Abstract
The pathogenesis in chickens of the apicomplexan Eimeria praecox was compared with that of Eimeria acervulina, using intestinal lesions, mucosal integrity, body weight gain (BWG) and the feed conversion ratio (FCR) as criteria. Characteristics of each species were described by combinations of polymerase chain reaction assays and classic parasitological signs. There were considerable overlaps in lengths, breadths, shape indices and volumes of the oocysts of each species. Both species caused statistically significant reductions in BWG at the lowest inocula tested (500,000 sporulated oocysts per bird of E. praecox and 250,000 of E. acervulina). E. praecox was observed for the first time to cause actual body weight loss and marked increases in FCR, as did E. acervulina. E. acervulina caused gross, white pathognomonic lesions, but E. praecox caused micro-lesions, visible in fresh tissue only with a dissecting microscope. Occasionally, lesions of the Houghton strain of E. acervulina were observed to be rounded, rather than typically “ladder-like”. Both species caused villous erosion and atrophy. No mortality occurred in birds receiving up to 1 million sporulated oocysts of either species. Using BWG and FCR as criteria, the virulence of recent field strains of E. praecox from Wales (Tynygongl) and the USA (Raleigh) was compared with English laboratory strains of E. praecox (Houghton) and E. acervulina (Houghton). E. praecox (Tynygongl) was markedly more virulent than E. acervulina (Houghton), which was more virulent than E. praecox (Raleigh) and E. praecox (Houghton).
Introduction
The apicomplexan genus Eimeria comprises at least 1160 species that parasitize vertebrate animals (Levine, Citation1988). Those found in farm animals cause serious financial losses worldwide, with poultry perhaps being the worst affected. In chickens, Eimeria acervulina, Eimeria brunetti, Eimeria maxima, Eimeria mitis, Eimeria necatrix, Eimeria praecox and Eimeria tenella each causes a separately recognizable disease resulting from the fundamental differences among the pathogenic effects characteristic of each species.
Although often used synonymously, the terms pathogenicity (characteristic capacity to cause disease, and its course of development) and virulence (degree of pathogenicity) should be clearly distinguished (Williams, Citation2006). Whilst the characteristic pathogenicity of each particular species is a constant feature, virulence may be regarded as an intraspecific variation. Thus the inherent virulence of any strain (as opposed to the pathogenicity of a species) may be greatly influenced by exogenous factors. In the present study, the size of parasite inoculum and the breed, age and diet of the host were consistent throughout.
There has been continuing controversy over the pathogenesis and virulence of E. praecox in relation to other Eimeria species in the chicken, with particular regard to the necessity of their inclusion in live vaccines (Williams, Citation2002). However, there seem to have been no studies published on field strain variation in E. praecox, and there is little on its clinical effects, namely Long (Citation1967a, Citation1968), Oikawa & Kawaguchi (Citation1976), Gore & Long (Citation1982), Salisch (Citation1990), Jorgensen et al. (Citation1997), Williams (Citation1998, Citation2001), Williams & Catchpole (Citation2000), Jenkins et al. (Citation2008), in contrast with hundreds of papers on E. acervulina. Therefore, the present study was carried out to confirm the specific pathogenic effects of E. praecox and to investigate the difference in virulence of recent field strains from Europe and the USA. Comparisons were made with standard laboratory strains of E. praecox and E. acervulina. E. acervulina was chosen for comparison with E. praecox because of its similar development site in the intestine and because it is generally accepted as being capable of causing serious disease, whereas E. praecox is often considered to be relatively innocuous.
Materials and Methods
Provenance of parasites
The strains of E. praecox and E. acervulina used were named and characterized as far as possible according to the recommendations and terminology of Joyner et al. (Citation1978). They were as follows:
| 1. | E. praecox (H): the Houghton strain, previously described by Shirley et al. (Citation1984). The line used was kindly provided by E. del Cacho, University of Zaragoza, Spain, who initially obtained it from J. Catchpole, Veterinary Laboratories Agency (VLA), Weybridge, UK in 2001, since when it had been maintained in liquid nitrogen. | ||||
| 2. | E. praecox (T): the Tynygongl strain, isolated at the VLA for the present study from an oocyst sample collected from a broiler flock near Tynygongl, Wales, in January 2007. The strain was established from this sample by collecting the earliest oocysts produced from the first passage, then further multiplying them by in vivo passage in chickens at the VLA. | ||||
| 3. | E. praecox (R): the Raleigh strain, isolated at the Laboratorios Hipra from an oocyst sample collected from a broiler flock near Raleigh, North Carolina, USA, in February 2005, and kindly supplied by M. Jenkins (USDA, Beltsville, USA). The strain was established from the stock derived from this sample by a passage initiated by a single oocyst, and maintained since then frozen in liquid nitrogen. | ||||
| 4. | E. acervulina (H): the Houghton strain (Long, Citation1967b). The line used was established at the Laboratorios Hipra from a stock kindly provided by J. H. Morgan (Institute of Animal Health, Compton, UK) in 2001, and maintained since then frozen in liquid nitrogen. | ||||
Characterization of parasites
Characteristics of each species were described by a combination of polymerase chain reaction (PCR) assays and classic parasitological signs. This part of the work was carried out at the Laboratorios Hipra (Amer, Spain).
DNA analyses
The identity of each Eimeria species was confirmed by PCR before use in the in vivo experiments. Clean suspensions of sporulated oocysts were prepared, and the oocyst walls were then disrupted by grinding each sample with a mini-pestle in a 1.5-ml Eppendorf tube (Haug et al., Citation2007). DNA was extracted from homogenates using a commercial kit according to the manufacturer's instructions (DNeasy; Qiagen). Identification of species was carried out using published methods for PCR assays of species-specific ITS-1 sequences of rDNA. Primers, sequences and methodologies for E. praecox and E. acervulina are presented in . PCR assays with positive and negative controls were also carried out on each strain of E. praecox and E. acervulina with specific primers for E. tenella, E. brunetti, E. necatrix, E. mitis and E. maxima. No bands corresponding to those species occurred in any of the resulting gels. All assays were carried out separately, since diagnostic multiplex PCR systems are less sensitive and less reproducible than simplex systems (Haug et al., Citation2007). In general, the assays could detect <100 oocysts; or <10 for E. acervulina and E. tenella. Every PCR assay was carried out at least three times to confirm reproducibility of the results.
Table 1. Species-specific primers and methods for PCR assays of E. praecox and E. acervulina used in the present study
Parasitological methods
The PCR results were corroborated (detailed results not shown here) by classic parasitological methods following infection of birds with 500,000 sporulated oocysts of all three E. praecox strains, or 250,000 sporulated oocysts of E. acervulina (H). In all cases endogenous parasites were seen in the duodenum and upper part of the jejunum, but nowhere else in the intestine, 4 to 6 days after infection. In the case of E. acervulina (H), pathognomonic lesions were observed in the duodenum and upper jejunum. No gross discrete lesions were seen for E. praecox strains, but the intestinal wall was thickened and the duodenum had mucoid contents. Oocyst measurements were also carried out on each strain (see below).
Oocyst measurements, shapes and volumes
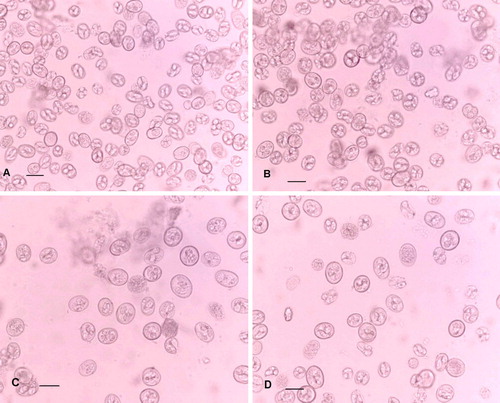
Freshly sporulated oocysts of each strain of each species, suspended in 2.5% w/v potassium dichromate, were measured at random. An Olympus CX31 microscope with an Olympus Altra 20 camera attachment was used, computer-controlled by an Olympus AnalySIS getIT program to take the photographs and an Olympus CellD program to measure the oocysts. The time between placing a cover slip over a drop of oocyst suspension on a microscope slide and taking the necessary multiple photographs was less than 3 min in order to avoid distortion of oocysts by evaporation of the suspension medium. By observing the position of the sporocysts in an oocyst, it was ensured that each oocyst was correctly orientated before it was measured. Photographs of several fields were taken randomly at ×200 magnification until images of about 80 oocysts were obtained, from which the length and breadth of each oocyst were derived. Each oocyst length was related to the breadth of the same oocyst, so that individual shape indices and volumes could be calculated validly. The shape index of each oocyst was calculated as: length/breadth. The approximate volume of each oocyst was calculated as that of an ellipsoid: 4/3×π×(length/2)×(breadth/2)2.
Experimental design of main study
The in vivo experimental work was carried out at the VLA. Replicate groups of 10 chicks were infected (except the uninfected controls, which received sterile distilled water as a sham infection) with E. praecox (1×106 or 0.5×106 oocysts) at 14 days of age (day 0) or with E. acervulina (1×106, 0.5×106, or 0.25×106 oocysts) on day 2. Duplicate groups infected with E. praecox were individually weighed on each of days 0 to 7 and 14, whilst duplicate groups infected with E. acervulina were similarly weighed on days 2 to 9 and 16. The daily weights of the uninfected controls were obtained on the same days as the E. praecox-infected birds, and, being 2 days out of synchrony with E. acervulina-infected birds, were adjusted to provide the control data for the E. acervulina infections, estimated from mean daily weight gains. Having carried out the adjustment of the uninfected controls for E. acervulina, the results from E. acervulina-infected birds were expressed for the same time periods as those from the E. praecox-infected birds.
The total feed consumed during days 0 to 7, days 7 to 14 and days 0 to 14 by each cage of 10 birds was weighed, and the feed conversion ratios (FCRs) were calculated by dividing the weight of feed consumed by the total body weight gain (BWG) of the birds in the same cage during the same time period. FCRs of the uninfected controls for E. acervulina were estimated from the feed consumption data from the uninfected controls for E. praecox, adjusted in a way similar to that for BWGs.
Clinical signs and mortality for every cage of birds were recorded on each of days 0 to 14 after infection. The intestines of all of the 10 birds in each of two further cages per treatment were examined for lesions and mucosal integrity; the birds in one cage were killed 4.5 days after infection (for E. praecox) or 5 days after infection (for E. acervulina), and those in the remaining cage for each species were killed on day 14.
Chickens and husbandry
Specific-pathogen-free chicken eggs (SPAFAS) were hatched in a specific-pathogen-free incubator. The newly hatched chicks were reared coccidia-free in a strictly controlled isolation building. They were accommodated in large wire-floored cages, and given access to feed and water ad libitum. The feed was the standard VLA formula (CDG Shipston), milled on premises where no anticoccidial drugs are employed. The feed contains wheat (42%), maize (25%), soya (20%) and fish meal (10%); protein 23% and energy 12.76 MJ/kg.
At 14 days of age (day 0) the mixed-sex birds were wing-tagged, weighed, randomized and allocated to 40 groups of 10 chicks each, in wire-floored cages (1 m2 floor area) in another disinfected building, where they were inoculated. Heating was by forced, filtered air with an average temperature of approximately 21°C, and the daily lighting programme was 23 h followed by 1 h of darkness. Animal welfare and experimental treatments were compliant with the prevailing UK legislation. All birds were inspected at least once a day by an animal technician, and also periodically by laboratory staff.
Administration of parasites
Chicks were infected by gavage with sporulated oocysts of E. praecox on day 0 or of E. acervulina on day 2. Infective material of each parasite was less than 1 week old when inoculated. To confirm accurate dosing, each bulked inoculum was weighed before and after administration, and the weight dispensed was calculated and compared with the weight expected to have been used for the number of birds infected. None of the weights dispensed deviated by more than 1.7% from that expected for any inoculum.
Lesion scoring and mucosal integrity
Birds were killed by injection into a brachial vein of approximately 0.2 ml pentobarbitone (“Solution 20% for Euthanasia”; J. M. Loverage, Southampton, UK), this being the method of choice for avoiding spontaneous disruption of intestinal epithelium during euthanasia (Rose et al., Citation1975). Gross coccidial lesions due to E. acervulina were assessed immediately after death, and were graded according to the scoring system of Johnson & Reid (Citation1970), with a potential range of scores 0 to 4. Since E. praecox does not cause consistently recognizable macroscopic lesions, such a system is not appropriate for that species; hence an unpublished scoring system (Laboratorios Hipra) was used for E. praecox, with a potential range of grades of 0 to 2. Grade 0 indicates normal intestine and contents; Grade 1 indicates a normal intestinal wall with no thickening, but with some liquid contents in the mid-gut zone, and perhaps even more liquid in the duodenum; and Grade 2 indicates a slightly thickened and wrinkled duodenal wall, with marked whitish mucoid and liquid duodenal contents, and undigested food in the caudal small intestine. This scoring system grades the gut condition, rather than coccidial lesions, resulting from an E. praecox infection; it is not comparable with the scoring of E. acervulina lesions.
After lesion scoring, some segments of gut were excised from selected birds for closer examination of the mucosal integrity under physiological saline with a dissecting microscope (Williams, Citation2005). Some samples of mucoid gut contents adhering to those segments were then transferred with a needle point onto a glass slide for examination with a Nikon Eclipse 50i microscope. Photographs were obtained through both microscopes with a Nikon E4500 Coolpix digital camera.
Statistical analyses
Oocyst dimensions, daily body weights, BWGs and FCRs were analysed by analysis of variance, followed by Tukey's Honestly Significant Difference (HSD) test where appropriate. The experimental unit for BWGs and FCRs was a cage. Before analysis, oocyst dimensions were transformed to log10 then back-transformed after analysis.
Results
Characterization of parasites
DNA analyses and parasitological observations
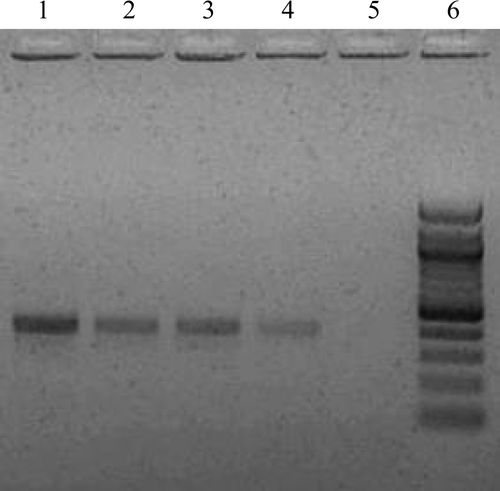
Simplex PCR assays confirmed the identities of the E. praecox and E. acervulina strains previously determined by classic parasitological characteristics. Furthermore, combinations of PCR assays and parasitological methods did not reveal contamination of any strains with E. tenella, E. brunetti, E. necatrix, E. mitis or E. maxima (gels not shown). The confirmatory PCR assays for diagnosis and purity of each strain were all reproducible. illustrates the specific identity of the three strains of E. praecox used in this study.
Figure 1. PCR assay gel confirming specific identification of E. praecox strains used in this study. Lane 1, E. praecox (Houghton); lane 2, E. praecox (Tynygongl); lane 3, E. praecox (Raleigh); lane 4, positive control (“Hipracox Broilers”, a vaccine that includes E. praecox); lane 5, negative control; lane 6, DNA marker (100 base pair ladder; Qiagen).
Oocyst measurements, shapes and volumes
Oocyst measurements and derived dimensions are presented in . The mean lengths of all the E. praecox strains are statistically significantly greater than those of E. acervulina (H). However, the lengths of all the E. praecox strains also differ significantly from each other. The breadths of E. praecox (R) and E. praecox (H) are similar, but both are smaller than E. praecox (T). The mean breadths of all the E. praecox strains are greater than that of E. acervulina (H). The shape index of E. acervulina (H) is greater than that of all the E. praecox strains, but those of E. praecox (R) and E. praecox (H) are different from each other (unlike in the case of their breadths), although both are similar to E. praecox (T). E. acervulina (H) has a significantly smaller volume than any of the E. praecox strains, but E. praecox (T) has a significantly greater volume than either E. praecox (R) or E. praecox (H).
Table 2. Means and extremes of oocyst dimensions of Eimeria strains used in the present study (n = 80)
In summary, there are statistically significant differences between all of the parameters of E. acervulina and E. praecox oocysts, but there are also differences among those of the E. praecox strains. It is notable that E. praecox (R) and E. praecox (H) oocysts have closely similar volumes, but significantly different shapes. Furthermore, the shape index of E. praecox (R) oocysts approaches that of E. acervulina (H), although they are statistically significantly different. In all the strains of E. praecox and E. acervulina, subspherical oocysts with shape indices of 1.01 or 1.02 are not uncommon. a to 2d illustrate the range of sizes and shapes of oocysts in the inocula used here (not the same samples as were used for measuring the oocysts at the Laboratorios Hipra).
Clinical effects
Lesion scores and mucosal integrity
All uninfected control birds had normal intestines and gut contents. The scores for gut condition of all birds given each inoculum of E. praecox were maximal (score 2) at 4.5 days post infection (d.p.i.). Mean scores of E. praecox groups at 14 d.p.i. were 0.5 to 0.65. Mean gross lesion scores for E. acervulina groups were relatively low, being only 1.0 to 1.1 at 5 d.p.i., very few birds having lesions scored as 2 or 3. No birds infected with E. acervulina had lesions after 14 days. There were no clearly dose-related differences within any strain or species. The many E. praecox results with a standard deviation of zero and the scarcity of lesions due to E. acervulina rendered statistical analysis inappropriate.
Palpation and naked-eye examination of unopened intestines 4.5 d.p.i. with 1×106 or 0.5×106 oocysts E. praecox revealed thickened walls with a somewhat wrinkled appearance (a). On opening the gut, the contents were somewhat liquid, with varying degrees of viscosity. The Tynygongl strain appeared to cause more mucoid contents than did the Houghton and Raleigh strains. The colours of the gut contents were quite variable, even for the same strain. The higher dose of the Houghton and Tynygongl strains induced a homogeneous greyish mucus, whilst the mucus resulting from the Raleigh strain at the same dose was greyish with yellowish flecks. At the lower dose, the Houghton strain induced greyish mucus, but the Tynygongl strain induced stringy, yellowish mucus. The mucus induced by the Raleigh strain was greyish in some birds and yellowish in others. The intestines of most birds appeared normal 14 d.p.i., whichever strain of E. praecox they had received.
Figure 3. 3a: Unopened duodenum of bird infected with 106 sporulated oocysts of E. praecox (Tynygongl) showing wrinkled appearance. 3b: Duodenal mucosa under saline of bird infected with 106 sporulated oocysts of E. praecox (Tynygongl), showing mucus veil (m), atrophied villi (a), and “ghosts” (g) of the coria of eroded villi. 3c: Smear of duodenal contents from bird infected with 0.5 × 106 sporulated oocysts of E. praecox (Houghton), showing plant material (p), patches of mucus (m), and fragmented villi. 3d: Smear of duodenal mucosa of bird infected with 0.5 × 106 sporulated oocysts of E. praecox (Tynygongl), showing fragmented villi containing oocysts (o).
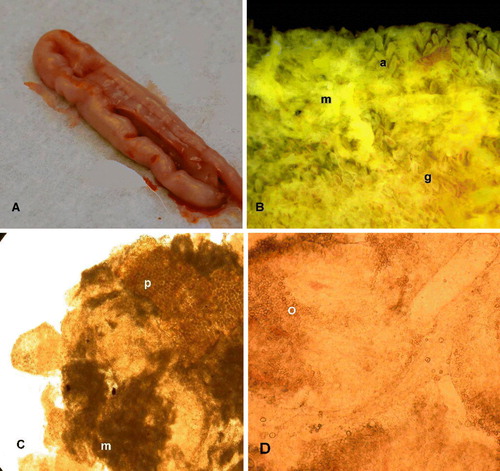
Direct examination of the mucosa with a dissecting microscope showed that in birds infected with E. praecox a greyish mucoid exudate occurred in veil-like patches. Beneath those patches, the villi were severely eroded, leaving “ghosts” of the villar coria (b); but where exudate was absent, complete villi remained, although often atrophied in comparison with villi of uninfected birds. The additional flecks of cream-coloured to yellow viscous material frequently present in areas of erosion were smeared onto a microscope slide and examined with a high-power objective. The material consisted of a mixture of minute particles of plant matter, viscous mucus and detached pieces of villi loaded fairly uniformly with parasites (gametocytes and oocysts) (c and 3d). Sometimes, material of similar gross appearance was also seen in uninfected birds, but it was much less viscous, and contained only plant matter and rather less mucus.
Five d.p.i. with 1×106 or 0.5×106 oocysts of E. acervulina (H), palpation and naked-eye examination of unopened intestines revealed rather thickened walls with a somewhat wrinkled appearance (a). The wrinkling was noticeably less in birds infected with 0.25×106 oocysts. In all cases, the opened guts contained slightly viscous liquid occasionally tinged with green (probably bile) and the mucosa was sometimes congested. The intestines of most birds appeared normal 14 d.p.i., however many oocysts they had received.
Figure 4. 4a: Unopened duodenum of bird infected with 106 sporulated oocysts of E. acervulina (Houghton) showing wrinkled appearance. 4b: Jejunal mucosa under saline of bird infected with 0.25×106 sporulated oocysts of E. acervulina (Houghton) showing rounded lesions. 4c: Smear of duodenal mucosa of bird infected with 106 sporulated oocysts of E. acervulina (Houghton), showing villi containing oocysts (o). 4d: Muscularis mucosae of the duodenum, under saline, of bird infected with 106 sporulated oocysts of E. acervulina (Houghton), showing inflammation, and “ghosts” of the coria of the stripped-off villi.
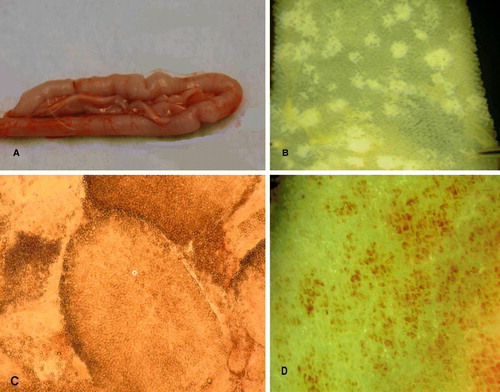
When examined with a dissecting microscope, the villi of birds infected with E. acervulina were seen to be reduced in height compared with those of uninfected birds. The discrete, whitish, pathognomonic lesions were sometimes transversely orientated across the gut, but were often more or less rounded (b). The healthy-looking villi surrounding the lesions could be gently moved aside with a needle-point without causing damage; but when a lesion was directly prodded, the white area disintegrated into a mass of loose tissue and mucus, demonstrating its fragility. In an individual villus, a white area was seen to be concentrated in the mucosa of the tip of the spatulate villus in a horseshoe shape. When examining material from disintegrated lesions with a high-power objective, the mucosa of the villus tips was packed with mature oocysts (c), in contrast to the usually more uniform distribution of E. praecox parasites along the villi. Very heavy infections completely stripped off the mucosa from the muscularis mucosae, leaving large areas of inflammation and the “ghosts” of the villar coria (d). Parts of the intestine so affected exhibited no discrete gross lesions, showing only uniform pale pinkish serosal and mucosal surfaces.
Weight gains
The mean daily body weights of birds infected with different doses of each E. praecox strain were lower than the uninfected controls from 3 to 4 d.p.i. until 14 d.p.i. These body weight differences were, however, statistically significant (P≤0.05) only in the cases of E. praecox (T) (both doses until day 14) and E. praecox (R) (1×106 oocysts per bird until day 7; and 0.5×106 oocysts per bird until day 6). All doses of E. acervulina (H) caused statistically significantly lower (P≤0.05) mean body weights than those of uninfected controls, beginning between 3 and 4 d.p.i., and continuing until 14 d.p.i.Most notably, with both doses of E. praecox (T), there was an actual loss of body weight from 3 d.p.i. until at least 6 or 7 d.p.i. In the case of E. acervulina (H), body weight loss occurred with all doses from 3 d.p.i., but it persisted only until 6 d.p.i. and was generally less than half as great as that caused by E. praecox (T).
presents the mean BWGs of birds during different periods up to 14 d.p.i. Preliminary analyses of covariance indicated no statistically significant effects of initial bird weights (day 0) on their BWGs (days 0 to 7, P=0.506; days 7 to 14, P=0.313; days 0 to 14, P=0.318). Therefore, comparisons of treatment groups were carried out by one-way analysis of variance for each species for each time period. During the acute phase (days 0 to 7), the mean BWGs of all infected birds were less than those of the uninfected controls for each species, but only E. praecox (T) (both doses) and E. acervulina (H) (all doses) produced statistically significant reductions in BWG. During days 7 to 14, there were no statistically significant differences between any treatments within each species. Overall (days 0 to 14), only E. praecox (T) (both doses) and E. acervulina (H) (all doses) caused statistically significantly lower BWGs than those of the respective uninfected controls. During days 0 to 7 and 0 to 14, the mean BWGs of birds infected with either dose of E. praecox (T), as percentages of uninfected controls, were lower than those of birds infected with the corresponding doses of E. acervulina (H).
Table 3. Mean body weight gain for three time periods after infection: each species analysed separately
Feed conversion ratios
presents the mean FCRs of infected and uninfected birds during different periods up to 14 d.p.i. Despite some quite large numerical differences between the FCRs during the period of acute disease (days 0 to 7), most were not statistically significant, because of the inherent variability among the duplicate cages of birds. Nevertheless, the two higher doses of E. acervulina produced FCRs about 2.4 to 2.6 times greater than those of the controls, which is as expected from the BWG results. Only the higher dose of E. praecox (T) produced a statistically significantly greater FCR than that of the control birds, but all the remaining E. praecox inocula resulted in numerically greater FCRs than the controls. There were no statistically significant differences between FCRs of any treatments during the recovery period (days 7 to 14) or overall.
Table 4. Mean feed conversion ratios for three time periods after infection: each species analysed separately
Discussion
Despite a common but mistaken notion that E. praecox is practically innocuous, its effect is clinically similar to that of E. acervulina, which is generally accepted to be damaging to commercial chickens. The reported variability in the effects of either species may often result from differences in the numbers of oocysts administered or other exogenous factors, but may also reflect differences in strain virulence. Therefore, in the present study various infective doses of laboratory and field strains of E. praecox from different continents were compared with a laboratory strain of E. acervulina as a standard, under identical conditions. The objective was to demonstrate the pathogenesis of E. praecox in comparison with E. acervulina and to elucidate any possible differences in strain virulence for E. praecox.
The infective doses employed of both E. praecox and E. acervulina caused adverse effects on BWG and FCR. The inocula of 0.5×106 and 1×106 sporulated oocysts of E. praecox (T) reduced BWG by 55% and 78%, respectively, during 1 week—far exceeding the greatest reductions (20 to 25%) previously recorded (Long, Citation1968; Gore & Long, Citation1982; Williams, Citation1998; Williams & Catchpole, Citation2000). Similar inocula increased FCRs by 55% (H), 105% (R) or 300% (T), exceeding the greatest previously recorded increases of 4 to 29% (Williams & Catchpole, Citation2000).
Regarding E. acervulina (H), BWG reductions of up to 57% occurred during 1 week after infection, which fall into the expected range of 4 to 64% reduction caused by about 0.5 to 1.0×106 oocysts (Hein, Citation1968; Long, Citation1968; Reid & Johnson, Citation1970; Michael & Hodges, Citation1971; Williams, Citation1998, Citation2006; Williams & Catchpole, Citation2000). An increase in FCR of 162% occurred after infection with 0.5×106 oocysts, greatly exceeding the 1 to 34% increases with a similar inoculum recorded by Williams & Catchpole (Citation2000) and Williams (Citation2006).
No birds infected with either species died, but E. acervulina produced gross pathognomonic lesions, whilst E. praecox caused only microscopic lesions associated with reductions in viscosity of gut contents. The pathogenic effects in the gut due to E. praecox were all maximal during the acute phase and persisted, although much reduced, 14 days after infection. This contrasted with the gross lesions of E. acervulina, which were rather milder than expected during the acute phase, possibly because they were scored a few hours too early for maximal effects to be discernible. No gross E. acervulina lesions were visible 14 days after infection.
It is important to note that considerable variation in the form of the intestinal lesions in birds infected with E. acervulina was observed in this study. Thus, in the widely-used Houghton strain, lesions may be rounded (see b) as well as “ladder-like” (as they are almost invariably described). Edgar & Seibold (Citation1964) considered such rounded lesions to be caused by their newly described nominal species E. mivati in contrast to the typical transverse E. acervulina lesions.
The combined methods used here to characterize the parasites are particularly effective for accurate species identification. Thus, the PCR assays supported the identification of each species based upon pathogenic effects in chickens, and as a result the amplicon sizes of the gel bands can now be correlated with the classic parasitological characteristics of each species. This increases confidence in the conclusion that E. acervulina is the species responsible for the rounded white lesions that are sometimes found in the duodenum and jejunum of chickens. We are not aware of any previous publications in which PCR assays are correlated with classic parasitological characteristics of Eimeria species.
To assess differences in virulence of the E. praecox strains, bird growth was regarded as the primary criterion. There were clear differences between the effects on BWG during the acute phase of infection. The Tynygongl strain was the most virulent, followed by the Raleigh and Houghton strains. In comparison with E. acervulina (H), which caused 37 to 57% BWG reductions (P<0.05), E. praecox (T) was more virulent, whilst the Raleigh and Houghton strains were less virulent.
In previous studies, heavy primary infections of E. acervulina usually caused body weight loss in young chicks (Hein, Citation1968; Long, Citation1968; Williams, Citation1998), but similar infections in older birds sometimes only reduced daily BWGs (Long, Citation1968). In contrast, E. praecox has hitherto been found only to reduce daily BWGs (Oikawa & Kawaguchi, Citation1976; Gore & Long, Citation1982; Shirley et al., Citation1984; Williams, Citation1998), as did the Houghton and Raleigh strains in the present study. Hence, our findings with the Tynygongl strain apparently constitute the first demonstration that E. praecox can cause actual body weight loss in chicks, as E. acervulina may do. E. praecox (T) also caused a significant increase in FCR.
Volume is the most meaningful measure of oocyst size. Morphometric results confirmed that E. acervulina oocysts are significantly smaller than E. praecox oocysts, but there was considerable overlap between all the dimensions measured, both between species and among the E. praecox strains. The means of the shape indices of the E. praecox strains were similar to each other but were significantly different from those of E. acervulina. However, the observed extremes within the data sets of all groups completely overlapped. In this study, the shape index of individual oocysts therefore proved to be an extremely unreliable species identifier, particularly as all strains of both species contained subspherical oocysts. Despite the convenience of the shape index, which is suggestive in species identification, volume remains the most reliable measurable characteristic of oocysts.
The present study has demonstrated that laboratory and field strains of E. praecox may exhibit a much wider range of virulence than previously thought. Furthermore, the virulence of E. praecox may not only equal but may exceed that of E. acervulina. Incidentally, important variation in the form of E. acervulina lesions has been observed and considerable overlaps in oocyst dimensions and shape indices of E. praecox and E. acervulina have been demonstrated.
Acknowledgements
The authors thank Robin Sayers (VLA) for carrying out the statistical analyses, and Jeremy H. Morgan (Institute of Animal Health, Compton, UK) and Mark Jenkins (USDA, Beltsville, USA) for providing samples of oocysts. Skilled technical assistance was provided at the VLA by Jackie A. Marshall, Michael Haslam and Margaret Newlands; and at Laboratorios Hipra, by Natalia Dewe, Natalia Bello and Noelia Lopez.
References
- Edgar , S.A. and Seibold , C.T. 1964 . A new coccidium of chickens, Eimeria mivati sp. n. (Protozoa: Eimeriidae) with details of its life history . Journal of Parasitology , 50 : 193 – 204 .
- Gore , T.C. and Long , P.L. 1982 . The biology and pathogenicity of a recent field isolate of Eimeria praecox Johnson, 1930 . Journal of Protozoology , 29 : 82 – 85 .
- Haug , A. , Thebo , P. and Mattson , J.G. 2007 . A simplified protocol for molecular identification of Eimeria species in field samples . Veterinary Parasitology , 146 : 35 – 45 .
- Hein , H. 1968 . The pathogenic effects of Eimeria acervulina in young chicks . Experimental Parasitology , 22 : 1 – 11 .
- Jenkins , M. , Allen , P. , Wilkins , G. , Klopp , S. and Miska , K. 2008 . Eimeria praecox infection ameliorates effects of Eimeria maxima infection in chickens . Veterinary Parasitology , 155 : 10 – 14 .
- Johnson , J. and Reid , W.M. 1970 . Anticoccidial drugs: lesion scoring techniques in battery and floor–pen experiments with chickens . Experimental Parasitology , 28 : 30 – 36 .
- Jorgensen , W.K. , Stewart , N.P. , Jeston , P.J. , Molloy , J.B. , Blight , G.W. and Dalgliesh , R.J. 1997 . Isolation and pathogenicity of Australian strains of Eimeria praecox and Eimeria mitis . Australian Veterinary Journal , 75 : 592 – 595 .
- Joyner , L.P. , Canning , E.U. , Long , P.L. , Rollinson , D. and Williams , R.B. 1978 . A suggested terminology for populations of coccidia (Eimeriorina), particularly of the genus Eimeria (Protozoa: Apicomplexa) . Parasitology , 77 : 27 – 31 .
- Levine , N.D. 1988 . The Protozoan Phylum Apicomplexa , Boca Raton , FL : CRC Press, Inc .
- Long , P.L. 1967a . Studies on Eimeria praecox Johnson, 1930, in the chicken . Parasitology , 57 : 351 – 361 .
- Long , P.L. 1967b . Studies on Eimeria mivati in chickens and a comparison with Eimeria acervulina . Journal of Comparative Pathology and Therapeutics , 77 : 315 – 325 .
- Long , P.L. 1968 . The pathogenic effects of Eimeria praecox and E. acervulina in the chicken . Parasitology , 58 : 691 – 700 .
- Michael , E. and Hodges , R.D. 1971 . The pathogenic effects of Eimeria acervulina: a comparison of single and repeated infections . The Veterinary Record , 89 : 329 – 333 .
- Oikawa , H. & Kawaguchi , H. 1976 Effect of mode of infection on manifestation of symptom and oocyst production in chicken coccidiosis. IV. Eimeria praecox and E. mitis . Japanese Journal of Parasitology , 25 , 141 – 147 . [In Japanese]
- Reid , W.M. and Johnson , J. 1970 . Pathogenicity of Eimeria acervulina in light and heavy coccidial infections . Avian Diseases , 14 : 166 – 171 .
- Rose , M.E. , Long , P.L. and Bradley , J.W.A. 1975 . Immune responses to infections with coccidia in chickens: gut hypersensitivity . Parasitology , 71 : 357 – 368 .
- Salisch , H. 1990 . A study of the life cycle of Eimeria praecox, Johnson 1930 . Journal of Veterinary Medicine B , 37 : 363 – 368 .
- Schnitzler , B.E. , Thebo , P. , Mattsson , J.G. , Tomley , F.M. and Shirley , M.W. 1998 . Development of a diagnostic PCR assay for the detection and discrimination of four pathogenic Eimeria species of the chicken . Avian Pathology , 27 : 490 – 497 .
- Schnitzler , B.E. , Thebo , P. , Tomley , F.M. , Uggla , A. and Shirley , M.W. 1999 . PCR identification of chicken Eimeria: a simplified read–out . Avian Pathology , 28 : 89 – 93 .
- Shirley , M.W. , McDonald , V. , Chapman , H.D. and Millard , B.J. 1984 . Eimeria praecox: selection and characteristics of precocious lines . Avian Pathology , 13 : 669 – 682 .
- Williams , R.B. 1998 . Epidemiological aspects of the use of live anticoccidial vaccines for chickens . International Journal for Parasitology , 28 : 1089 – 1098 .
- Williams , R.B. 2001 . Quantification of the crowding effect during infections with the seven Eimeria species of the domesticated fowl: its importance for experimental designs and the production of oocyst stocks . International Journal for Parasitology , 31 : 1056 – 1069 .
- Williams , R.B. 2002 . Anticoccidial vaccines for broiler chickens: pathways to success . Avian Pathology , 31 : 317 – 353 .
- Williams , R.B. 2005 . Direct assessment of physical gut integrity by mucosal examination . World Poultry , 21 ( 10 ) : 43 – 45 .
- Williams , R.B. 2006 . Relative virulences of a drug–resistant and a drug–sensitive strain of Eimeria acervulina, a coccidium of chickens . Veterinary Parasitology , 135 : 15 – 23 .
- Williams , R.B. and Catchpole , C. 2000 . A new protocol for a challenge test to assess the efficacy of live anticoccidial vaccines for chickens . Vaccine , 18 : 1178 – 1185 .