Abstract
A case of nervous signs in red-legged partridges (Alectoris rufa) associated with a severe otitis and osteomyelitis is reported. The outbreak was characterized by abnormal head position, torticollis and difficulty in standing, walking and flying. Pathological, microbiological and molecular genetic data supported an association with Ornithobacterium rhinotracheale (ORT) infection. Clinical signs persisted for several days and were accompanied by weight loss leading to death. Morbidity was approximately 20% and most birds died if untreated. Lesions were mainly characterized by a severe osteomyelitis of the cranial bones and purulent inflammation of the external, middle and inner ears. O. rhinotracheale was isolated from ear samples, skull and brain stem in pure culture. Genetic characterization by pulsed-field gel electrophoresis of the clinical isolates showed that the outbreak was caused by a single strain of ORT. This appears to be the first report of otitis associated with ORT in an avian species.
We would like to dedicate this article to E. Gracia who passed away during its writing
Introduction
Ornithobacterium rhinotracheale (ORT) is a recently described bacterial pathogen of birds and causes mainly respiratory tract infections (van Empel & Hafez, Citation1999; Chin et al., Citation2003). It is reported mostly in chickens and turkeys and less frequently, in other species such as duck, goose, pheasant, partridge, quail, guinea fowl, ostrich, pigeon, gull and rook (Charlton et al., Citation1993; van Empel & Hafez, Citation1999; Ley et al., Citation2000; Chin et al., Citation2003; Bisschop, Citation2005).
ORT was first described in 1991 in South Africa as the cause of respiratory disease in broilers (van Empel & Hafez, Citation1999). It was further characterized by Charlton et al. (Citation1993) as Pasteurella-like or Kingella-like, and the name Ornithobacterium rhinotracheale was proposed by Vandamme et al. (Citation1994). Retrospective studies revealed that ORT had been isolated as early as 1981 and since then it has emerged as an important pathogen of poultry worldwide (Pagés et al., Citation1995; van Empel & Hafez, Citation1999; Chin et al., Citation2003; Bisschop, Citation2005). Serological studies, which have recognized 18 serotypes of ORT, have demonstrated its high prevalence (Chin et al., Citation2003; Ozbey et al., Citation2004; Bisschop, Citation2005; Türkyilmaz, Citation2005). The first and only report of ORT in Spain was by Pagés et al. (Citation1995) who isolated it from 4–6 week-old broilers, from breeder hens and from pheasants with respiratory signs.
Clinically, the infection is associated mainly with nasal discharge, sneezing, wet eyes, facial oedema, and depression, anorexia and weight loss. The infection has also been associated with increased condemnations at slaughter (van Veen et al., Citation2000), decreased egg production and quality, increased embryo mortality (El-Gohary, Citation1998), green diarrhoea and head tremors (Sakai et al., Citation2000). Travers et al. (Citation1996) described “three ORT associated syndromes” in broilers: an upper respiratory tract syndrome; a severe peritonitis; and few respiratory signs and respiratory signs with joint disease and lameness. In all cases, growth retardation was also observed. The infection has occasionally been associated with hepatitis, arthritis, osteomyelitis or encephalitis (van Empel & Hafez, Citation1999; Szalay et al., Citation2002) and Goovaerts et al. (Citation1998) described significant losses in broilers associated with an osteitis and encephalitis syndrome with no respiratory involvement. Lesions associated with the disease in chickens and turkeys are fibrinopurulent or necrotic pneumonia, tracheitis, airsacculitis, hepatomegaly with necrosis, splenomegaly, arthritis and pericarditis (De Rosa et al., Citation1996; van Empel & Hafez, Citation1999; Sakai et al., Citation2000; Bisschop, Citation2005). In other species, such as partridges, the few existing references are mainly associated with respiratory pathology (Leroy-Sétrin et al., Citation1998; Chin et al., Citation2003).
The severity of the disease is affected by environmental factors such as poor management and hygiene, high stocking density and inadequate ventilation. It may also be enhanced by co-infection with other pathogens such as avian pneumovirus in turkeys or Newcastle disease virus in broilers and, less effectively, by some bacteria such as Escherichia coli or Bordetella avium (van Empel & Hafez, Citation1999).
This report describes an outbreak of nervous signs in red-legged partridges due to severe otitis but with no respiratory involvement and which was associated with infection of O. rhinotracheale.
Materials and Methods
Case history
Seven adult red-legged partridges with nervous signs were referred to the Diagnostic Service of Exopol in 2006. They were from a farm that supplied birds mainly for hunting. A group of unsold birds was kept for replacement of breeders. The farm had 500 pairs of breeders, which were located in separate areas to the growing partridges. The growing birds were kept in several groups of 400 separated by fences. In these groups 50% of partridges were brought in from other farms. The outbreak was seen in three groups of replacement birds at 15 to 16 weeks of age.
Affected partridges exhibited mainly nervous signs, although depression, anorexia and weight loss were also seen. The birds presented with abnormal head positions with head tilt, tremors and circling and were unable to walk or fly correctly. The disease persisted for some days and such birds were unable to eat, lost weight and finally died. Morbidity in the three affected groups (a total of 1200 partridges) was approximately 20%. Mortality during the outbreak was almost 20% and approximately 240 partridges died. Morbidity and mortality were similar because most of the affected birds died if they were not treated. Following antimicrobial susceptibility tests, the partridges were treated with doxycyline plus enrofloxacin in the drinking water for 8 days and no further clinical cases were observed.
Pathology
Partridges were first examined for clinical signs and then euthanatized and a complete necropsy performed. Samples of the ear, skull, brain, trachea, lung, heart, liver, spleen and kidney and from each part of the digestive system were collected from three birds for histopathological studies. To examine the ear structures, the brain was removed and several transverse sections of approximately 2 mm were cut from the skull base. Sections were taken from the anterior area of the external canal of the ear to the atlanto-occipital joint. Tissues were fixed in 10% buffered neutral formalin for 3 days, embedded in paraffin wax and cut at 4 µm. Bone samples were previously decalcified with 3% nitric acid. Serial sections were made from the paraffin blocks and stained with haematoxylin and eosin. Some were also stained with Gram′s stain.
Biochemistry and haematology
Tests were performed on two diseased and two clinically normal birds from the same farm. A blood sample was taken into ethylenediamine tetraacetic acid (EDTA) K3 anticoagulant and another for serum production before euthanasia. Haematology analysis was performed with a haematology counter (Medonic CA 620/530, Boule Sweden). Parameters measured were total counts of red blood cells (RBC) and white blood cells (WBC), the mean corpuscular volume (MCV) and the mean corpuscular haemoglobin (MCH). Biochemical analysis included determination of alkaline phosphatase, creatine kinase, uric acid, creatinine, blood urea nitrogen, glucose, alanine aminotransferase, total protein, albumins, globulins and albumin:globulin ratio. The parameters were measured with a dry chemistry automated analyser, Spotchem SP-4430 system and Spotchem II strips (Arkray Inc., Japan).
Haematological and biochemical values were compared with the reference values for this species of partridge (Rodríguez et al., Citation2004; Lloyd & Gibson, Citation2006) and others (ISIS, Citation2002).
Bacterial isolation and identification
Samples of the brain, skull, ear canal, lung and digestive system from five partridges were cultured on MacConkey agar (Oxoid PO5002A) and Columbia 5% sheep blood agar (Oxoid PB 5039A) in aerobic, anaerobic and microaerobic (5 to 10% carbon dioxide) conditions at 37°C for 48 h. Preliminary identification of bacterial isolates followed standard procedures (van Empel, Citation1997; Hafez, Citation2002). Biochemical identification was also made by use of the commercial API 20E system (bioMérieux, Spain).
Biochemical identification of ORT was confirmed by sequencing the 16S rRNA gene of six clinical isolates. The gene was amplified by polymerase chain reaction and sequenced as described previously (Vela et al., Citation2006). The determined sequences, consisting of about 1400 nucleotides, were compared with those of other Gram-negative, catalase-negative and oxidase-positive species available in the GenBank database, using the FASTA program (Pearson, Citation1994).
Antimicrobial susceptibility
This was determined by the disc diffusion method using commercially prepared discs (Oxoid Ltd), according to the protocol of the Clinical and Laboratory Standards, Wayne, PA, with the following antimicrobial agents: amoxycillin, amoxycillin + clavulonic acid, ampicillin, apramycin, bacitracin, cephalexin, ceftiofur, cloxacillin, doxycycline, colistin sulphate, enrofloxacin, erythromycin, lincomycin, neomycin, gentamicin, streptomycin, oxytetracycline, spectinomycin, penicillin G, spiramycin, tetracycline, sulphamethoxazole + trimetroprim. The agar plates were examined after 24 h incubation at 37°C. Antimicrobial susceptibility results were interpreted using the break points recommended by the manufacturer.
Genetic typing
Genetic typing of ORT strains was performed by pulsed-field gel electrophoresis (PFGE). The method was adapted from Fernández-Garayzál et al. (Citation1998) and Vela et al. (Citation2000) and performed as follows: ORT strains were incubated on Columbia agar (bioMérieux España, S.A) in aerobic conditions for 24 h at 37°C. DNA plugs were prepared from fresh colonies of bacteria suspended into 1ml of TE1x buffer (1 mol/l Tris-HCl, 0.5 mol/l EDTA, pH 8) with a transmittance of 20%. The suspension was incubated at 37°C for 10 min, 60 µl of lysozyme (Sigma) was added. Then the suspension was mixed with equal volume of 2% molten agarose (low EEO, Pronadisa, Spain), 10% sodium dodecyl sulphate PB (Panreac, Spain) and proteinase K recombinant (Roche, Spain). The DNA plugs were incubated at 56°C for 2 h in TE1X buffer with 30 µl of proteinase K recombinant (Roche, Spain) and lysis buffer (1 mol/l Tris-HCl, 0.5 mol/l EDTA, 10% N-lauroyl sarcosine, pH 8) and afterwards were washed six times. The restriction enzymes ApaI (Promega Co. Southampton, UK) and SmaI (MBI Fermentas, Vilnius, Lithuania) were used according to the manufacturer's recommendations.
DNA fragments were resolved in a 1% gel on a PFGE apparatus, CHEF-DR III (Bio-Rad, Spain), at 6 V/cm for 21 h, with switching times ramped from 0.1 to 25 sec at 14°C, with an angle of 120°. A Salmonella enterica serovar Braendorup H9812 restricted with XbaI (MBI Fermentas, Quimigen, Spain) was included as the internal molecular ladder, and Mid Range PFG Marker II (New England BioLabs) was included as a molecular weight ladder for size determinations. The gel was stained with 100 µl/l ethidium bromide for 10 min and destained in distilled water for 24 h. The PFGE patterns were examined visually and the genetic relationship among isolates was evaluated following the criteria of Tenover et al. (Citation1995). In addition to the clinical isolates an epidemiologically unrelated pheasant strain of O. rhinotracheale that had been isolated in France was typed by PFGE.
Results
Pathology
Gross lesions observed in the head of all partridges consisted of thick brown exudates in both ear canals. In some a slight yellowish discoloration of the bone of the ventral cranial cavity close to the ear zone was seen.
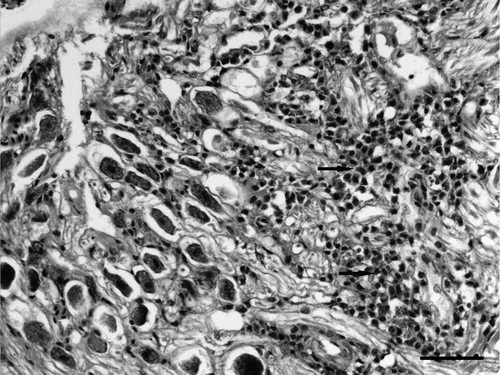
Microscopically, a severe subacute to chronic fibrinopurulent osteomyelitis of the cranial bone and otitis affecting the external, middle and inner parts of the ear was found. In the external ear there was thick purulent exudate occupying the lumen () and the mucosa and submucosa were infiltrated by numerous heterophils, macrophages and lymphocytes that occasionally formed lymphoid aggregates.
Figure 1. Section of ear of a diseased partridge. Several structures can be seen: external ear (asterisk), four semicircular canals (stars), the beginning of the cochlea with remnants of vascular and sensorial epithelium (arrow) and the cochlear ganglia (arrow head). Heterophilic exudate (diamonds) can be seen in the external ear canal, the vestibular apparatus, surrounding bony labyrinth of the cochlea and in the network of branching trabeculae of the temporal bone (upper left area of image). Haematoxylin and eosin. Bar = 800 µm.
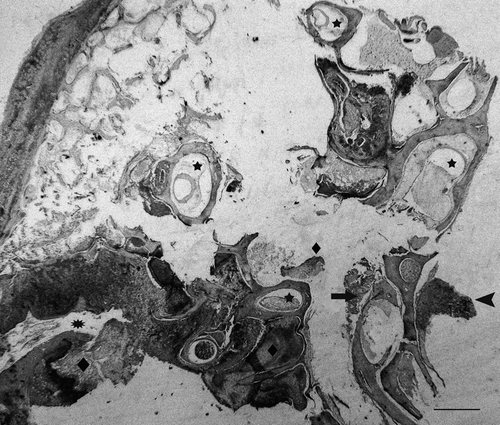
A severe purulent inflammation had also infiltrated the middle ear and several structures of the inner ear (). In general, the bony labyrinth was more severely affected than the membranous labyrinth with mild to severe heterophilic periostitis occasionally causing osteolysis ( and ), sometimes accompanied by multinucleated cells compatible with osteoclasts. The membranous labyrinth was well preserved in some areas (), while in others a slight infiltrate of heterophils in the perilymph was detected (). Vasculitis was also seen in some vessels of the bony labyrinth and the membranous labyrinth (). In some cases the membranous labyrinth was completely destroyed (). The cochlea was generally well preserved with a light infiltrate of heterophils in the vessels and nerve fibres of the basilar papilla being observed occasionally (). In some areas, the exudate was surrounded by macrophages and multinucleated cells (). Gram-negative bacteria were seen in the inflammatory exudate, always associated with severe heterophil infiltration (). In the network of branching trabeculae of the temporal bone, an acute and severe heterophilic osteomyelitis, occasionally associated to osteolysis was detected (). Slight to moderate inflammation, mainly mononuclear, was also detected in the muscle surrounding the cranial bones () and in the cochlear ganglia ().
Figure 2. Magnified view of . Bony labyrinth of the cochlea destroyed by severe heterophilic exudate (arrows). Haematoxylin and eosin. Bar = 20 µm.
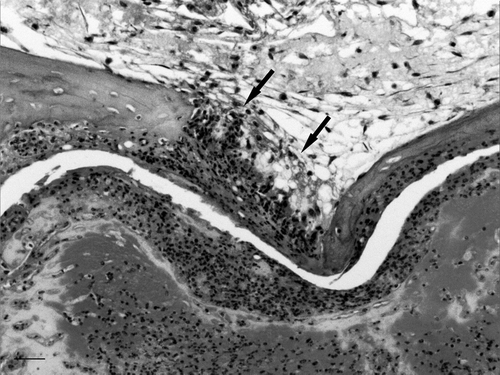
Figure 3. Magnified view of . Semicircular canal with inflammation of bony labyrinth and well preserved membranous labyrinth. Several degrees of periostitis (asterisks) with inflammation penetrating through blood vessels can be seen (arrow). Inset: detail of periostitis penetrating the bone. Bar = 20 µm. BL, Bony labyrinth; PL, perilymph; ML, membranous labyrinth; EL, endolymph. Haematoxylin and eosin. Bar = 200 µm.
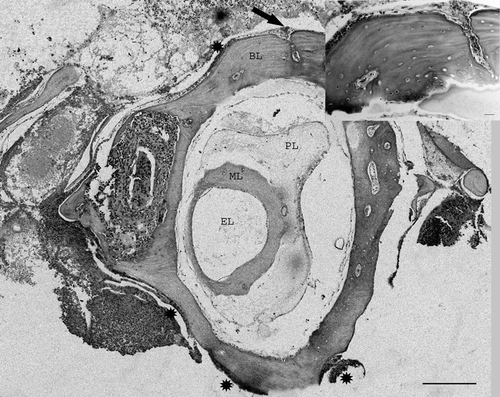
Figure 4. Magnified view of . Semicircular canal with heterophilic inflammation of the perilymph (asterisks). Vasculitis in vessels of the membranous and bony labyrinths (arrows). Inset: vasculitis in bony labyrinth and heterophils in perilymph and endosteal surface (Bar = 10 µm). BL, Bony labyrinth; PL, perilymph; ML, membranous labyrinth; EL, endolymph. Haematoxylin and eosin. Bar = 20 µm.
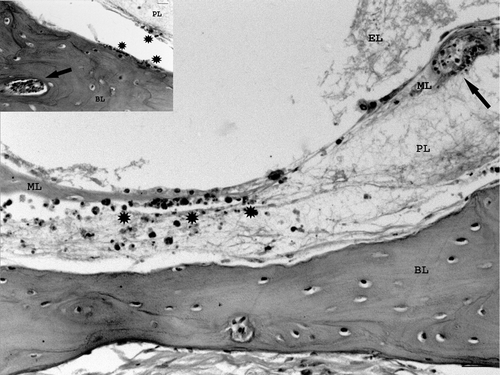
Figure 5. Magnified view of . Semicircular canal with moderate heterophilic inflammation and destruction of the membranous labyrinth (ML). A severe exudate surrounds the canal with destruction of bony labyrinth (also shown in ). Remnants of cochlear sensorial and vascular epithelium can be seen on the right. Haematoxylin and eosin. Bar = 200 µm.
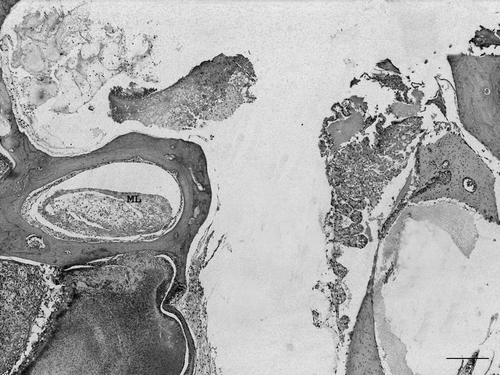
Figure 6. Magnified view of and . Heterophilic vasculitis in two vessels (bv) and neuritis in a nerve (nv) in the basilar papilla of the cochlea. Upper inset: details of vasculitis and neuritis with heterophilic infiltration (ht) (bar = 10 µm). Lower inset: area of the same bird with a non-affected cochlear canal to identify normal structures. TV, Tegmentum vasculosum; TM, tectorial membrane; BP, basilar papilla; HC, hair cells; CG, cochlear ganglia (bar = 50 µm). Haematoxylin and eosin. Bar = 80 µm.
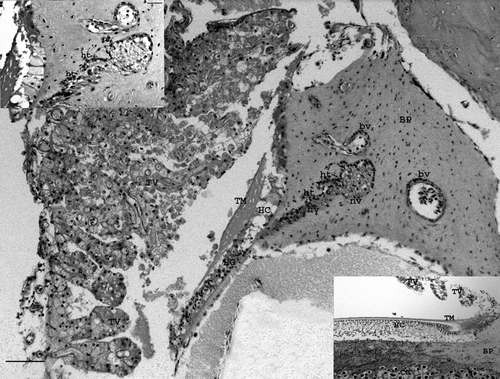
Figure 7. Partridge ear showing dense heterophilic exudate adjacent to bone which is surrounded by macrophages and multinucleated cells (asterisks). Bacterial colonies can be seen within the exudate (arrows). Haematoxylin and eosin. Bar = 20 µm.
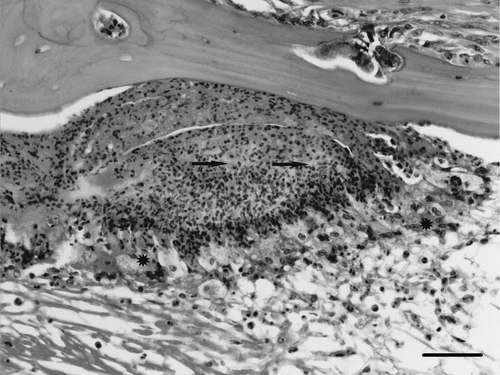
Figure 8. Magnified view of . Inflammation of temporal bone (left) and surrounding muscle (right). Haematoxylin and eosin. Bar = 50 µm.
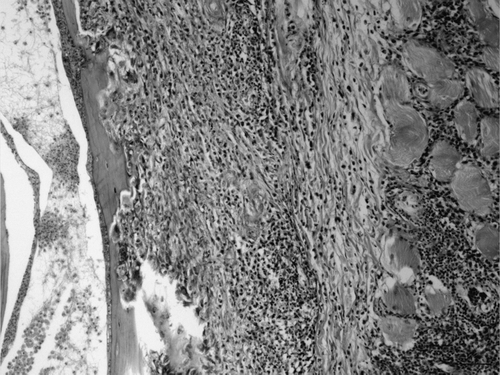
Figure 9. Magnified view of . Cochlear ganglia infiltrated with mononuclear cells (arrows). Haematoxylin and eosin. Bar = 20 µm.
In the brain of two birds, slight and focal mononuclear pachymeningitis was detected in the ventral areas of the caudal brain steam, close to the vestibulocochlear nerve area. No significant lesions were detected in the respiratory, digestive, urinary or haematological systems.
Biochemistry and haematology
Haematology results showed lower values of red blood cells (<1.5×1012/mm3) and haematocrit (<17) than the reference values for this species (). The anaemia was normocytic and normochromic being characteristic of nutritional deficiencies. The rest of the haematological parameters were within normal ranges.
Table 1. Haematology and biochemistry results
Biochemical analysis () revealed a decrease in serum glucose, indicating an energy deficiency, and an increase of serum concentration of creatinine (1.5× the maximum values of references ranges), uric acid (2×), creatine kinase (2×) and ALP (1.5–2×). The increases of these parameters were characteristic of muscle damage and cachexia. In general, haematological and biochemical alterations were compatible with a chronic consumptive disease associated to an impaired capacity for food intake.
Bacterial isolation and identification
Gram-negative pleomorphic, non motile, non spore forming, oxidase-positive, catalase-negative, pleomorphic rods were isolated in pure culture from the ear canal and brain of five animals, and the skull of three of them. No other bacteria were isolated from lungs or the digestive system. Optimal growth of isolates was obtained in microaerobic conditions after 48 h of incubation at 37°C, although they were also able to grow in aerobic and anaerobic conditions. In blood agar, isolates grew as small, grey and non haemolytic colonies, and they did not grow on MacConkey agar. Tests for production of indole and citrate were negative.
All isolates showed an identical biochemical profile (numerical code 1004046) with the commercial API 20E identification system, which gave a doubtful identification as Pasteurella pneumotropica/Mannheimia haemolytica; however, their morphological and biochemical characteristics were not consistent with the provisional assignment to these genera.
Definitive molecular identification was achieved by sequence analysis of the almost complete 16S rRNA gene (> 1400 nucleotides) of each isolate and showed that the six isolates were identical, displaying 99.9% similarity with that of the type strain of O. rhinotracheale CCUG 23171T (accession number AY162321).
Antimicrobial susceptibility
All isolates were sensitive to amoxycillin, amoxycillin + clavulonic acid, ampicillin, bacitracin, cephalexin, ceftiofur, cloxacillin, doxycycline, enrofloxacin, lincomycin, oxytetracycline, penicillin G and tetracycline.
Genetic typing
PFGE revealed that all the ORT isolates displayed indistinguishable macrorestriction patterns with the enzymes SmaI and ApaI (data not shown), while the non-epidemiologically related French isolate exhibited a different restriction pattern.
Discussion
This study reports the first isolation of ORT from farmed partridges with nervous signs due to otitis and osteomyelitis with no respiratory involvement. There appears to be only one other report of ORT infection associated with a nervous syndrome. The case involved chickens (Goovaerts et al., Citation1998) and, in contrast to their findings, the partridges in the present outbreak showed no significant encephalitis with only slight meningitis being found in two birds. The nervous signs could be explained by the severe otitis with cranial bone involvement detected in all birds. The signs were compatible with a vestibular syndrome which may be associated with otitis interna, encephalitis affecting the vestibular nucleus (as in listeriosis) or traumatic lesions in the vestibular apparatus (i.e. fracture of temporal or occipital bones) (Radostits et al., Citation2002).
Otitis is rarely reported in birds (Shivaprasad et al., Citation2006). Otitis media is more often described and is mainly associated with Pasteurella multocida infection. However, otitis interna in birds is very rare. Only recently did Shivaprasad et al. (Citation2006) report the first documentation of otitis interna caused by bacteria in an avian species (the turkey). These authors considered the lesion to be a consequence of a systemic infection by Salmonella enterica subsp. arizonae and secondary to a meningoencephalitis. In our case, in contrast, the slight meningeal lesions as well as the inner otitis might be considered as an extension of the severe otitis media and cranial osteomyelitis that had spread to all parts of the ears and the meninges.
The role of ORT as a primary pathogen has been questioned because it is frequently isolated together with other pathogens such as E. coli or P. multocida and can be aggravated by previous viral infections (Pagés et al., Citation1995; De Rosa et al., Citation1996; van Empel and Hafez, Citation1999: Bisschop, Citation2005). Although no virus detection was attempted in the present study and virus participation could not therefore be ruled out, no lesions suggesting viral involvement were observed. Moreover the microbiological and the histopathological data, together with the fact that no other bacterial pathogens were isolated and no more clinical cases were observed after the appropriate antimicrobial treatment, suggest a causal relationship between ORT and the disease. These data suggest also that this bacterium should be considered in differential diagnosis in cases of avian otitis and nervous signs. Further studies are necessary to confirm if ORT is a primary pathogen in such cases.
Epidemiological studies have shown that the infection can be transmitted vertically and also horizontally by direct or indirect contact. It is usually introduced to a farm by animal vectors, contact with wild birds or is airborne (De Rosa et al., Citation1996; Amonsin et al., Citation1997; van Empel and Hafez, Citation1999; Bisschop, Citation2005). The identity of the PFGE macro restriction patterns of the clinical ORT isolates indicates that they represent a single strain, suggesting a common source of the infection, possibly by horizontal transmission from subclinically infected partridges introduced onto the farm. Subclinical ORT infections have been observed in young birds (Zorman-Rojs et al., Citation2000) and although mortality usually ranges between 3 and 15%, other factors can aggravate the infection (van Empel and Hafez, Citation1999; Lopes et al., Citation2002). No obvious complicating factors were observed in the present study.
Differences in pathogenicity have been reported between clinical isolates of ORT (Devriese et al., Citation1995; Travers et al., Citation1996; van Veen et al., Citation2000; Koga & Zavaleta, Citation2005) so the high mortality in the partridges might have been related to high pathogenicity of the strain involved. Alternatively, partridges could differ from other birds in their susceptibility to ORT.
In conclusion, this study represents the first association of ORT with an internal otitis in partridges and highlights its possible role as a pathogen.
Acknowledgements
The authors would like to thank Professor J. A. Bascuas and Professor H. L. Shivaprasad for their help in histological evaluation of ear lesions and A. Casamayor for his technical assistance in PFGE analysis.
Notes
We would like to dedicate this article to E. Gracia who passed away during its writing
References
- Amonsin , A. , Wellehan , J.F. , Li , L.L. , Vandamme , P. , Lindeman , C. Edman , M. 1997 . Molecular epidemiology of Ornithobacterium rhinotracheale . Journal of Clinical Microbiology , 35 : 2894 – 2898 .
- Bisschop , S.P.R. 2005 The use of a bacterin vaccine in broiler breeders in the control of Ornithobacterium rhinotracheale in commercial broilers . Thesis. University of PretoriaSouth Africa
- Charlton , B.R. , Channing-Santiago , S.E. , Bickford , A.A. , Cardona , C.J. , Chin , R.P. Cooper , G.L. 1993 . Preliminary characterization of a pleomorphic gram-negative rod associated with an avian respiratory disease . Journal of Veterinary Diagnostic Investigation , 5 : 47 – 51 .
- Chin , R.P. , van Empel , P.C.M. & Hafez , H.M. 2003 Ornithobacterium rhinotracheale infection . In Y.M. Saif , H.J. Barnes , J.R. Glisson , A.M. Fadly , L.R. McDougald , & D.E. Swayne 2003 Diseases of Poultry , 11th edn 683 – 690 Ames : Iowa State Press .
- De Rosa , M. , Droual , R. , Chin , R.P. , Shivaprasad , H.L. and Walter , R.L. 1996 . Ornithobacterium rhinotracheale infection in turkey breeders . Avian Diseases , 40 : 865 – 874 .
- Devriese , L. , Hommez , J. , Vandamme , P. , Kerster , K. and Haesebruck , F. 1995 . In vitro antibiotic sensitivity of Ornithobacterium rhinotracheale strains from poultry and wild birds . The Veterinary Record , 137 : 435 – 436 .
- El-Gohary , A.A. 1998 . Ornithobacterium rhinotracheale (ORT) associated with hatching problems in chicken and turkey eggs . Veterinary Medical Journal Giza , 46 : 183 – 191 .
- Fernández-Garayzábal , J.F. , Fernández , E. , las Heras , A. , Pascual , C. , Collins , M.D. and Domínguez , L. 1998 . Streptococcus parasanguinis: new animal pathogen associated with asymptomatic mammary gland infections in sheep . Emerging Infectious Diseases , 4 : 645 – 647 .
- Goovaerts , D. , Vrijenhoek M. & van Empel , P.C.M. 1998 Immunohistochemical and bacteriological investigation of the pathogenesis of Ornithobacterium rhinotracheale infection in South Africa in chickens with osteitis and encephalitis syndrome . In Proceedings of the 16th meeting of the European Society of Veterinary Pathology 81 Lillehammer Norway
- Hafez , H.M. 2002 . Diagnosis of Ornithobacterium rhinotracheale . International Journal of Poultry Science , 1 : 114 – 118 .
- ISIS 2002 Rollulus roulroul, Crested Wood Partridge . In J. Andrew Teare Reference Ranges for Physiological values in Captive Wildlife . Apple Valley : International Species Information System .
- Koga , Y. and Zavaleta , A.I. 2005 . Intraspecies genetic variability of Ornithobacterium rhinotracheale in commercial birds in Peru . Avian Diseases , 49 : 108 – 111 .
- Leroy-Sétrin , S. , Flaujac , G. , Thénaisy , K. and Chaslus-Dancla , E. 1998 . Genetic diversity of Ornithobacterium rhinotracheale strains isolated from poultry in France . Letters in Applied Microbiology , 26 : 189 – 193 .
- Ley , E.C. , Morishita , T.Y. , Harr , B.S. , Mohan , R. and Brisker , T. 2000 . Serologic survey of slaughter-age ostriches (Struthio camelus) for antibodies to selected avian pathogens . Avian Diseases , 44 : 989 – 992 .
- Lloyd , S. and Gibson , J.S. 2006 . Haematology and biochemistry in healthy young pheasants and red-legged partridges and effects of spironucleosis on these parameters . Avian Pathology , 35 : 335 – 340 .
- Lopes , V.C. , Velayudhan , B. , Harvolson , D.A. and Nagaraja , K.V. 2002 . Survival of Ornithobacterium rhinotracheale in sterilized poultry litters . Avian Diseases , 46 : 1011 – 1014 .
- National Committee for Clinical Laboratory Standards 2004 . Performance Standards for Antimicrobial Disk and Dilution Susceptibility Test for Bacteria Isolated from Animals: Informational Supplement, 1st edn, NCCLS document M31-S1 National Committee for Clinical Laboratory Standards , Wayne, PA , , USA .
- Ozbey , G. , Ongor , H. , Balik , D.T. , Celik , V. , Kilic , A. and Muz , A. 2004 . Investigations on Ornithobacterium rhinotracheale in broilers flocks in Elazig province located in the East of Turkey . Veterinarni Medicina Praha , 49 : 305 – 311 .
- Pagés , A. , Foix , A. , March , R. and Artigas , C. 1995 . Estudio bacteriológico de un agente asociado a problemas respiratorios en aves de producción: Ornithobacterium rhinotracheale . Medicina Veterinaria , 12 : 585 – 588 .
- Pearson , W.R. 1994 . Using the FASTA program to search protein and DNA sequence databases . Methods in Molecular Biology , 24 : 307 – 331 .
- Radostits , O.M. , Gay , C.C. , Blood , D.Ch. & Hinchcliff , K.W. 2002 Enfermedades del Sistema Nervioso In: O.M. Radostits , C.C. Gay , D.Ch. Blood , & K.W. Hinchcliff Medicina Veterinaria. Tratado de las enfermedades del ganado bovino, ovino, porcino, caprino y equino , 9th edn 595 – 596 Madrid McGraw-Hill, Interamérica de España, S.A.U. Aravaca .
- Rodríguez , P. , Tortosa , F.S. , Millán , J. and Gortázar , C. 2004 . Plasma chemistry reference values from captive red-legged partridges (Alectoris rufa) . British Poultry Science , 45 : 565 – 567 .
- Sakai , E. , Tokuyama , Y. , Nonaka , F. , Ohishi , S. , Ishikawa , Y. Tanaka , M. 2000 . Ornithobacterium rhinotracheale infection in Japan: preliminary investigations . The Veterinary Record , 146 : 502 – 503 .
- Shivaprasad , H.L. , Cortes , P. and Crespo , R. 2006 . Otitis interna (labyrinthitis) associated with Salmonella enterica arizonae in turkey poults . Avian Diseases , 50 : 135 – 138 .
- Szalay , D. , Glavits , R. , Nemes , C. , Kosa , A. and Fodor , L. 2002 . Clinical signs and mortality caused by Ornithobacterium rhinotracheale in turkey flocks . Acta Veterinaria Hungarica , 50 : 297 – 305 .
- Tenover , F.C. , Arbeit , R.D. , Goering , R.V. , Mickelsen , P.A. , Murray , B.E. , Persing , D.H. and Swaminathan , B. 1995 . Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing . Journal of Clinical Microbiology , 33 : 2233 – 2239 .
- Travers , A.F. , Coetzee , L. and Gummow , B. 1996 . Pathogenicity differences between South African isolates of Ornithobacterium rhinotracheale . The Onderstepoort Journal of Veterinary Research , 63 : 197 – 207 .
- Türkyilmaz , S. 2005 . Isolation and serotyping of Ornithobacterium rhinotracheale from poultry . Turkish Journal of Veterinary Animal Sciences , 29 : 1299 – 1304 .
- Vandamme , P. , Segers , P. , Vancanneyt , M. , van Hove , K. , Mutters , R. Hommez , J. 1994 . Ornithobacterium rhinotracheale gen. nov., sp. nov., isolated from the avian respiratory tract . International Journal of Systematic Bacteriology , 44 : 24 – 37 .
- van Empel , P. and Hafez , H.M. 1999 . Ornithobacterium rhinotracheale: a review . Avian Pathology , 28 : 217 – 227 .
- van Empel , P. , van den Bosch , H. , Loeffen , P. and Storm , P. 1997 . Identification and serotyping of Ornithobacterium rhinotracheale . Journal of Clinical Microbiology , 35 : 418 – 421 .
- van Veen , L. , Gruys , E. , Frik , K. and van Empel , P. 2000 . Increased condemnation of broilers associated with Ornithobacterium rhinotracheale . The Veterinary Record , 147 : 422 – 423 .
- Vela , A.I. , Vázquez , J. , Gibello , A. , Blanco , M.M. , Moreno , M.A. Liébana , P. 2000 . Phenotypic and genetic characterizationof Lactococcus garvieae isolated in Spain from lactococcosis outbreaks and comparison with isolates of other countries and sources . Journal of Clinical Microbiology , 38 : 3791 – 3795 .
- Vela , A.I. , Gracia , E. , Fernández , A. , Domínguez , L. and Fernández-Garayzábal , J.F. 2006 . Isolation of Corynebacterium xerosis from animal clinical specimens . Journal of Clinical Microbiology , 44 : 2242 – 2243 .
- Zorman-Rojs , O. , Zdovc , I. , Benčina , D. and Mrzel , I. 2000 . Infection of turkeys with Ornithobacterium rhinotracheale and Mycoplasma synoviae . Avian Diseases , 44 : 1017 – 1022 .