Abstract
Consensus polymerase chain reaction was used to identify a novel adenovirus from two psittacine birds: a plum-headed parakeet (Psittacula cyanocephala) with lethargy, weight loss, and marked leukocytosis; and an umbrella cockatoo (Cacatua alba) with lethargy, weight loss, and feather abnormalities. Phylogenetic and comparative sequence analysis suggested that this virus is a member of the genus Siadenovirus, and is here termed psittacine adenovirus 2. This extends the characterized adenoviruses of psittacine birds beyond Aviadenovirus to include the genus Siadenovirus. Identification and further study of adenoviral types and species will provide useful diagnostic, prognostic, and epidemiologic information for the clinician. Like other known members of the genus Siadenovirus, Psittacine adenovirus 2 is AT-rich over the region sequenced, and it is hypothesized that this may be associated with shorter host–virus evolutionary association.
Introduction
Members of the family Adenoviridae are non-enveloped double-stranded DNA viruses with a medium-sized genome of 26 to 45 kbp. Like many DNA viruses, phylogenetic analyses of adenoviruses indicate that many elements in the branching patterns of these viruses are congruent with branching patterns for the corresponding host species (Benkö & Harrach, Citation2003). This suggests that these viruses have often co-evolved along with their hosts, although host switches have certainly occurred. Adenoviruses are classified into four genera; Mastadenovirus, Aviadenovirus, and the two recently accepted genera Atadenovirus and Siadenovirus (Benkö et al., Citation2005). A fifth genus is proposed for fish adenoviruses (Benkö et al., Citation2005). Members of Mastadenovirus are found in mammals, and aviadenoviruses are found in birds. Atadenoviruses have been found in birds (Harrach et al., Citation1997) and mammals (Dán et al., Citation1998; Thomson et al., Citation2002), but it appears that their most probable origin is in squamates (Harrach, Citation2000; Benkö et al., Citation2002; Farkas et al., Citation2002; Wellehan et al., Citation2004). Siadenoviruses have been found in birds (Pitcovski et al., Citation1998), tortoises (Rivera et al., Citation2009), and amphibians (Davison et al., Citation2000). The origin of Siadenovirus is not clear. Three of four accepted genera of adenoviruses are found in avian hosts.
Lesions consistent with adenoviral disease have been found in many psittacine species, including budgerigars (Melopsittacus undulatus) (McFerran et al., Citation1976), cockatiels (Nymphicus hollandicus) (Scott et al., Citation1986), lovebirds (Agapornis spp.) (Pass, Citation1987; Jacobson et al., Citation1989), parakeets (Psittacula krameri) (Desmidt et al., Citation1991), eclectus parrots (Eclectus roratus) (Ramis et al., Citation1992), Amazon parrots (Amazona spp.) (Gómez-Villamandos et al., Citation1992), African grey parrots (Psittacus erithacus) (Droual et al., Citation1994), cockatoos (Cacatua spp.) (Latimer et al., Citation1997), Poicephalus spp. (Latimer et al., Citation1997; Wellehan et al., Citation2005), and lorikeets (Trichoglossus spp.) (Mackie et al., Citation2003). Lesions associated with adenoviruses in psittacine birds include hepatitis, (Scott et al., Citation1986; Pass, Citation1987; Ramis et al., Citation1992; Gómez-Villamandos et al., Citation1992; Droual et al., Citation1994), conjunctivitis (Jacobson et al., Citation1989), interstitial pneumonia (Desmidt et al., Citation1991; Ramis et al., Citation1992), enteritis (Droual et al., Citation1994; Mackie et al., Citation2003), and splenic lymphoid depletion (Gómez-Villamandos et al., Citation1995).
Most methods that have been reported for diagnosis of adenoviral infection in psittacine birds do not identify adenoviruses beyond the family level. The degree of cross-reactivity of antibodies to psittacine adenoviruses is not known, complicating interpretation of antibody-based diagnostics. Serology also requires that a virus be previously cultured, making this a poor method for novel virus discovery. Attempts at further classification of psittacine adenoviruses have previously been made in five reports. Spleens from psittacine birds were positive on immunohistochemistry using an antibody against turkey adenovirus 3, a siadenovirus (Gómez-Villamandos et al., Citation1995), and this is the only evidence to date suggestive of the presence of this genus in psittacines. Tissues from African grey parrots containing adenovirus-like particles on electron microscopy were negative on immunohistochemistry using an antibody against turkey adenovirus 3 (Droual et al., Citation1994). Isolates from African grey parrots and Cape parrots (Poicephalus robustus) were classified by serological and restriction enzyme methods as being consistent with fowl adenovirus 4, an aviadenovirus (Soike et al., Citation1998). The first available sequence information on psittacine adenoviruses described the DNA-dependent DNA polymerase (pol) of Meyer's parrot adenovirus 1, an Aviadenovirus (Wellehan et al., Citation2005), from Poicephalus meyeri. Shortly following this, the hexon gene of an aviadenovirus found in a Senegal parrot (Poicephalus senegalus) was described, which may be the same virus type found in the Meyer's parrot (Raue et al., Citation2005). An identical hexon sequence has recently been amplified from an Alexandrine parrot (Psittacula alexandri), and a specific real-time polymerase chain reaction (PCR) also resulted in product from a blue-fronted Amazon parrot (Amazona aestiva) (Lüschow et al., Citation2007).
Materials and Methods
Cases
Case 1
An 8-year-old male Plum-headed parakeet (Psittacula cyanocephala) was presented for concerns of depression and weight loss. On physical examination, the bird weighed 70 g, which was lower than 75 to 80 g seen on previous examinations. No other abnormalities were identified on physical examination. No abnormalities were identified on radiographs. Laboratory reference values were not available for this species, so reference values from ring-necked parakeets (P. krameri), a congeneric species, were used (Fudge, Citation2000). A complete blood count (CBC) revealed a marked leukocytosis (51.26×109/l, reference 8 to 14×109/l) with heterophilia (39×109/l, reference 4.5 to 7.6×109/l), lymphocytosis (7.2×109/l, reference 3.6 to 6.3×109/l), monocytosis (4.6×109/l, reference 0 to 0.1×109/l), and basophilia (0.5×109/l, reference 0 to 0.1×109/l). Plasma biochemistry found a mild hypoglycaemia (10.9 mmol/l, reference 12.21 to 19.65 mmol/l). Reference values were not available for creatine kinase (CK), but it was interpreted as elevated (3282 u/l). Direct faecal examination revealed a large number of flagellates. A cloacal swab was collected for culture, and was negative for Campylobacter, Salmonella, and Yersinia. The bird was started on courses of metronidazole (50 mg/kg per orally every 24 h×5 days), enrofloxacin (13 mg/kg per orally every 24 h×7 days), and meloxicam (0.2 mg/kg per orally every 24 h×3 days) and was hospitalized.
On recheck 1 week later, the bird had held weight at 70 g. No abnormalities were identified on physical examination. A CBC revealed continued marked leukocytosis (39.4×109/l) with heterophilia (26×109/l), lymphocytosis (8.7×109/l), and monocytosis (5.1×109/l), with a mild anaemia (38%, reference 45 to 54%), and hypochromia was noted. Plasma biochemistry found a mild elevation in AST (424 u/l, reference 152 to 386 u/l). Hypochromia was also noted on the CBC. Faecal examination found no flagellates or other parasites, and a faecal acid-fast stain found no evidence of Mycobacterium spp. A cloacal swab was submitted for adenoviral consensus PCR and sequencing.
Thirteen days after initial presentation, liver biopsies were taken surgically for histology and Mycobacterium avium and Mycobacterium genavense PCR.
Twenty days after presentation, the bird was noted to have dyspnoea with an auditory click, tail-bob, and increased respiratory rate. The weight had decreased to 66 g. Radiographs were consistent with an infiltrative lung pattern. A CBC revealed continued marked leukocytosis (35.3×109/l) with heterophilia (18×109/l), lymphocytosis (7.1×109/l), and monocytosis (7.4×109/l), with toxic heterophils and a continued mild anaemia (41%). Plasma was submitted for Chlamydophila psittaci serology and was negative. Fluid therapy and itraconazole therapy were initiated (14 mg/kg per orally every 12 h), and enrofloxacin therapy was reinitiated (5 mg/kg intramuscularly every 12 h). The patient died the following day.
Case 2
An approximately 20-year-old female umbrella cockatoo (Cacatua alba) was presented for a beak trim to manage chronic mandibular malocclusion. On physical examination, the bird was noted to have a decreased amount of powder down, dry skin on the feet, feathers that appeared chewed over the breast and dorsum, and retained feather sheaths at the crown. The right second and third toe had mild dermal proliferation distally. A CBC showed a mild heterophilia (9.2×109/l, reference 5.8 to 9.1×109/l) with a left shift (10 to 15% of the heterophils were immature), and plasma biochemistry found an elevated CK (1012 u/l, reference 152 to 444 u/l), lactate dehydrogenase (548 u/l, reference 208 to 405 u/l) and hyperuricemia (886 µmol/l, reference 208 to 648 µmol/l) (Fudge, Citation2000). A psittacine beak and feather disease virus-specific PCR test of whole blood and an immunofluorescence assay (IFA) test on serum for C. psittaci were both negative.
One year later, the bird presented again with a recent history of inactivity and difficulty walking. On physical examination, the bird was moderately thin (380 g compared with 426 g the previous year), with feathers that lacked powder down and had dysfunctional barbules giving the feathers an “unzipped” appearance. When standing, the bird exhibited instability of the head. When approached, the bird exhibited an intention tremor and ataxia. A weak toe grip was present. A cloacal swab was submitted for adenoviral consensus PCR and sequencing. Seven months after adenoviral testing, the neurological signs progressed, and the owners elected euthanasia since the bird could no longer stand unassisted.
PCR amplification and sequencing
DNA was extracted from samples using the DNeasy Kit (Qiagen, Valencia, California, USA). Adenoviral consensus nested PCR amplification of a partial sequence of the DNA-dependent DNA polymerase gene was performed using previously described methods (Wellehan et al., Citation2004). Products were resolved on 1% agarose gels, and bands of the expected size were excised and purified using the QIAquick Gel Extraction Kit (Qiagen). Direct sequencing was performed using the Big-Dye Terminator Kit v3.1 (Perkin-Elmer, Branchburg, New Jersey, USA) and was analysed on ABI 3130 automated DNA sequencers at the University of Florida Interdisciplinary Center for Biotechnology Research DNA Sequencing Facilities. All products were sequenced in both directions. Primer sequences were edited out prior to further analyses.
Phylogenetic analysis
The sequences were compared with those in the GenBank (National Center for Biotechnology Information, Bethesda, Maryland, USA), EMBL (Cambridge, UK), and Data Bank of Japan (Mishima, Shizuoka, Japan) databases using TBLASTX for protein coding sequence or BLASTN for non-protein coding sequence (Altschul et al., Citation1997). Predicted homologous 89 to 91 amino acid sequences of adenoviral DNA-dependent DNA polymerase were aligned using three methods: ClustalW (Thompson et al., Citation1994), T-COFFEE (Notredame et al., Citation2000), and MUSCLE (Edgar, Citation2004).
Bayesian analyses of each amino acid alignment were performed using MrBayes 3.1 (Ronquist & Huelsenbeck, Citation2003) with gamma-distributed rate variation and a proportion of invariant sites, and mixed amino acid substitution models. Statistical convergence was assessed by looking at the standard deviation of split frequencies as well as potential scale reduction factors of parameters. The first 10% of 1,000,000 iterations were discarded as a burn in.
Maximum likelihood analyses of each amino acid alignment were performed using PHYLIP (Phylogeny Inference Package, version 3.66) (Felsenstein, Citation1989), running each alignment in proml with amino acid substitution models JTT (Jones et al., Citation1992), PMB (Veerassamy et al., Citation2003), and PAM (Kosiol & Goldman, Citation2005) further set with global rearrangements, five replications of random input order, less rough, gamma plus invariant rate distributions, and unrooted. The values for the gamma distribution were taken from the Bayesian analysis. Sturgeon adenovirus was designated as the outgroup for bootstrapping. The combination of alignment producing the most likely tree was then used to create data subsets for bootstrap analysis to test the strength of the tree topology (200 re-samplings) (Felsenstein, Citation1985), which was analysed using the amino acid substitution model producing the most likely tree.
Sequence submission
The sequence was submitted to GenBank under accession number EU056825.
Results
Cases
Case 1
The ante-mortem liver biopsies found scattered small numbers of heterophils, lymphocytes, and rare plasma cells present in portal areas. The Mycobacterium PCR was negative.
Gross necropsy revealed evidence of fibrinous coelomitis and airsacculitis, and aerobic culture of the coelom at necropsy grew heavy growth of 100% Staphylococcus aureus. Routine tissue sections of all organs were collected in 10% neutral buffered formalin, processed and sectioned by routine methods, and stained with haematoxylin and eosin. Histopathology revealed severe acute fibrinous and heterophilic coelomitis with intralesional Gram-positive cocci, acute heterophilic pneumonia, acute heterophilic hepatitis, and focal necrotizing and heterophilic encephalitis. These lesions were attributed to bacterial coelomitis and sepsis following the liver biopsy procedure. In the liver, random nodular infiltrates of lymphocytes, plasma cells and heterophils with individual hepatocyte necrosis and multifocal karyomegaly were also seen. These lesions were more subacute to chronic in nature and appeared unrelated to the acute septic process.
Case 2
Gross necropsy revealed thickened meninges on the left side of the brain and small scattered white foci on the liver. Routine tissue sections of all organs were collected in 10% neutral buffered formalin, processed and sectioned by routine methods, and stained with haematoxylin and eosin. Histopathology revealed extensive primarily mononuclear meningoencephalitis with scattered neuronal necrosis. There was also evidence of diffuse hepatic lipidosis; random nodular infiltrates of lymphocytes, plasma cells and few heterophils in the liver; chronic moderate myocardial degeneration and fibrosis; and atherosclerosis of the distal aorta. The aetiology of the brain and inflammatory liver lesions was not apparent based upon histopathologic examination. The heart and aortic lesions were interpreted as age-related degenerative lesions.
PCR amplification and sequencing
Adenovirus PCR products from both cases was positive. Adenovirus PCR amplification yielded a 269 base pair product after primers were edited out. The sequence of the PCR products was identical.
Phylogenetic analysis
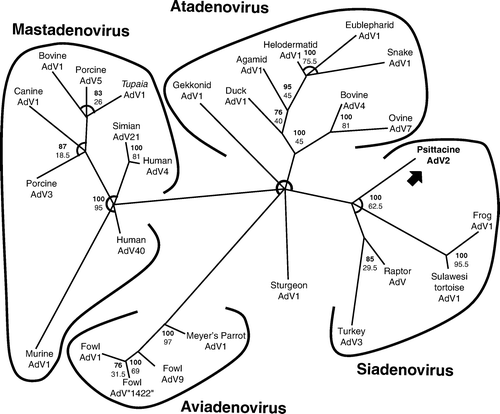
Comparison with other sequences using TBLASTX revealed that the adenovirus PCR product sequence is similar to, but distinct from. other adenoviruses present in the available databases. The highest score obtained was with Raptor adenovirus (GenBank accession number DQ022750). Raptor adenovirus is in the genus Siadenovirus (Zsivanovits et al., Citation2006). As it is unclear which species is the original host of the virus described in this study, we hereafter refer to this virus as psittacine adenovirus 2. In addition to being a new type, this virus probably represents a new species, which would be called Psittacine adenovirus B.
Psittacine adenovirus 2 sequence is A and T rich (62.1%), as are the other siadenoviruses with available comparable sequence (frog adenovirus 1, 62.5%; raptor adenovirus, 62.9%; Sulawesi tortoise adenovirus 1, 64.7%; Turkey adenovirus 3, 65.8%).
Bayesian phylogenetic analysis of the adenovirus showed the greatest harmonic mean of estimated marginal likelihoods using the T-COFFEE alignment. The rtREV model of amino acid substitution was found to be most probable with a posterior probability of 1.000 (Dimmic et al., Citation2002). A Bayesian tree using the T-COFFEE alignment is shown in .
Figure 1. Bayesian phylogenic tree of partial adenoviral DNA polymerase amino acid sequences using T-COFFEE alignment. Psittacine adenovirus 2 is in bold font with an arrow. Sturgeon adenovirus 1 was used as an outgroup of the unrooted tree. Bayesian posterior probability values for branchings are in bold, and bootstrap values for the maximum likelihood tree are below. Branchings with Bayesian posterior probability < 70 are not shown, and areas where multifarcations occurred are shown as arcs. Sequences retrieved from GenBank include frog adenovirus 1 (GenBank accession number AF224336), turkey adenovirus 3 (AF074946), raptor adenovirus 1 (DQ022750), Meyer's parrot adenovirus 1 (AY644731), fowl adenovirus 1 (AAU46933), fowl adenovirus 9 (AF083975), fowl adenovirus “1422” (DQ159938), Agamid adenovirus 1 (AY576678), Eublepharid adenovirus 1 (AY576677), Gekkonid adenovirus 1 (AY576681), Helodermatid adenovirus 1 (AY576680), snake adenovirus 1 (ABA47232), duck adenovirus 1 (AAK68691), ovine adenovirus 7 (PC4394), bovine adenovirus 4 (AAK13183), murine adenovirus 1 (AP000342), Tupaia adenovirus 1 (AAN84890), porcine adenovirus 3 (YP009201), canine adenovirus 1 (NP044189), bovine adenovirus 1 (YP094032), porcine adenovirus 5 (AP000236), human adenovirus 4 (AAS16276), human adenovirus 40 (NP040853), and simian adenovirus 21 (AP000266). AdV, adenovirus.
Discussion
The present study extends the characterized adenoviruses of psittacine birds beyond Aviadenovirus to include the genus Siadenovirus. Given their presence in galliform and anseriform birds, it is plausible that future studies may find the genus Atadenovirus in psittacine birds as well. Identification of adenoviral types and species will provide useful diagnostic, prognostic, and epidemiologic information for clinicians. The adenoviruses are a diverse group; in the best-studied host species, humans, 52 adenoviral types classified into seven species are currently recognized (Jones et al., Citation2007). Given that there are over 300 psittacine species, significant diversity should be expected. Significant clinical differences exist between adenoviral types and species, and further investigation of adenoviral diversity is expected to have clinical relevance.
Large DNA viruses generally have more complex host–virus interactions and a more limited host range. The congruence of adenoviral phylogenetic branching patterns with those of the corresponding host species implies host/virus co-divergence (Benkö & Harrach, Citation2003). Inherent in this is the implication of a narrow host range. The majority of characterized adenoviruses found in non-avian reptiles (which are mostly atadenoviruses) have only been found in one host species (Wellehan et al., Citation2004). An aviadenovirus of falcons grew in falcon cells but not chicken cells (Oaks et al., Citation2005), and an aviadenovirus of psittacines grew in psittacine cells but not chicken cells (Lüschow et al., Citation2007). However, a siadenovirus of raptors infects both falconiform and strigiform birds (Zsivanovits et al., Citation2006). Psittacine adenovirus 2 has shown the ability to infect birds in the subfamily Cacatuinae as well as the subfamily Psittacinae. Cacatuinae are a basal divergence within the psittaciformes, and it has been suggested that the order psittaciformes should be divided into the families Cacatuidae and Psittacidae (Astuti et al., Citation2006). It is possible that barriers to infection of novel species may be lesser for siadenoviruses than for other adenoviral genera.
Psittacine adenovirus 2 and other characterized siadenoviruses are A and T rich. The atadenoviruses were originally named because the first atadenoviruses characterized, from mammals and birds, were AT rich, and range from 52.6 to 67.7% AT over the sequence homologous to that in our study. The atadenoviruses of squamates range from 41.9 to 56.3% over this region. It has been hypothesized that squamates are the original Atadenovirus hosts and the AT bias seen in atadenoviruses of mammals and birds may be due to a recent host shift (Benkö & Harrach, Citation2003; Wellehan et al., Citation2004). DNA containing CG dinucleotides is recognized by the innate immune system (Aderem & Hume, Citation2000). In the absence of a previous host-adapted function of a sequence, there could be strong selective pressure away from CG dinucleotides. Canine parvovirus, which has recently switched to canine hosts from felines, shows strong selection at synonymous sites away from G and C (Shackelton et al., Citation2006). Feline immunodeficiency virus shows strong G to A selection in the polymerase upon entering a new host species (Poss et al., Citation2006). If an AT bias is indicative of a recent host switch, then none of the known siadenoviruses may share a long association with their known hosts.
The clinical significance of psittacine adenovirus 2 remains to be determined. The clinical signs shared by these cases were weight loss and lethargy, which are fairly non-specific signs. The acute inflammatory response to S. aureus was temporally consistent with surgical infection secondary to the liver biopsies. Flagellates were seen in Case 1, but the signs persisted after apparently successful treatment of flagellates. It is not known whether the marked leukocytosis seen in Case 1 was present in Case 2 at the time of virus sampling. Healthy birds were not surveyed for this virus. Further studies are indicated.
The prevalence of adenoviral infection and disease in psittacine birds is not known. Adenoviruses have been found to be the most common cause of infectious conjunctivitis in humans (Ishii et al., Citation1987; Woodland et al., Citation1992). With the advent of improved antemortem diagnostics, a marked increase in diagnoses of human adenoviral disease has occurred (Rocholl et al., Citation2004). Few attempts have been made at identification of adenoviruses found in psittacine birds. It is likely that increased surveillance would result in the recognition of additional disease syndromes and associated adenoviral types.
Acknowledgements
The authors thank Balázs Harrach for his comments on the manuscript.
References
- Aderem , A. and Hume , D.A. 2000 . How do you see CG? . Cell , 103 : 993 – 996 .
- Altschul , S.F. , Madden , T.L. , Schäffer , A.A. , Zhang , J. , Zhang , Z. Miller , W. 1997 . Gapped BLAST and PSI-BLAST: a new generation of protein database search programs . Nucleic Acids Research , 25 : 3389 – 3402 .
- Astuti , D. , Azuma , N. , Suzuki , H. and Higashi , S. 2006 . Phylogenetic relationships within parrots (Psittacidae) inferred from mitochondrial cytochrome-b gene sequences . Zoological Science , 23 : 191 – 198 .
- Benkö , M. and Harrach , B. 2003 . “ Molecular evolution of adenoviruses ” . In Adenoviruses: Model and Vectors in Virus Host Interactions. Current Topics in Microbiology and Immunology , Edited by: Doerfler , W. and Böhm , P. Vol. 272 , 3 – 35 . New York : Springer .
- Benkö , M. , Élö , P. , Ursu , K. , Ahne , W. , LaPatra , S.E. Thomson , D. 2002 . First molecular evidence for the existence of distinct fish and snake adenoviruses . Journal of Virology , 76 : 10056 – 10059 .
- Benkö , M. , Harrach , B. , Both , G.W. , Russell , W.C. , Adair , B.M. Adam , E. 2005 . “ Family Adenoviridae ” . In Virus Taxonomy. Eighth Report of the International Committee on Taxonomy of Viruses , Edited by: Fauquet , C.M. , Mayo , M.A. , Maniloff , J. , Desselberger , U. and Ball , L.A. 213 – 228 . San Diego , CA : Academic Press .
- Dán , Á. , Ruzsics , Z. , Russell , W.C. , Benkö , M. and Harrach , B. 1998 . Analysis of the hexon gene sequence of bovine adenovirus type 4 provides further support for a new adenovirus genus (Atadenovirus) . Journal of General Virology , 79 : 1453 – 1460 .
- Davison , A.J. , Wright , K.M. and Harrach , B. 2000 . DNA sequence of frog adenovirus . Journal of General Virology , 81 : 2431 – 2439 .
- Desmidt , M. , Ducatelle , R. , Uyttebroek , E. , Charlier , G. and Hoorens , J. 1991 . Respiratory adenovirus-like infection in a rose-ringed parakeet (Psittacula krameri) . Avian Diseases , 35 : 1001 – 1006 .
- Dimmic , M.W. , Rest , J.S. , Mindell , D.P. and Goldstein , R.A. 2002 . rtREV: an amino acid substitution matrix for inference of retrovirus and reverse transcriptase phylogeny . Journal of Molecular Evolution , 55 : 65 – 73 .
- Droual , R. , Woolcock , P.R. , Nordhausen , R.W. and Fitzgerald , S.D. 1994 . Inclusion body hepatitis and hemorrhagic enteritis in two African grey parrots (Psittacus erithacus) associated with adenovirus . Journal of Veterinary Diagnostic Investigation , 7 : 150 – 154 .
- Edgar , R.C. 2004 . MUSCLE: multiple sequence alignment with high accuracy and high throughput . Nucleic Acids Research , 32 : 1792 – 1797 .
- Farkas , S.L. , Benkö , M. , Élö , P. , Ursu , K. , Dán , Á. Ahne , W. 2002 . Genomic and phylogenetic analyses of an adenovirus isolated from a corn snake (Elaphe guttata) imply common origin with the members of the proposed new genus Atadenovirus . Journal of General Virology , 83 : 2403 – 2410 .
- Felsenstein , J. 1985 . Confidence limits on phylogenies: an approach using the bootstrap . Evolution , 39 : 783 – 791 .
- Felsenstein , J. 1989 . PHYLIP-Phylogeny inference package . Cladistics , 5 : 164 – 166 .
- Fudge , A.M. 2000 . “ Laboratory reference ranges for selected avian, mammalian and reptilian species ” . In Laboratory Medicine Avian and Exotic Pets , Edited by: Fudge , A.M. 375 – 400 . Philadelphia , PA : W.B. Saunders Company .
- Gómez-Villamandos , J.C. , Mozos , E. , Sierra , M.A. , Pérez , J. and Mendez , A. 1992 . Inclusion bodies containing adenovirus-like particles in the intestine of a psittacine bird affected by inclusion body hepatitis . Journal of Wildlife Diseases , 28 : 319 – 322 .
- Gómez-Villamandos , J.C. , Bautista , M.J. , Carrasco , L. , Hervás , J. and Sierra , M.A. 1995 . Electron microscopic evidence for infection of splenic dendritic cells by adenovirus in psittacine birds . Research in Virology , 146 : 389 – 395 .
- Harrach , B. 2000 . Reptile adenoviruses in cattle? . Acta Veterinaria Hungarica , 48 : 485 – 490 .
- Harrach , B. , Meehan , B.M. , Benkö , M. , Adair , B.M. and Todd , D. 1997 . Close phylogenetic relationship between egg drop syndrome virus, bovine adenovirus serotype 7, and ovine adenovirus strain 287 . Virology , 229 : 302 – 308 .
- Ishii , K. , Nakazono , N. , Fujinaga , K. , Fujii , S. , Kato , M. Ohtsuka , H. 1987 . Comparative studies on aetiology and epidemiology of viral conjunctivitis in three countries of East Asia—Japan, Taiwan and South Korea . International Journal of Epidemiology , 16 : 98 – 103 .
- Jacobson , E.R. , Gardiner , C. and Clubb , S. 1989 . Adenovirus-like infection in white-masked lovebirds (Agapornis personata) . Journal of the Association of Avian Veterinarians , 1 : 32 – 34 .
- Jones , D.T. , Taylor , W.R. and Thornton , J.M. 1992 . The rapid generation of mutation data matrices from protein sequences . Computer Applications in the Biosciences , 8 : 275 – 282 .
- Jones , M.S. 2nd Harrach , B. , Ganac , R.D. Gozum , M.M. dela Cruz , W.P. , Riedel , B. et al. 2007 New adenovirus species found in a patient presenting with gastroenteritis . Journal of Virology , 81 , 5978 – 5984 .
- Kosiol , C. and Goldman , N. 2005 . Different versions of the Dayhoff rate matrix . Molecular Biology and Evolution , 22 : 193 – 199 .
- Latimer , K.S. , Niagro , F.D. , Williams , O.C. , Ramis , A. , Goodwin , M.A. Ritchie , B.W. 1997 . Diagnosis of avian adenovirus infections using DNA in situ hybridization . Avian Diseases , 41 : 773 – 782 .
- Lüschow , D. , Prusas , C. , Lierz , M. , Gerlach , H. , Soike , D. and Hafez , H.M. 2007 . Adenovirus of psittacine birds: investigations on isolation and development of a real-time polymerase chain reaction for specific detection . Avian Pathology , 36 : 487 – 494 .
- Mackie , J.T. , Black , D. and Prior , H. 2003 . Enteritis associated with adenovirus-like particles in captive lorikeets . Australian Veterinary Journal , 81 : 293 – 295 .
- McFerran , J.B. , Connor , T.J. and McCracken , R.M. 1976 . Isolation of adenoviruses and reoviruses from avian species other than domestic fowl . Avian Diseases , 20 : 519 – 524 .
- Notredame , C. , Higgins , D.G. and Heringa , J. 2000 . T-COFFEE: a novel method for fast and accurate multiple sequence alignment . Journal of Molecular Biology , 302 : 205 – 217 .
- Oaks , J.L. , Schrenzel , M. , Rideout , B. and Sandfort , C. 2005 . Isolation and epidemiology of falcon adenovirus . Journal of Clinical Microbiology , 43 : 3414 – 3420 .
- Pass , D.A. 1987 . Inclusion bodies and hepatopathies in psittacines . Avian Pathology , 16 : 581 – 597 .
- Pitcovski , J. , Mualem , M. , Rei-Koren , Z. , Krispel , S. , Shmueli , E. Peretz , Y. 1998 . The complete DNA sequence and genome organization of the avian adenovirus, hemorrhagic enteritis virus . Virology , 249 : 307 – 315 .
- Poss , M. , Ross , H.A. , Painter , S.L. , Holley , D.C. , Terwee , J.A. Vandewoude , S. 2006 . Feline lentivirus evolution in cross-species infection reveals extensive G-to-A mutation and selection on key residues in the viral polymerase . Journal of Virology , 80 : 2728 – 2737 .
- Ramis , A. , Marlasca , M.J. , Majo , N. and Ferrer , L. 1992 . Inclusion body hepatitis (IBH) in a group of eclectus parrots (Eclectus roratus) . Avian Pathology , 21 : 165 – 169 .
- Raue , R. , Gerlach , H. and Müller , H. 2005 . Phylogenetic analysis of the hexon loop 1 region of an adenovirus from psittacine birds supports the existence of a new psittacine adenovirus (PsAdV) . Archives of Virology , 150 : 1933 – 1943 .
- Rivera , S. , Wellehan , J.F.X. , McManamon , R. , Innis , C.J. , Garner , M.M. Raphael , B.L. 2009 . An epizootic in Sulawesi tortoises (Indotestudo forstenii) caused by a novel Siadenovirus . Journal of Veterinary Diagnostic Investigation , 21 : 415 – 426 .
- Rocholl , C. , Gerber , K. , Daly , J. , Pavia , A.T. & Byington , C.L. 2004 Adenoviral infections in children: the impact of rapid diagnosis . Pediatrics , 113 , e51 – e56 .
- Ronquist , F. and Huelsenbeck , J.P. 2003 . MrBayes 3: Bayesian phylogenetic inference under mixed models . Bioinformatics , 19 : 1572 – 1574 .
- Scott , P.C. , Condron , R.J. and Reece , R.L. 1986 . Inclusion body hepatitis associated with adenovirus-like particles in a cockatiel (Psittaciformes; Nymphicus hollandicus) . Australian Veterinary Journal , 63 : 337 – 338 .
- Shackelton , L.A. , Parrish , C.R. and Holmes , E.C. 2006 . Evolutionary basis of codon usage and nucleotide composition bias in vertebrate DNA viruses . Journal of Molecular Evolution , 62 : 551 – 563 .
- Soike , D. , Hess , M. , Prusas , C. and Albrecht , K. 1998 . Adenovirusinfektion bei Psittaziden . Tierarztliche Praxis Ausgabe K Klientiere Heimtiere , 26 : 354 – 359 .
- Thompson , J.D. , Higgins , D.G. and Gibson , T.J. 1994 . CLUSTAL W: improving the sensitivity of progressive multiple sequence alignments through sequence weighting, position specific gap penalties and weight matrix choice . Nucleic Acids Research , 22 : 4673 – 4680 .
- Thomson , D. , Meers , J. and Harrach , B. 2002 . Molecular confirmation of an adenovirus in brushtail possums (Trichosurus vulpecula) . Virus Research , 83 : 189 – 195 .
- Veerassamy , S. , Smith , A. and Tillier , E.R. 2003 . A transition probability model for amino acid substitutions from blocks . Journal of Computational Biology , 10 : 997 – 1010 .
- Wellehan , J.F.X. , Johnson , A.J. , Harrach , B. , Benkö , M. , Pessier , A.P. Johnson , C.M. 2004 . Detection and analysis of six lizard adenoviruses by consensus primer PCR provides further evidence of a reptilian origin for the atadenoviruses . Journal of Virology , 78 : 13366 – 13369 .
- Wellehan , J.F.X. , Johnson , A.J. , Latimer , K.S. , Bischoff , K. , Lafortune , M. and Jacobson , E.R. 2005 . Initial characterization of an adenovirus associated with fatal hepatic and lymphoid necrosis in a Meyer's parrot (Poicephalus meyeri) . Journal of Avian Medicine and Surgery , 19 : 191 – 197 .
- Woodland , R.M. , Darougar , S. , Thaker , U. , Cornell , L. , Siddique , M. Wania , J. 1992 . Causes of conjunctivitis and keratoconjunctivitis in Karachi, Pakistan . Transactions of the Royal Society of Tropical Medicine and Hygiene , 86 : 317 – 320 .
- Zsivanovits , P. , Monks , D.J. , Forbes , N.A. , Ursu , K. , Raue , R. and Benkö , M. 2006 . Presumptive identification of a novel adenovirus in a Harris hawk (Parabuteo unicinctus), a Bengal eagle owl (Bubo bengalensis), and a Verreaux's eagle owl (Bubo lacteus) . Journal of Avian Medicine and Surgery , 20 : 105 – 112 .