Abstract
High mortalities in 17 canary flocks from different regions of Tehran, Iran, were reported. Necropsy and histopathologic examination revealed necrotic hepatitis and overall congestive septicaemia in carcasses. Salmonella enterica was isolated from 34 examined samples, two samples from each flock, including visceral organs of carcasses and droppings of live diseased birds. All isolates were typed as Salmonella enterica serovar Typhimurium by conventional serotyping. Antibiotic resistance profiling using 33 antibiotics and random amplification of polymorphic DNA differentiation by three primers were performed and showed an identical clonal relationship between these isolates and S. Typhimurium isolated from a sample of feedstuffs. Changing the feed ingredients along with antibiotic therapy via the drinking water by enrofloxacin solution controlled the outbreaks, and mortalities ceased. The zoonotic nature of S. Typhimurium and close contact of bird owners with pet birds in the home environment made the case significant in relation to public health.
Introduction
Infections with serovars of Salmonella enterica are among the most frequent causes of bacterial infections in animals and humans (Herikstad et al., Citation2002). However, the host susceptibility and development of carrier states vary widely among species. Paratyphoid (PT) salmonella infections have long been known to cause significant disease losses in young poultry (Gast, Citation2008). Avian species without caeca, like the canary, appear to be more susceptible to salmonella infections than birds with fully functioning caeca (Gerlach, Citation1994). Salmonella infection formerly has been found in many captive aviary or pet birds (Faddoul & Fellows, Citation1966; Harrington et al., Citation1975; Shima & Osborn, Citation1989; Battisti et al., 1995; Grimes & Arizmendi, Citation1995; Sato & Wada, Citation1995; Reche et al., Citation2003; Ward et al., Citation2003; Allgayer et al., Citation2008; Sareyyüpoglu et al., Citation2008; Vigo et al., Citation2009), and PT infection is one of the important bacterial diseases of canaries and other pet birds (Harrington et al., Citation1975).
High mortalities were reported in numerous flocks of multi-age canaries in different regions of Tehran. This study was performed in order to confirm an epornitic by a single causative agent. Furthermore, to our knowledge, this is the first study to include molecular typing of Salmonella enterica serovar Typhimurium isolated from canaries.
Materials and Methods
Samples
Diseased and dead canaries were referred to Faculty of Veterinary Medicine, University of Tehran. The birds belonged to 17 different collections located in Tehran (). The number of canaries in each collection and mortalities are presented in .
Figure 1. Geographical distribution of affected canary flocks in Tehran (black squares). Numbers refer to regions of municipality of Tehran.
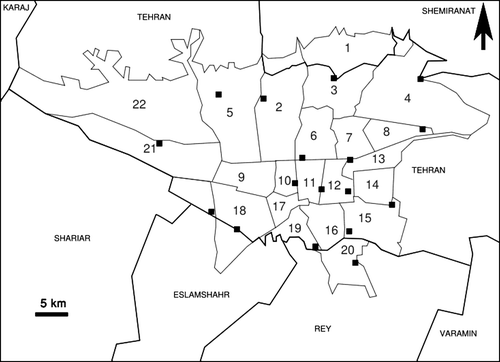
Table 1. Mortality in each flock
Pathology
All carcasses were examined macroscopically. Tissue samples were obtained in 10% buffered formalin for histopathologic examination after haematoxylin and eosin staining.
Isolation of bacteria
Samples from viscera of dead birds and/or droppings of diseased birds were obtained aseptically in each flock for bacteriologic tests. As almost all canary fanciers in Tehran purchase canary seed from the same downtown market, feedstuffs and water supply were also cultured by conventional microbiological methods and biotyping was performed by conventional biochemical tests (Quinn et al., Citation1994). Two isolates from each flock in addition to a feed isolate were selected for further characterization.
Serotyping of the isolated salmonellas was performed by commercial antisera (Difco, Detroit, Michigan, USA).
Antibiotic susceptibility test
The antibiotic susceptibility test was performed by the standard disk diffusion method in Mueller − Hinton agar (Difco) and the results were interpreted in accordance with the criteria of the National Committee for Clinical Laboratory Standards (Citation2000 ,Citation2001). The isolates were screened for resistance to the following antibiotics: Tiamulin (30 g), Tylosin (30 g), Lincospectin (Lincomycin 15 g/Spectinomycin 200 g), Flumequine (30 g), Difloxacin (25 g), Neomycin (30 g), Sulphamethoxazole (100 g), Florfenicol (30 g), Trimethoprim (5 g), Enrofloxacin (5 g), Cloxacillin (1 g), Oxytetracycline (30 g), Furazolidone (100 g), Chloramphenicol (30 g), Ampicillin (10 g), Ceftriaxone (30 g), Co-amoxiclav (30 g), Imipenem (10 g), Clarithromycin (15 g), Ceftazidime (30 g), Cefotaxime(30 g), Kanamycin (30 g), Cephazolin (30 g), Ciprofloxacin (5 g), Cefixime (5 g), Piperacillin (100 g), Norfloxacin (10 g), Carbenicillin (100 g), Ofloxacin (5 g), Cefuroxime (30 g), Vancomycin (30 g) and Ticarcillin (75 g).
Randomly amplified polymorphic DNA analysis
A single colony of each isolate from an agar plate was picked and suspended in 200 µl distilled water. After vortexing, the suspension was boiled for 5 min, and 50 l supernatant was collected after centrifuging for 10 min at 25,000×g. The DNA concentration of boiled extracts was determined with a spectrophotometer.
The polymerase chain reaction (PCR) was conducted in a 25 l volume containing 40 ng total S. Typhimurium DNA (extracted by boiling), 1.5 mM MgCl2, 0.5 M primer, 1 U Smartaq DNA polymerase (Fermentas, LT) and 200 M dNTPs mix in 1×PCR buffer (Fermentas, LT). The PCR programme and electrophoresis condition were conducted as previously described by Lin et al. (Citation1996).
The selected primers for this study were P1254 (5′-CCGCAGCCAA-3′), 23L (5′-CCGAAGCTGC-3′) and OPA-4 (5′-AATCGGGCTG-3′) (Lin et al., Citation1996; Madadgar et al., Citation2008)
Treatment and follow-up
The suspect feed materials were exchanged for new fresh sources. Isolation of diseased birds from the rest of the flock, cleaning and disinfection of cages, water and feed containers, and perches with 10% (vol/vol) solution of commercial bleach (containing 5% sodium hypochlorite) and/or commercial disinfectants such as Virkon® S (Antec™ International, Sudbury, UK) were recommended to the bird owners. Treatment of the remaining live birds in flocks was conducted by 10% (w/v) enrofloxacin solution supplied as 200 mg/l drinking water for 5 to 7 days. Three weeks to 6 weeks after treatment, bacteriological examination of a pooled faecal sample was performed to evaluate therapy success.
Results
Clinical presentation and pathology
The mean mortality rate was 51% in the referred collections (). The flocks had no geographical relationship with each other and were distributed in a vast area of Tehran (approximately 700 km2) (). All fanciers purchased feed ingredients from the same live bird market located of downtown Tehran. The affected birds were lethargic, and had watery or mucoid diarrhoea with excess urate deposits. Almost all bird owners reported a sudden increase in mortality a week before the mortalities reported in .
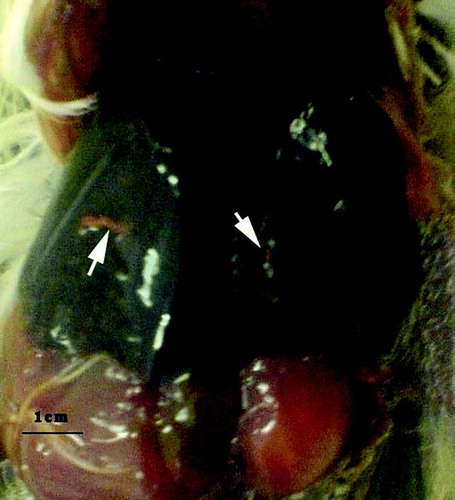
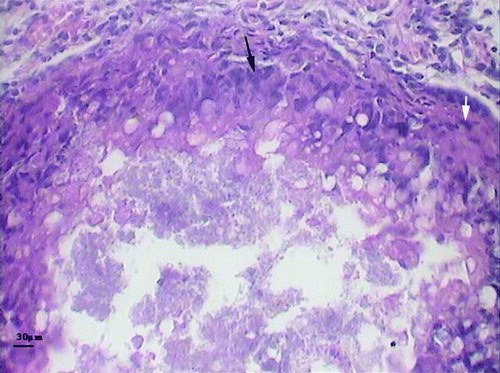
Overall congestion of visceral organs and necrotic foci on the liver with a nodular and miliary appearance were the most significant and consistent macroscopic lesions in dead birds (). Lung congestion was seen variably in many cases. Dark bloody intestinal contents were also obvious in many carcasses and were probably the result of haemorrhagic diathesis (as previously described by Dorrestein, Citation2000). Mild to moderate splenomegaly with varying degrees of discoloration was also seen. Microscopic inspection of histopathologic sections revealed disseminated and nodular necrosis of parenchymatous organs, particularly the liver with variable degrees of granulocyte and mononuclear leukocyte infiltration and fibrin deposition. A typical granulomatous reaction with a necrotic centre surrounded by foamy macrophages, multinucleated giant cells and fibroblasts was observed in some affected livers (). Pneumonia with typical heterophilic and mononuclear infiltration and even massive necrosis was confirmed in a few cases.
Bacteriology and serology
Salmonellas were isolated from all carcasses and almost all faecal samples. The same bacteria were isolated from fresh feedstuffs that had not been in contact with bird collections or droppings, but not from the water supply of the flocks mentioned.
All canary isolates and a tested feed isolate proved to be S. Typhimurium, which were identified by O:4, O:5, O:12, H1:i and H2:1, H2:2 antisera (Difco).
Antibiotic resistance profiling
All isolated S. Typhimurium revealed the same resistance pattern and were susceptible to all antibiotics with the exception of Sulphamethoxazole, Tylosin, Cloxacillin, Tiamulin and Vancomycin.
Random amplification of polymorphic DNA
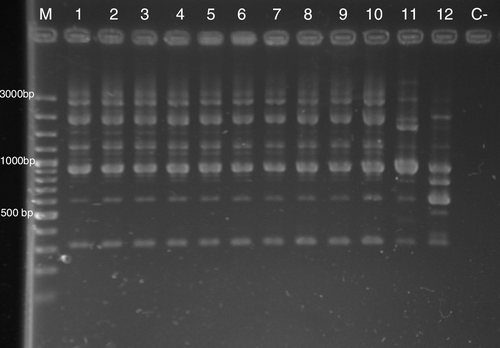
Random amplification of polymorphic DNA (RAPD) by P1254, 23L and OPA-4 primers revealed identical patterns in all 36 isolates of S. Typhimurium. RAPD profiles of selected isolates are shown in . A high similarity between S. Typhimurium ATCC 14028 and the canary isolates was observed.
Figure 4. Results of RAPD of selected S. Typhimurium isolates of this study generated with the P1254 primer. Notice the similarity between S. Typhimurium ATCC 14028 and outbreak isolates. Lane M, 100 base pair (bp) ladder; lane 1, S. Typhimurium ATCC 14028; lane 11, S. Typhimurium bovine isolate; lane 12, Salmonella Senftenberg parrot isolate; lanes 2 to 7, S. Typhimurium isolates from canaries in the present study; lanes 8 to 10, S. Typhimurium isolates from feedstuff in the present study.
Treatment
No new cases of diseased birds were reported in the affected collections after initiation of antibacterial therapy, but some bird fanciers had faced heavy losses before treatment was initiated. Diseased birds responded variably to treatment and some less affected birds could be cured. The therapeutic regimen could prevent development of new cases of PT and mortality ceased in most flocks. Three weeks to 6 weeks after treatment, all faecal samples were negative for salmonella infection.
Discussion
Diseases caused by non-host-adapted salmonella infection are uncommon in poultry, and are usually seen in chicks, poults or ducklings younger than 2 weeks of age and rarely in birds over 4 weeks of age. The morbidity and mortality varies considerably, and deaths are usually less than 20% of the affected group (Lister & Barrow, Citation2008). In very susceptible young chicks and poults, PT infection can sometimes lead to illness and death at high frequencies. Older birds are considerably less susceptible to the lethal effects of PT salmonellas and may experience intestinal colonization and even systemic dissemination without significant morbidity or mortality (Gast, Citation2008). Infection with S. Typhimurium in small passerines appears identical with pseudotuberculosis, both clinically and at necropsy (Dorrestein, Citation2000 ,Citation2005).
The outbreaks we observed occurred in multi-age canaries with mortality rates that varied from 20% to 94%. These results indicate high virulence of the S. Typhimurium isolates or high susceptibility of the canary. Keyvanfar (Citation1968) reported a lethal systemic outbreak of S. Typhimurium in a canary flock in Tehran with more than 50% mortality in 100 birds. Raidal (Citation1998) described mortalities in two non-related flocks of canary in Australia due to S. Typhimurium infection with almost the same pathological findings as ours. Some authors have reported more chronic courses of PT in canary (Dorrestein, Citation2000 ,Citation2005). Refsum et al. (Citation2003) reported that necrotic oesophagitis/ingluvitis was the most consistent lesion in wild, free-ranging passerine birds in Norway, while we did not see such lesions in this study.
Madadgar et al. (Citation2008) reported that a combination of pulsed field gel electrophoresis, RAPD and R-typing had high discriminatory ability for S. Typhimurium isolate differentiation. The use of this method revealed identical profiles of isolates in the outbreak investigated. In addition, high similarity between S. Typhimurium ATCC 14028 and the outbreak isolates was observed in RAPD with all primers. Genetic relatedness of salmonellae isolates from wild passerine birds in the United States was previously shown by pulsed field gel electrophoresis by Hudson et al. (Citation2000).
Feed contamination with bacteria originating from bird droppings was the suspected cause of this outbreak as all bird fanciers had purchased canary seeds from the same live bird market and the feed isolate was identical to the bird isolates with respect to molecular typing and drug resistance patterns. It seems likely that some predisposing factors and environmental situations, such as breeding or shipping, could affect the severity of PT outbreaks in the canary (Harrington et al., Citation1975; Raidal, Citation1998). As canaries are kept indoors, feed contamination or recently bought birds are the most probable sources of infection to collections. The significance of newly imported birds on the introduction of salmonellosis to a bird collection was previously reported (Shima & Osborn, Citation1989).
Death caused by non-typhoid salmonellad occurs primarily in humans with serious underlying disease such as HIV infection, cancer or leukaemia and cirrhosis (Janda & Abbott, Citation2006). The human risk for salmonellosis in close association with passerine birds infection was previously reported (Alley et al., Citation2002). Although there is no official report of PT transmission from canaries to humans considering the zoonotic multi-host nature of PT salmonellas, increasing numbers of bird fanciers in Iran and indoor rearing of these kinds of birds, the risk of human infection could be significant. It is obvious that identification and characterization of isolates lethal to birds could be very important for human health surveillance systems.
Feed contamination possibly played an important role in PT spread in both the current case and that reported by Keyvanfar (Citation1968). Free-ranging or other pet birds can be subclinical carriers and serve as a reservoir for the aviary (Gerlach, Citation1994; Shima & Osborn, Citation1989). Sato & Aoyagi (Citation1996) showed that S. Typhimurium infection persisted for 22 days in zebra finches (Poephila guttata). In addition, rats, flies and other vermin may also serve as vectors of Salmonella spp. (Gast, Citation2008). A reptile has also been suspected as a source of infection in an aviary (Ward et al., Citation2003).
This appears to be the first molecular epidemiological study of S. Typhimurium in canaries. It is suggested that further studies on canary salmonellosis with more isolates from different areas are performed.
Acknowledgements
The authors are grateful to the Ministry of Science, Research and Technology, Research Council of University of Tehran and Research Council of Faculty of Veterinary Medicine for financial support and project no. 7504001/6/1.
References
- Alley , M.R. , Connolly , J.H. , Fenwick , S.G. , Mackereth , G.F. , Leyland , M.J. Rogers , M.E. 2002 . An epidemic of salmonellosis caused by Salmonella Typhimurium DT160 in wild birds and humans in New Zealand . New Zealand Veterinary Journal , 50 : 170 – 176 .
- Allgayer , M.C. , Lima-Rosa , C.A. , Weimer , T.A. , Rodenbusch , C.R. , Pereira , R.A. Streck , A.F. 2008 . Molecular diagnosis of Salmonella species in captive psittacine birds . The Veterinary Record , 162 : 816 – 819 .
- Battisti , A. , Di Guardo , G. , Agrimi , U. and Bozzano , A.I. 1998 . Embryonic and neonatal mortality from salmonellosis in captive bred raptors . Journal of Wildlife Diseases , 34 : 64 – 72 .
- Dorrestein , G.M. 2000 . “ Passerines and exotic soft bills ” . In Avian Medicine , Edited by: Tully , T.N. , Lawton , M.P.C. and Dorrestein , G.M. 144 – 179 . Edinburgh : Elsevier Science .
- Dorrestein , G.M. 2005 . Passerine and soft bills medicine and surgery In Proceedings of 8th European AAV Conference and 6th Scientific ECAMS Meeting 409 424 . Arles, France .
- Faddoul , G.P. and Fellows , G.W. 1966 . Five year survey of the incidence of salmonellae in avian species . Avian Diseases , 10 : 296 – 304 .
- Gast , R.K. 2008 . “ Paratyphoid infections ” . In Diseases of Poultry , 12th edn , Edited by: Saif , Y.M. , Fadly , A.M. , Glisson , J.R. , McDougald , L.R. , Nolan , L.K. and Swayne , D.E. 636 – 665 . Ames : Iowa State Press .
- Gerlach , H. 1994 . “ Bacteria ” . In Avian Medicine, Principles and Application , Edited by: Ritchie , B.W. , Harrison , G.J. and Harrison , L.R. 949 – 983 . Lake Worth , FL : Wingers Publishing .
- Grimes , J.E. Arizmendi , F. 7 1995 270 . Salmonella Typhimurium agglutinins in exotic bird sera in the USA . Journal of Veterinary Diagnostic Investigation
- Harrington , R. , Blackbum , B.O. and Cassidy , D.R. 1975 . Salmonellosis in canaries . Avian Diseases , 19 : 827 – 829 .
- Herikstad , H. , Motarjemi , Y. and Tauxe , R.V. 2002 . Salmonella surveillance: a global survey of public health serotyping . Epidemiology and Infection , 129 : 1 – 8 .
- Hudson , C.R. , Quist , C. , Lee , M.D. , Keyes , K. , Dodson , S.V. Morales , C. 2000 . Genetic relatedness of Salmonella isolates from nondomestic birds in southeastern United States . Journal of Clinical Microbiology , 38 : 1860 – 1865 .
- Janda , J.M. and Abbott , S.L. 2006 . The Enterobacteria , 2nd edn , Washington , DC : American Society for Microbiology Press .
- Keyvanfar , H. 1968 . A lethal outbreak of Salmonella Typhimurium in canary and parrots . The Journal of Faculty of Veterinary Medicine of Univercity of Tehran , 24 : 101 – 108 .
- Lin , A.W. , Usera , M.A. , Barrett , T.J. and Godsby , R.A. 1996 . Application of random amplified polymorphic DNA analysis to differentiate strains of Salmonella enteritidis . Journal of Clinical Microbiology , 34 : 870 – 876 .
- Lister , S.A. and Barrow , P. 2008 . “ Enterobacteriaceae ” . In Poultry Diseases , 6th edn , Edited by: Pattison , M. , McMullin , P.F. , Bradbury , J.M. and Alexander , D.J. 110 – 145 . Edinburgh : Saunders .
- Madadgar , O. , Tadjbakhsh , H. , Zahraei Salehi , T. , Mahzounieh , M. and Feizabadi , M.M. 2008 . Evaluation of random amplified polymorphic DNA analysis and antibiotic susceptibility application in discrimination of Salmonella Typhimurium isolates in Iran . New Microbiologica , 31 : 211 – 216 .
- National Committee for Clinical Laboratory Standards 2000 . Performance Standards for Antimicrobial Disks Susceptibility Tests. Approved Standard , 7th edn vol. 20, no. 1, M2-A7 . Wayne , PA : NCCLS .
- National Committee for Clinical Laboratory Standards 2001 . Performance Standards for Antimicrobial Susceptibility Testing; Eleventh Informational Supplement , vol. 21, no. 1, M100-S11 . Wayne , PA : NCCLS .
- Quinn , P.J. , Carter , M.E. , Markey , P.K. and Carter , G.R. 1994 . Enterobacteriaceae In Clinical Veterinary Microbiology , 209 – 236 . London : Wolfe Publishing .
- Raidal , S.R. 28 1998 58 Salmonellosis in two canary (Serinus canaria) flocks . Australian Veterinary Practitioner
- Reche , M.P. , Jiménez , P.A. , Alvarez , F. , Garcia de los Rios , J.E. , Rojas , A.M. and de Pedro , P. 2003 . Incidence of salmonellae in captive and wild free-living raptorial birds in central Spain . Journal of Veterinary Medicine B, Infectious Diseases and Veterinary Public Health , 50 : 42 – 44 .
- Refsum , T. , Vikøren , T. , Handeland , K. , Kapperud , G. and Holstad , G. 2003 . Epidemiologic and pathologic aspects of Salmonella Typhimuriun infection in passerine birds in Norway . Journal of Wildlife Diseases , 39 : 64 – 72 .
- Sareyyüpoğlu , B. , Cantekin , Z. , Yardimci , A.H. , Akan , M. and Akacay , A. 2008 . Polymerase chain reaction detection of Salmonella spp. in fecal samples of pet birds . Avian Diseases , 52 : 163 – 167 .
- Sato , Y. and Aoyagi , T. 1996 . Infectivity and persistence of Salmonella Typhimurium for zebra finches (Poephila guttata) isolated from the same species . Journal of Veterinary Medical Science , 58 : 845 – 848 .
- Sato , Y. and Wada , K. 1995 . Isolation of Salmonella Typhimurium from zebra finches (Poephila guttata) . Journal of Veterinary Medical Science , 57 : 137 – 138 .
- Shima , A.L. and Osborn , K.G. 1989 . An epornitic of Salmonella typhimurium in a collection of lories and lorikeets . Journal of Zoo and Wildlife Medicine , 20 : 373 – 376 .
- Vigo , G.B. , Origlia , J. , Gornatti , D. , Piscopo , M. , Salve , A. Caffer , M.I. 2009 . Isolation of Salmonella Typhimurium from dead blue and gold macaws (Ara ararauna) . Avian Diseases , 53 : 135 – 138 .
- Ward , M.P. , Ramer , J.C. , Proudfoot , J. , Garner , M.M. , Juan-Sallés , C. and Wu , C.C. 2003 . Outbreak of salmonellosis in a zoologic collection of lorikeets and lories (Trichoglossus, Lorius, and Eos spp.) . Avian Diseases , 47 : 493 – 498 .