV H5N1 (Perkins & Swayne, Citation2002,Citation2003; Keawcharoen et al., Citation2004; J. Liu et al., Citation2005). Interestingly, we and others have shown that, using infectious doses likely to be encountered naturally, pigeons are not susceptible to different subtypes of AIV including Asian lineage HPAIV H5N1 and do not transmit virus to chickens (Panigrahy et al., Citation1996; Kaleta & Honicke, Citation2004; Fang et al., Citation2006; Y. Liu et al., Citation2007). However, the molecular mechanism of the observed resistance is not clear.
Sialic acid is a well-recognized receptor for influenza A viruses. The binding of haemagglutinin on the surface of influenza virus particles to sialic acid molecules on the epithelial cell surfaces initiates virus entry and subsequent virus replication cycle in susceptible cells. Both virus haemagglutinin mutations and the adaptation of the host's receptors can influence the binding specificities. Accumulative evidence suggests that the linkage of sialic acid to galatose, the species of sialic acid, and the anatomic distribution of sialic acids in the airway of animals all play important roles in determining the host's susceptibility and the transmission efficiency of specific influenza viruses. For AIVs, including the recently emerged HPAIV subtype H5N1 virus, one of the major receptors located in the chicken airway is sialic acid linked to galatose by an α2,3 linkage (SAα2,3Gal) (Gambaryan et al., Citation2002). A number of avian and mammalian species that are highly susceptible to AIVs such as ducks, pigs, horses, and others apparently contain predominantly SAα2,3Gal in the upper respiratory tract or in the intestines (Connor et al., Citation1994; Ito et al., Citation1998; Ito et al., Citation2000; Suzuki et al., Citation2000). In contrast, humans have primarily sialic acid linked to galatose by an α2,6 linkage (SAα2,6Gal) in the upper respiratory tract and are relatively insusceptible to AIV infection (Rogers & Paulson, Citation1983; Shinya et al., Citation2006). Pigs are unusual in that they have receptors for both AIVs, SAα2,3Gal, and human influenza viruses, SAα2,6Gal, in their respiratory tracts, suggesting that pigs serve as a mixing vessel for generating human influenza virus and AIV re-assortment strains, which could result in pandemic outbreaks of influenza virus (Ito et al., Citation1998). We have recently shown that pigeons are resistant to Asian lineage HPAIV subtype H5N1 isolates (Y Liu et al., Citation2007). The presence and distribution of SAα2,3Gal and SAα2,6Gal in the respiratory and intestine tracts of pigeons have not been reported. We hypothesized that the sialic acid receptors and their distribution in the pigeon airway may contribute to the insusceptibility. In the present study, we tested this hypothesis by determining the distribution of the two receptors in the respiratory and intestinal tracts of pigeons using lectins specific for SAα2,3Gal or SAα2,6Gal.
In addition to the sialic acid linkages, the species of sialic acid has also been reported to be a determinant for the susceptibility animals to influenza A viruses (Suzuki et al., Citation2000). It is well recognized that different animal species exhibit quite distinct distributions of the two main sialic acid species, N-acetylneuraminic acid (NeuAc) and N-glycolylneuraminic acid (NeuGc) (Hara et al., Citation1987). We intended to determine the distribution of these two sialic acids in the trachea and intestines of pigeons and to compare the difference between pigeons and chickens.
Materials and Methods
Animals
Specific-pathogen-free chickens were purchased from Beijing Experimental Animal Research Center (Beijing, China). Healthy racing pigeons were provided by the Beijing Conference Center. Animal protocols used in this study were approved by Beijing Animal Welfare Committee (SYXK, Beijing, 2002-0010).
Detection of SAα2,3Gal and SAα2,6Gal in animal tissues
The larynx, trachea (including upper, middle, and lower parts), lungs, duodenum, ileum, and rectum were collected from four to six healthy pigeons of different ages (1, 5, 15, 30, 56, and 112 days old) and from chickens (45 and 160 days old). Tissues were fixed with 10% neutral formalin for 24 h. The tissues were then trimmed and fixed for an additional 12 h in fresh 10% neutral formalin. Tissues were dehydrated and processed for paraffin embedding. Embedded tissues were cut into 3 µm sections using a microtome. The mounted sections were deparaffinized in xylene and immersed in water. To detect sialyloligosaccharides reactive with SAα2,3 Gal-specific or SAα2,6Gal-specific lectins, the sections were incubated with 1:50 dilution of biotinylated Maackia amurensis lectin (Vector Laboratories Inc., Burlingame, California, USA) and 1:50 dilution of FITC-labelled Elderberry bark lectin (Vector Laboratories Inc.) at 35°C for 1 h. After washing three times with Tris-buffered saline (pH 7.6), the sections were incubated with 1:200 dilution of Alexa Fluor 568-conjugated streptavidin (Molecular Probes Inc., Eugene, Oregon, USA) at 35°C for 25 min. After washing three times with Tris-buffered saline, Vectashield Mounting Medium with DAPI (Vector Laboratories Inc.) was added dropwise and the sections were sealed. The sections were observed under an Olympus 1X51F + DP70 fluorescence microscope.
Determination of distributions of NeuAc and NeuGc in trachea and intestinal tracts of pigeons and chickens using Reverse-Phased High-Performance Liquid Chromatography
The epithelial surfaces of tracheas were directly scraped with scissors and used for the extraction of sialic acids. The intestines were washed three times with distilled water before scraping with scissors. Ten milligrams of collected epithelial cells were hydrolysed in 25 mM H2SO4 at 80°C for 2 h to release the free sialic acids. Cooled samples were spun at 13 200×g (Sigma 3K15 centrifuge) for 10 min. Forty microlitres of supernatant were removed and added to 200 µl 1,2-diamino-4, 5-methyleneoxybenzene (Takara Bio Inc.) and heated at 50°C for 2.5 h in the dark. The mixture was placed in ice-cold water to stop the reaction. Ten microlitres of 1,2-diamino-4, 5-methyleneoxybenzene derived sialic acids were separated by reverse-phase high-performance liquid chromatography using a C-18 column (250 mm x 4.6 mm; particle size, 5 mm) and examined by a Shimadza RF-535 fluorescence spectromonitor (Shimadzu, Kyoto, Japan) (emission:excitation = 448 nm:373 nm). The mobile phase was a mixture of acetonitrile–methanol–water (9:7:84) at a flow rate of 1 ml/min. The amount of sialic acids was determined using the methods described previously (Hara et al., Citation1987).
Statistical analysis
Statistical analysis was performed using the Student t test. Differences were considered statistically significant with P<0.05.
Results and discussion
We have previously shown that pigeons are resistant to Asian lineage HPAIV subtype H5N1 (Y. Liu et al., Citation2007). We hypothesized that pigeons might not possess the specific receptors for the initial attachment and subsequent entry into the host of Asian lineage HPAIVs. To test this hypothesis, we first examined the distribution of two known influenza virus receptors, SAα2,3Gal and SAα2,6Gal, in the respiratory and intestinal tracts of pigeons. It has been shown that SAα2,3Gal is one of the major receptors for AIVs including the H5N1 subtype, while SAα2,6Gal is the major known receptor for human influenza viruses but not AIVs (Connor et al., Citation1994; Shinya et al., Citation2006). However, the presence and distribution of these two receptors in the respiratory and intestinal tracts of pigeons have not been reported. We collected the pharynx, trachea (upper, middle, lower), lungs, duodenum, ileum, and rectum from healthy pigeons of different age groups, including 1, 5, 15, 30, 56 and 112 days old. The presence of influenza virus receptors was detected using lectins specific for SAα2,3Gal and SAα2,6Gal as described elsewhere (Connor et al., Citation1994; Shinya et al., Citation2006). The results obtained showed that the epithelial surfaces of the pharynx, trachea, bronchus, and bronchiole of pigeons of different ages contained mainly SAα2,6Gal ( and ); that is, the receptor for human influenza viruses. Little or no SAα2,3Gal was found in the pigeon respiratory tract except in lung alveolar cells, which occasionally showed positive staining. This is in direct contrast to the predominant SAα2,3Gal distribution in the respiratory tracts of chickens ( and ). Interestingly, the anatomic distribution of SAα2,6Gal and SAα2,3Gal in the respiratory tract of pigeons is very similar to their distribution in the human respiratory tract (Shinya et al., Citation2006). These data suggest that the dominant presence of SAα2,6Gal in the respiratory tract of pigeons may partly contribute to the resistance of pigeons to Asian lineage HPAIV H5N1. We speculate that virus failed to replicate efficiently in pigeons and did not transmit virus to chickens in experimentally infected animals due to the restriction in compatible receptor availability (Y. Liu et al., Citation2007).
Figure 1. Influenza virus receptor profiles of the respiratory and intestinal tracts of pigeons. Green, staining with a lectin specific for SAα2,6Gal; red, staining with a lectin specific for SAα2,3Gal; blue, nuclear staining (DAPI). br, bronchiole. Samples from different ages of pigeons and chickens were examined and similar results were obtained.
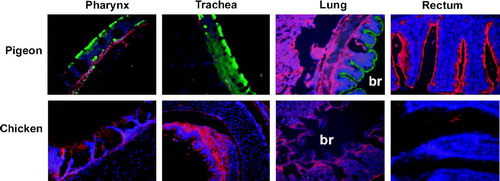
Table 1. Distributions of SAα2,3Gal and SAα2,6Gal in the respiratory and intestinal tracts of pigeon and chicken
Despite the predominant distribution of SAα2,6Gal in the respiratory tracts of pigeons, we observed that the rectum of pigeons contained predominantly SAα2,3Gal. Interestingly, little or no SAα2,3Gal was detected in the rectum of chickens ( and ). Furthermore, very few or no SAα2,3Gal and SAα2,6Gal receptors were detected in duodenum or ileum samples of chickens and pigeons (). Previous studies have reported that ducks contained abundant SAα2,3Gal in their intestines, which is assumed to be the major site for AIV replication (Ito et al., Citation2000). However, we did not detect any virus replication in pigeons when they were inoculated with different Asian lineage HPAIV H5N1 isolates via different inoculation routes (Y. Liu et al., Citation2007). We recognize that multiple viral and host factors may contribute to the susceptibility to influenza virus. For example, both the nucleoprotein and matrix proteins of influenza viruses are reported to be responsible for the restriction of virus replication in squirrel monkeys (Tian et al., Citation1985). Polymerase genes have also been shown to affect the generation of reassortant viruses, which may exhibit a different host range (Subbarao et al., Citation1993). It is possible that multiple host and viral factors contribute to the resistance of pigeons to Asian lineage HPAIVs. Further studies are needed to fully characterize the molecular mechanisms of the resistance of pigeons to AIV infections. It is interesting to note that the receptor profile in pigeons mimics those of human respiratory tracts, but is very different from chickens. Future studies will be focused on characterizing the binding of AIVs and human influenza viruses to the pigeon airway and rectum epithelial cells, which will provide further evidence on the specificity of the lectin staining profile we observed here and the definitive role of receptors in determining the resistance of pigeons to AIV H5N1 infections.
Previous studies suggested that the sialic acid species also serves as one of the determinants of the host's susceptibility to influenza A virus. The difference in distribution of sialic acids in different animal species has been well recognized. Most animal species possess both NeuAc and NeuGc, but others such as humans contain very little NeuGc (Suzuki et al., Citation2000). It has been reported that specific haemagglutinin molecules on an influenza virus recognize NeuGcα2,3Gal, but not NeuAcα2,6Gal (Suzuki et al., Citation2000). To determine whether the distribution of sialic acids, NeuAc and NeuGc, play any role in the resistance of pigeons to HPAIV H5N1 isolates, we examined the contents of both sialic acids in the trachea and intestines of different ages of pigeons and chickens. As shown in , both types of sialic acid were detected from the trachea and intestines of chicken and pigeons. However, considerably higher levels of NeuAc than NeuGc were observed in both tracheas and intestines of pigeons and chickens; with a NeuAc/NeuGc ratio varying from 1.28 to 67.53. Additionally, a significantly higher level of NeuAc was observed in the trachea, duodenum, jejunum, and rectum samples from chickens compared with those of pigeons (P<0.05, P <0.01). Nonetheless, a similar distribution pattern was observed in both species. We conclude that the species of sialic acids may not play a significant role in establishing influenza virus infection in pigeons.
Table 2. Distributions of NeuGc and NeuAc in the trachea and intestines of pigeons and chickens
Acknowledgements
The project was supported by grants from Beijing Natural Science Foundation (Grant No. 6072011), National Science Foundation (Grant No. 30740028), Beijing Municipal Homecoming Personnel, and New Millennium Specialist.
References
- Connor , R.J. , Kawaoka , Y. , Webster , R.G. and Paulson , J.C. 1994 . Receptor specificity in human, avian, and equine H2 and H3 influenza virus isolates . Virology , 205 : 17 – 23 .
- Fang , T.H. , Lien , Y.Y. , Cheng , M.C. and Tsai , H.J. 2006 . Resistance of immune-suppressed pigeons to subtypes H5N2 and H6N1 low pathogenic avian influenza virus . Avian Diseases , 50 : 269 – 272 .
- Gambaryan , A. , Webster , R. and Matrosovich , M. 2002 . Differences between influenza virus receptors on target cells of duck and chicken . Archives of Virology , 147 : 1197 – 1208 .
- Hara , S. , Takemori , Y. , Yamaguchi , M. , Nakamura , M. and Ohkura , Y. 1987 . Fluorometric high-performance liquid chromatography of N-acetyl- and N-glycolylneuraminic acids and its application to their microdetermination in human and animal sera, glycoproteins, and glycolipids . Analytical Biochemistry , 164 : 138 – 145 .
- Ito , T. , Couceiro , J.N. , Kelm , S. , Baum , L.G. , Krauss , S. Castrucci , M.R. 1998 . Molecular basis for the generation in pigs of influenza A viruses with pandemic potential . Journal of Virology , 72 : 7367 – 7373 .
- Ito , T. , Suzuki , Y. , Suzuki , T. , Takada , A. , Horimoto , T. Wells , K. 2000 . Recognition of N-glycolylneuraminic acid linked to galactose by the 2,3 linkage is associated with intestinal replication of influenza A virus in ducks . Journal of Virology , 74 : 9300 – 9305 .
- Kaleta , E.F. and Honicke , A. 2004 . Review of the literature on avian influenza A viruses in pigeons and experimental studies on the susceptibility of domestic pigeons to influenza A viruses of the haemagglutinin subtype H7 . Deutsche Tierarztliche Wochenschrift , 111 : 467 – 472 .
- Keawcharoen , J. , Oraveerakul , K. , Kuiken , T. , Fouchier , R.A. , Amonsin , A. Payungporn , S. 2004 . Avian influenza H5N1 in tigers and leopards . Emerging Infectious Diseases , 10 : 2189 – 2191 .
- Li , K.S. , Guan , Y. , Wang , J. , Smith , G.J. , Xu , K.M. Duan , L. 2004 . Genesis of a highly pathogenic and potentially pandemic H5N1 influenza virus in eastern Asia . Nature , 430 : 209 – 213 .
- Liu , J. , Xiao , H. , Lei , F. , Zhu , Q. , Qin , K. Zhang , X.W. 2005 . Highly pathogenic H5N1 influenza virus infection in migratory birds . Science , 309 : 1206
- Liu , Y. , Zhou , J. , Yang , H. , Yao , W. , Bu , W. Yang , B. 2007 . Susceptibility and transmissibility of pigeons to Asian lineage highly pathogenic avian influenza virus subtype H5N1 . Avian Pathology , 36 : 461 – 465 .
- Panigrahy , B. , Senne , D.A. , Pedersen , J.C. , Shafer , A.L. and Pearson , J.E. 1996 . Susceptibility of pigeons to avian influenza . Avian Diseases , 40 : 600 – 604 .
- Perkins , L.E. and Swayne , D.E. 2002 . Pathogenicity of a Hong Kong-origin H5N1 highly pathogenic avian influenza virus for emus, geese, ducks, and pigeons . Avian Diseases , 46 : 53 – 63 .
- Perkins , L.E. and Swayne , D.E. 2003 . Comparative susceptibility of selected avian and mammalian species to a Hong Kong-origin H5N1 high-pathogenicity avian influenza virus . Avian Diseases , 47 : 956 – 967 .
- Rogers , G.N. and Paulson , J.C. 1983 . Receptor determinants of human and animal influenza virus isolates: differences in receptor specificity of the H3 hemagglutinin based on species of origin . Virology , 127 : 361 – 373 .
- Shinya , K. , Ebina , M. , Yamada , S. , Ono , M. , Kasai , N. and Kawaoka , Y. 2006 . Avian flu: influenza virus receptors in the human airway . Nature , 440 : 435 – 436 .
- Subbarao , E.K. , London , W. and Murphy , B.R. 1993 . A single amino acid in the PB2 gene of influenza A virus is a determinant of host range . Journal of Virology , 67 : 1761 – 1764 .
- Suzuki , Y. , Ito , T. , Suzuki , T. , Holland , R.E. Jr , Chambers , T.M. Kiso , M. 2000 . Sialic acid species as a determinant of the host range of influenza A viruses . Journal of Virology , 74 : 11825 – 11831 .
- Tian , S.F. , Buckler-White , A.J. , London , W.T. , Reck , L.J. , Chanock , R.M. and Murphy , B.R. 1985 . Nucleoprotein and membrane protein genes are associated with restriction of replication of influenza A/Mallard/NY/78 virus and its reassortants in squirrel monkey respiratory tract . Journal of Virology , 53 : 771 – 775 .
- Xu , X. , Subbarao Cox , N.J. and Guo , Y. 1999 . Genetic characterization of the pathogenic influenza A/Goose/Guangdong/1/96 (H5N1) virus: similarity of its hemagglutinin gene to those of H5N1 viruses from the 1997 outbreaks in Hong Kong . Virology , 261 : 15 – 19 .