Abstract
Factors influencing replication of serotype 1 Marek's disease vaccines in the lung of chickens within the first 10 days of age (doa) were evaluated. In particular, the effect of vaccine efficacy, age/route of vaccination, and vaccine dose were examined in three experiments. In the first experiment, three vaccine pairs, each pair consisting of a high protective (HP) and a low protective (LP) vaccine (CVI988/BP5 and CVI988-Clone C, 648A80 and 648A100, R2 and R2/23) were used to inoculate chickens subcutaneously (s.c.) with 2000 plaque-forming units (PFU) at hatch. DNA load in the lung was significantly higher in the HP vaccine group than the LP vaccine group at 5 and 10 doa in two of the three vaccine pairs. In the lung, at 5 doa, early MDV gene transcripts (ICP4 and pp38) were detected in most vaccine groups, whereas late MDV gene transcripts (gB and gI) were detected only in the HP vaccine group. In the second experiment, chickens were vaccinated in ovo or s.c. at hatch with 2000 PFU R2, R2/23, or CVI988/BP5. Compared with s.c. vaccination, in ovo vaccination resulted in higher MDV DNA load in the lung at 3 doa, lower or equal MDV DNA load at 5 doa, and lower MDV DNA load at 10 doa. In the third experiment, chickens were vaccinated s.c. at hatch with either 2000 or 10,000 PFU strain R2. There were no statistically significant differences in the load of MDV DNA in the lung after vaccination with R2 between the two doses. Our results showed that HP vaccines tend to replicate better than LP vaccines in the lung; and vaccine replication in the lung within the first 10 days of age was affected by the age/route of inoculation (in ovo versus s.c.) but not by the dose of vaccine administered.
Introduction
Marek's disease (MD) is a lymphoproliferative disease of chickens that, in the absence of control measures, is capable of causing devastating losses in commercial poultry flocks. The economic impact of MD on the world poultry industry is thought to be in the range of US$1 to 2 billion annually (Morrow & Fehler, Citation2004). MD has been successfully controlled by vaccination since 1969 (Churchill et al., Citation1969). However, vaccine efficacy has decreased concomitantly with the increase in virulence of Marek's disease virus (MDV) (Witter, Citation1997). Of particular importance, the most commonly utilized vaccine in the USA during the past 20 years (bivalent vaccine including serotypes 2 and 3) does not protect chickens against the most recently isolated strains of MDV, the very virulent plus strains (Witter, Citation1997). Attenuated serotype 1 vaccines seem to confer better protection against challenge with very virulent plus strains (Witter, Citation1987, Citation2001). Nonetheless, even serotype 1 vaccines have been shown to have reduced efficacy with time in some countries (Kross, Citation1996; Venugopal et al., Citation1996). Hence, the search for more efficient vaccines continues mainly due to the threat of emergence of MDV strains of higher virulence and the great economic impact of MD on the poultry industry. However, a major limitation in the development of more efficient vaccines against MD is the lack of information on the pathogenesis of vaccine strains and on the mechanisms of vaccine-induced immunity.
A major drawback of vaccination is the fact that introduction of MDV vaccines has driven the virus to an increase in its virulence, with each successive vaccine being followed by the emergence of more pathogenic strains (Witter, Citation1997). This continuous evolution of MDV could be explained by the nature of vaccine-induced immune response. MD vaccines induce non-sterile immunity that restricts viral replication within the host, rather than either blocking viral entry or shedding of infective virus into the environment (Gandon et al., Citation2001). A better understanding of the MDV pathogenesis in the lung (route of entry) and in the feather follicle epithelium (source of free infectious virus) will be necessary to address this problem.
Inhalation of chicken dander infected with MDV is the natural route of infection in chickens (Beasley et al., Citation1970; Calnek et al., Citation1970). However, little is known about the events of MDV infection during the first 2 to 3 days and until MDV is detected in the lymphoid organs. It is possible to detect viral genome as well as viral antigens in the lung following infection with oncogenic MDV either by inhalation or by the intraabdominal route (Purchase, Citation1970; Adldinger & Calnek, 1973; St Hill et al., Citation2004). It has also been demonstrated that intratracheal inoculation of MDV resulted in earlier replication of the virus in the lung and in more severe forms of disease than intraabdominal inoculation (Butter et al., Citation2007).
In the field, MDV challenge occurs in chickens as soon as they hatch. An early strong pulmonary immune response against MDV might reduce or delay the subsequent systemic replication. Unfortunately, the role of pulmonary immune response in protection against MD has not been studied. It has been recently reported that infection with oncogenic viruses by the respiratory route elicits a strong innate immune response in the lung (Abdul-Careem et al., Citation2009). There is evidence that MD vaccines can replicate in the lung and therefore might be able to elicit pulmonary immune responses. However, various factors seem to influence MD vaccine replication in the lung (i.e. vaccine serotype, age/route of vaccination, and vaccine strain). Lung is the first tissue where herpesvirus of turkey replicates following inoculation of 18-day-old embryos by the amniotic route (Sharma et al., Citation1984). Serotype 1 MD vaccines, however, did not replicate in the lung of embryos when the vaccine was administered by the amniotic route (Sharma, Citation1987a) but it did when administered by the intravenous route (St Hill & Sharma, Citation2000). On the other hand, serotype 1 MD vaccines exhibited pulmonary tropism after subcutaneous (s.c.) inoculation of 1-day-old chickens. In a previous work, we demonstrated that attenuated serotype 1 MDV vaccines can be detected in the lung as early as 3 days post s.c. inoculation (Gimeno et al., Citation2004). However, vaccine DNA load in the lung varied greatly among vaccines (Gimeno et al., Citation2004). Some vaccine strains seemed to have remarkable pulmonary tropism as vaccine DNA load in the lung was higher than that detected in the bursa of Fabricius, a target organ for early MDV replication (Gimeno et al., Citation2004).
Understanding the factors influencing MD vaccine replication in chicken lung is essential for a rational design of vaccines that can protect better at the pulmonary mucosal surfaces. The objective of this work was to study factors influencing the replication of serotype 1 MDV vaccines in the lung within the first 10 days of the chicken's life. In particular, our specific objectives were to elucidate the effect of vaccine efficacy, age/route of vaccination and dose of vaccine on the replication of MDV vaccines in the lung.
Materials and Methods
Chickens and embryonated eggs
Commercially available specific pathogen free SPAFAS chickens (Charles River SPAFAS, N Franklin, Connecticut, USA) were used.
Viruses
Three pairs of viruses were selected, each one including a high protective (HP) vaccine and a low protective (LP) vaccine. Relative protection of the vaccines used in this study has been reported elsewhere (Witter, Citation1987, Citation2002; Witter et al., Citation1995; Dudnikov & Witter, Citation2001; Gimeno et al., Citation2004; Witter & Kreager, Citation2004). Briefly, the two components of each pair originated from the same strain and differed in both chicken embryo fibroblast tissue culture (CEF) passage levels and protection ability. Pair A was derived from strain CVI988 (Rispens et al., Citation1972). The HP component was strain CVI988/BP5 (Witter et al., Citation1995), which was derived from a commercial source of CVI988 (42 CEF passages) after five back passages in chickens and five additional passages in CEF (Witter et al., Citation1995); the LP component was strain CVI988/C at 69 passages in CEF (de Boer et al., Citation1986). Pair B originated from 648A (Witter, Citation1997) and included strains 648A at 80 passages in CEF (648A80) as the HP vaccine and at 100 passages in CEF (648A100) as the LP vaccine (Witter, Citation2002). Pair C originated from strain Md11 and was composed of strain R2 at 89 passages in CEF (Witter, Citation1987) as the HP vaccine and strain R2/23 at 106 passages in CEF as the LP vaccine (Witter et al., Citation1995). Strain R2 induced greater protection than R2/23 (Witter, Citation1991; Witter et al., Citation1995) and it is considered an HP vaccine for the purpose of this work; however, it is less protective than CVI988/BP5 and 648A80 (Witter & Kreager, Citation2004).
Real-time PCR and real-time RT-PCR
The real-time polymerase chain reaction (PCR) assay was performed as previously described (Gimeno et al., Citation2005). Primer sets have been reported before (Kaiser et al., Citation2003; Levy et al., Citation2003; Gimeno et al., Citation2008a; Xu et al., Citation2008) or were designed using the OligoPerfect™ Designer software program (Invitrogen™, Carlsbad, California, USA) (). Briefly, DNA was extracted from lung and spleen samples using the Puregene™ DNA Isolation Kit (Gentra System, Minneapolis, Minnesota, USA). Each sample was amplified with two primer sets specific for the MDV glycoprotein B (gB) gene and the chicken GAPDH gene. Amplifications were done using the Mx3005 Stratagene (Stratagene, La Jolla, California, USA) in a 25 µl PCR reaction containing 50 ng DNA, 0.2 µM each primer and SYBR® green PCR master mix (Brilliant® SYBR® Green) that contains the appropriate buffers, nucleotides and Taq-polymerase (Biocrest-Stratagene, Cedar Creek, Texas, USA). The reaction was cycled 50 times at 95°C denaturation for 15 sec and a 60°C combined annealing/extension for 60 sec. Fluorescence was acquired at the end of the annealing/extension phase. The melting curve was obtained at the end of amplification by cooling the sample at 20°C/sec to 60°C and then increasing the temperature to 95°C at 0.1°C/sec. The threshold cycle parameter (Ct) was calculated for each PCR reaction by establishing a fixed threshold. Ct is defined as the fractional cycle number at which the fluorescence crosses the fixed threshold. Relative quantification of the amount of target in unknown samples was accomplished by the comparative Ct method (DDCT) (Gimeno et al., Citation2005). In addition to the Ct ratio of lung (absolute values), the vaccine DNA load in lung was also expressed as a relative value to the spleen. In that case, the Ct ratio of the spleen was subtracted from the Ct ratio of lung.
Table 1. Oligonucleotides used for real-time PCR and real-time RT-PCR.
Real-time reverse transcriptase (RT)-PCR was performed to evaluate transcription of various MDV genes. Each sample was amplified with five primer sets specific for the immediate MDV gene ICP4, the early gene pp38, the late MDV genes glycoprotein B (gB) and glycoprotein I (gI), and for the housekeeping gene 28S rRNA. Amplifications were carried out in 25 µl reactions using Brilliant® SYBR® Green Q-PCR Master Mix (Biocrest-Stratagene) in a Mx3005 Stratagene (Stratagene) machine. The PCR cycle profile was as follows: one cycle of 50°C for 30 min; one cycle of 95°C for 10 min; 50 cycles of 95°C for 10 sec and 56°C for 1 min; and one cycle of 95°C for 1 min, 55°C for 30 sec, and 95°C for 30 sec. Each sample was run in triplicate and Ct was averaged from triplicates. To correct for differences in RNA levels between samples within the experiment, the correction factor for each sample was calculated by dividing the mean Ct value for 28S rRNA-specific product for each sample, by the overall mean Ct value for the 28S rRNA-specific product from all samples (Swaggerty et al., Citation2008). The corrected MDV gene mean is calculated as follows: (average of each replicate x MDV gene slope) / (28S slope x 28S correction factor).
Samples that were amplified for the housekeeping gene (GAPDH for real-time PCR and 28S for real-time RT-PCR) but not for the other studied genes were considered negative and given a value of “zero”. Negative samples were included in the statistical analysis of the results.
Experimental design
Three experiments were conducted to study the effect of vaccine efficacy (Experiment 1), age/route of inoculation (Experiment 2), and vaccine dose (Experiment 3) on vaccine replication in the lung. For all experiments, the dose of inoculum was confirmed by titration at the time of vaccination. In Experiment 1, 1-day-old SPAFAS chickens were randomly divided into seven groups, 24 chickens each, and housed in Horsfall–Bauer (HB) isolation units under biosecurity level 2 (BSL2) containment. Each of six groups was inoculated by s.c. route with 2000 PFU of one of the six vaccine viruses. The remaining seventh group was an uninoculated control group. Seven chickens per treatment were euthanized at 3, 5, and 10 days of age (doa), and spleen and lung samples were collected for nucleic acid extraction. The samples were stored at –70°C until they were processed.
In the second experiment, three groups of 30 embryonated eggs each were vaccinated by amniotic route at 18 days of embryonation with 2000 PFU R2, R2/23, or CVI988/BP5. Inoculation was done as described earlier (Sharma & Burmester, Citation1982; Sharma et al., Citation1984). Briefly, the external surface of the broad end of the egg was swabbed with 70% ethyl alcohol, and a 1 mm hole was punched in the eggshell. The viral inoculum in 0.1 ml volume was injected by inserting the entire length of a 3.1cm long, #22-gauge needle through the hole. This method of inoculation resulted in deposition of the inoculum in amniotic fluid in most eggs (Sharma & Burmester, Citation1982). After hatch, chickens were housed in HB isolation units under BSL2 containment. In addition, unvaccinated 1-day-old SPAFAS chickens were randomly divided into four groups, 24 chickens each, and housed in HB isolation units under BSL2 containment. Three of the groups were inoculated by s.c. route with 2000 PFU strains R2, R2/23 or CVI988/BP5. The remaining group was an uninoculated control group. Seven chickens per treatment were euthanized at 3, 5, and 10 doa, and spleen and lung samples were collected for nucleic acid extraction. The samples were stored at –70°C until they were processed.
In the third experiment, 1-day-old SPAFAS chickens were randomly divided into three groups, 24 chickens each, and housed in HB isolation units under BSL2 containment. Two of the groups were inoculated by s.c. route with either 2000 PFU or 10,000 PFU strain R2. The remaining group was an uninoculated control group. Seven chickens per treatment were euthanized at 3, 5, and 10 doa, and spleen and lung samples were collected for nucleic acid extraction. The samples were stored at –70°C until they were processed.
Statistical analysis
Data were analysed using the statistical program Statistica® (Statsoft, Tulsa, Oklahoma, USA). Comparison between groups was conducted by Student's t test. The level of statistical significance was considered P <0.05.
Results
Effect of MD vaccine efficacy on MD vaccine replication in the lung (Experiment 1)
In all treatment groups, the vaccine virus DNA load in the lung was minimal or absent at 3 doa but tended to increase at 5 and 10 doa (). The frequency of chickens that were positive for vaccine virus DNA by real-time PCR was very low at 3 doa but increased at 5 and 10 doa (). Statistically significant differences were found between CVI988/BP5 versus Clone C (a) and between 648A80 versus 648A100 (c) at 5 and 10 doa. However, differences were not statistically significant in the pair R2 versus R2-23 (e).
Figure 1. Replication in the lung of HP vaccines (CVI988-BP5, 648A80, and R2) versus LP vaccines (Clone C, 648A100, and R2-23). DNA load of serotype 1 MDV vaccines was evaluated by real-time PCR. Results expressed as the relative Ct ratio of the amplification of the chicken GAPDH gene and the MDV gB (see Materials and Methods). Results are presented as the mean and the standard error. 1a: Load of DNA of CVI988/BP5 and Clone C in the lung at 3, 5, and 10 days of age (doa). 1b: Load of viral DNA in the lung relative to the load of viral DNA in the spleen (load of viral DNA in the lung – load of viral DNA in the spleen) for serotype 1 MDV vaccines CVI988/BP5 and Clone C. 1c: Load of DNA of 648A80 and 648A100 in the lung at 3, 5, and 10 doa. 1d: Load of viral DNA in the lung relative to the load of viral DNA in the spleen for serotype 1 MDV vaccines 648A80 and 648A100. 1e: Load of DNA of R2 and R2-23 in the lung at 3, 5, and 10 doa. 1f: Load of viral DNA in the lung relative to the load of viral DNA in spleen for serotype 1 MDV vaccines R2 and R2-23. Same letter above the bar indicates no statistically significant differences were detected between the members of each pair(P < 0.05). *Statistically significant differences between the load of vaccine DNA in the lung and in the spleen.
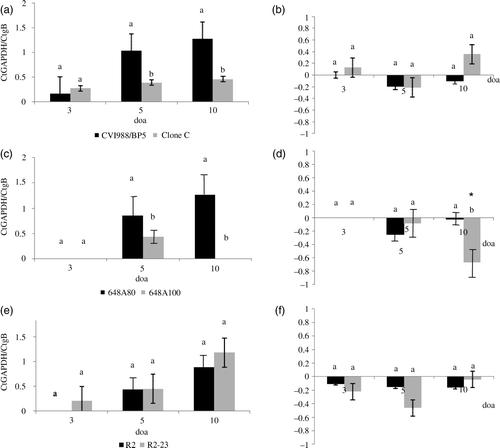
Table 2. Number of chickens positive for the vaccine DNA and which had transcription of MDV genes in the lung (Experiment 1).
No statistically significant differences were detected in the relative level of replication in the lung versus the spleen at any time point between HP and LP vaccines in any of the pairs with the exception of 648A80 and 648A100 pair at 10 doa (b, d, f). Statistically significant differences between DNA load in the lung and spleen were only detected in chickens vaccinated with 648A100 at 10 doa (d). Those chickens had significantly lower vaccine DNA load in the lung than in the spleen.
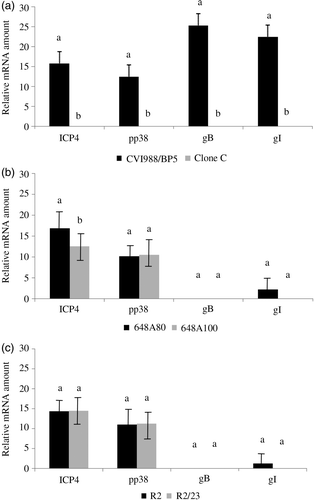
Transcription of immediate early gene ICP4, early gene pp38, and late genes gB and gI in the lung was evaluated by real-time RT-PCR. With the exception of the least protective CVI988–Clone C vaccine, all vaccines had transcription of ICP4 and pp38 genes at 5 doa in the lung ( and ). However, only CVI988/BP5 (the most protective vaccine used in this study) had transcription of gB in the lung at 5 doa. Transcription of gI was only found in chickens vaccinated with the HP vaccines CVI988/BP5, 648A80, and R2. The frequency of chickens that had transcripts of gI in the lung differed greatly among groups, being 100% in chickens inoculated with CVI988/BP5, 28.6% in chickens inoculated with 648A80, and 25% in chickens inoculated with 648A100 (). Statistically significant differences were found between HP and LP vaccines in the level of transcription of ICP4 in the lung at 5 doa. This was true for the pairs CVI988/BP5 versus Clone C (a) and 648A80 versus 648A100 (b). However, no differences were detected in the pair R2 and R2/23 (c). Statistically significant differences were found in the level of transcription of pp38 and gI between CVI988/BP5 and Clone C (a) but not between viruses of the other two pairs (b,c).
Figure 2. MDV gene transcripts in the lung at 5 doa with HP vaccines (CVI988/BP5, 648A80, and R2) and LP vaccines (Clone C, 648A100, and R2-23). Transcription of MDV genes ICP4, pp38, gB, and gI was measured by real-time RT-PCR (see Materials and Methods). Results presented as the mean and the standard error. Comparisons have been done between the two components of the same pair. 2a: Transcription of MDV genes in the pair formed by CVI988/BP5 and Clone C. 2b: Transcription of MDV genes in the pair formed by 648A80 and 648A100. 2c: Transcription of MDV genes in the pair formed by R2 and R2/23. Same letter above the bar indicates no statistically significant differences were detected (P < 0.05).
Effect of age/route of vaccination on MD vaccine DNA load in the lung (Experiment 2)
The effect of age/route of vaccination on serotype 1 MDV vaccine DNA load in lung after hatch was very remarkable ( and ). In ovo (i.o.) vaccination resulted in higher MDV DNA load in lung at 3 doa, lower or equal MDV DNA load at 5 doa, and lower MDV DNA load at 10 doa than s.c. administration of the vaccine (a,c,e). A similar trend was also observed in the frequency of chickens that were positive for vaccine virus DNA by real-time PCR. While the frequency of positive chickens was higher in chickens vaccinated i.o. versus those vaccinated at hatch (by s.c. route) at 3 doa, it was similar at 5 doa, and lower at 10 doa (). Note that since samples were collected based on the age of chickens, chickens vaccinated i.o. were 3 days ahead post vaccination compared with chickens vaccinated at hatch. The relative replication of serotype 1 MDV vaccines in the lung versus the spleen when administered i.o. tended to be higher than when administered s.c. Differences, however, were only statistically significant in chickens vaccinated with R2/23 at 5 doa (b, d, f). No statistically significant differences were found between vaccine DNA load in the lung and spleen in any of the treatment groups at any time point (f).
Figure 3. Effect of route of vaccination on the load of vaccine DNA in the lung when vaccines were administered in ovo at 18 days of embryonation (i.o.) and subcutaneously at day of age (s.c.). Load of serotype 1 MDV vaccines DNA was evaluated by real-time PCR. Results expressed as the relative Ct ratio of the amplification of the chicken GAPDH gene and the MDV gB (see Materials and Methods). Results presented as the mean and the standard error. 3a: Load of DNA of CVI988/BP5 in the lung when administered i.o. and s.c. at 3, 5, and 10 days of age (doa). 3b: Load of viral DNA in the lung relative to the load of viral DNA in the spleen (load of viral DNA in the lung – load of viral DNA in the spleen) when CVI988/BP5 was administered i.o. and s.c. 3c: Load of DNA of R2 in the lung when administered i.o. and s.c. at 3, 5, and 10 doa. 3d: Load of viral DNA in the lung relative to the load of viral DNA in the spleen when R2 was administered i.o. and s.c. 3e: Load of DNA of R2-23 in the lung when administered i.o. and s.c. at 3, 5, and 10 doa. 3f: Load of viral DNA in the lung relative to the load of viral DNA in the spleen when R2/23 was administered i.o. and s.c. Same letter above the bar indicates that no statistically significant differences were detected (P < 0.05). *Statistically significant differences between the load of vaccine DNA in the lung and in the spleen.
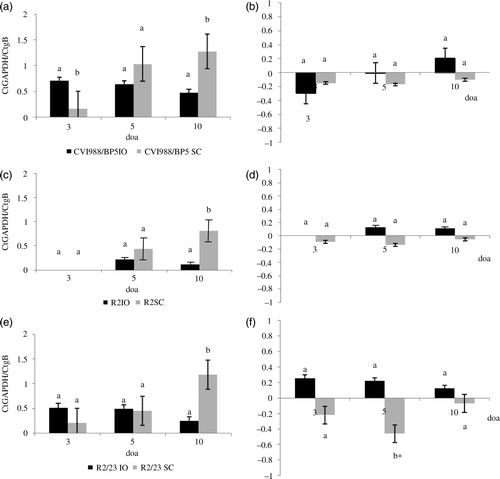
Table 3. Number of chickens positive for the vaccine DNA and which had transcription of MDV genes in the lung (Experiment 2).
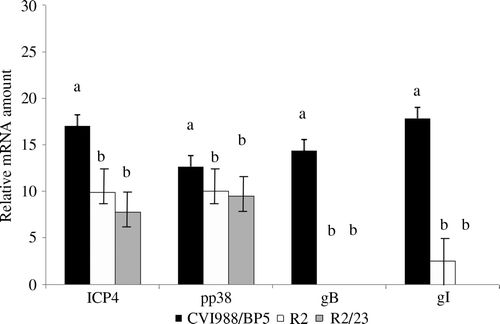
Transcription of immediate early gene ICP4, early gene pp38, and late genes gB and gI, when vaccines were administered i.o., was studied by real-time RT-PCR. Every chicken that had viral DNA in lungs also had transcripts of ICP4 and pp38 at 5 doa in the lung ( and ). However, only chickens inoculated with CVI988/BP5 had transcription of gB in the lung at 5 doa. Transcription of the gI gene was detected in lungs of chickens inoculated with CVI988/BP5 and with R2. However, the frequency of chickens with transcripts of gI in the lung greatly depends on the treatment, being 100% in the group inoculated with CVI988/BP5 and 33.3% in the group inoculated with R2. Transcription of all the evaluated genes was significantly higher in chickens vaccinated with CVI988/BP5 than in those chickens vaccinated with R2 or R2/23 when vaccine was administered i.o.
Figure 4. MDV gene transcripts in the lung of 5-day-old chickens after i.o. vaccination with R2, R2-23, and CVI988/BP5. Transcription of MDV genes ICP4, pp38, gB, and gI was measured by real-time RT-PCR (see Materials and Methods). Results presented as the mean and the standard error. Comparisons are done for each gene among the three vaccine strains. Same letter above the bar indicates that no statistically significant differences were detected (P < 0.05).
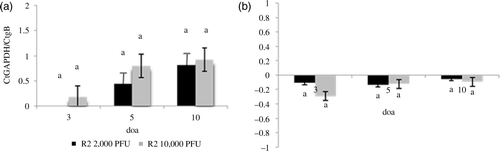
Effect of vaccine dose on MD vaccine DNA load in lung (Experiment 3)
There were no statistically significant differences in the MDV DNA load in the lung after vaccination with R2 at two different doses (10,000 PFU and 2000 PFU) (a). In addition, there were no significant differences in the relative vaccine virus DNA load in the lung versus the spleen when different doses were administered (b). No differences in the vaccine DNA load in the lung and spleen were detected at any time point in any of the treatment groups.
Figure 5. Effect of the dose of vaccine on the load of vaccine DNA in the lung. Load of serotype 1 MDV vaccines DNA was evaluated by real-time PCR. Results expressed as the relative Ct ratio of the amplification of the chicken GAPDH gene and the MDV gB (see Materials and Methods). 5a: Load of DNA of R2 in the lung at 3, 5, and 10 doa after administration of 2000 or 10,000 PFU of the vaccines by the s.c. route. 5b: Load of viral DNA in the lung relative to the load of viral DNA in the spleen (load of viral DNA in the lung – load of viral DNA in the spleen) after administration of 2000 or 10,000 PFU of vaccine R2 via s.c. route. Same letter above the bar indicates that no statistically significant differences were detected (P < 0.05). *Statistically significant differences between the load of vaccine DNA in the lung and in the spleen.
Discussion
In this work we have demonstrated that some serotype 1 MDV vaccines replicate in the lung as early as 3 to 5 doa. MDV vaccine replication was evaluated by vaccine DNA load in lung and by transcription of MDV ICP4, pp38, gB, and gI genes. Viral DNA load in lungs of chickens within the first 10 doa was affected by vaccine efficacy and by age/route of inoculation but not by vaccine dose. HP vaccines tended to have higher viral DNA loads in the lung than LP vaccines. The pair formed by R2 and R2/23 was the only exception, but this might be due to the lower protection that R2 conferred when compared with CVI988/BP5 or 648A80 (Witter & Kreager, Citation2004). Transcripts of immediate early gene ICP4 and early gene pp38 were found at 5 doa in lungs of chickens of every treatment group with the exception of those chickens vaccinated with Clone C. This was true regardless of the age/route of inoculation. However, transcripts of late gene gB were detected only in lungs of chickens vaccinated with the most protective vaccine CVI988/BP5. Transcripts of late gene gI were detected in lungs of chickens vaccinated with the three HP vaccines (CVI988/BP5, 648A80, 648A100), although the frequency of chickens with transcripts of gI varied greatly among groups.
MDV establishes various types of interaction with the host cells: cytolytic, latency, and transformation. During cytolytic infection, early and late antigen expression can be detected as seen in cell culture; lymphocytes during the early cytolytic infection; and feather follicle epithelium. However, abortive cytolytic infection has been described in lymphocytes infiltrating the feather pulp, brain, eye, and tumours (Cho et al., Citation1998; Gimeno et al., Citation2001; Pandiri et al., Citation2008). In those cases, transcripts and/or antigens of early genes ICP4 and pp38 can be detected but not those of the late gene gB. In this study, we demonstrated that infection in the lung with serotype 1 MDV vaccines can result in various types of cytolytic infection depending mainly on the vaccine strain. Every chicken vaccinated with strain CVI988/BP5 suffered a cytolytic infection in the lung where transcription of both early genes (ICP4 and pp38) and late genes (gB and gI) occurred. Few chickens inoculated with the other two HP vaccines used in this study (648A80 and R2) had transcripts of ICP4, pp38, and gI but lacked transcripts of gB. Most chickens vaccinated with the HP vaccines 648A80 and R2 and all chickens vaccinated with the LP vaccines 648A100 and R2/23 had transcripts of early genes (ICP4 and pp38) but not late genes, and therefore they were considered to suffer an abortive cytolytic infection. Finally, no transcripts of the evaluated genes were detected in lungs of chickens inoculated with Clone C and replication of this vaccine in the lung could not be confirmed. Previous studies have demonstrated that infection with oncogenic strains leads to cytolytic infection in the lung with expression of both early and late antigens (Butter et al., Citation2007). It is plausible that vaccines lose the ability to replicate cytolytically in the lung during attenuation since the least attenuated vaccines are able to complete a cytolytic infection. The relevance of the abortive cytolytic infection in lung is unknown at this point and the significance of having a complete versus an abortive cytolytic infection in the development of a pulmonary immune response warrants further studies.
Our results indicate that HP vaccines tend to replicate in the lung better than LP vaccines. We have recently demonstrated that there are also differences in the cytokine gene expression in the lungs between chickens vaccinated with HP vaccines and those vaccinated with LP vaccines (Gimeno et al., Citation2009). We have also found differences in the expression of major histocompatibility complex II in pulmonary endothelial cells and in the frequency of inflammatory infiltrates in the lung after inoculation with various serotype 1 MD vaccines (Cortes et al., Citation2009b). Our results suggest that MD vaccines elicit a pulmonary immune response that might greatly contribute to protection against MD. Pulmonary immune responses elicited by MD vaccines warrant further studies. Enhancing pulmonary immune response elicited by vaccination might lead to interference with infection or at least to decrease and delay of infection.
The age/route of vaccination greatly influenced MD replication in the lung of chickens within 10 days of age. The i.o. vaccination resulted in higher vaccine DNA load in the lung at 3 doa, a lower or equal load of vaccine DNA at 5 doa, and a lower load of vaccine virus DNA at 10 doa than at hatch/s.c. administration of the vaccine. A similar trend was also observed regarding the frequency of chickens that were positive for vaccine DNA in the lung by real-time PCR. The fact that chickens inoculated i.o. received the vaccine 3 days earlier than those vaccinated s.c. at hatch might have contributed to these differences. However, replication of serotype 1 MD vaccines in the embryonic lung after amniotic inoculation of 18-day-old embryos seems to be compromised (Sharma, Citation1987a; St Hill & Sharma, Citation2000; Zhang & Sharma, Citation2001). While we cannot be sure that in our experiment there was replication of vaccine virus in lungs of chickens during embryonic life, our results support the fact that lung is a target tissue of MDV vaccines and that i.o. inoculation results in higher vaccine DNA load in lung within the first few days of life. It is possible that i.o. vaccination elicits an earlier pulmonary immune response that aids in the control of MD. Differences in the dynamics of infection when vaccines are administered i.o. versus when they are administered s.c. could be used to our advantage to increase protection by combinations of both routes/ages (Gimeno et al., Citation2008b; Cortes et al., Citation2009a).
It remains unknown which types of cells support serotype 1 MDV vaccine infection in the lungs. From our results, it is not possible to evaluate whether the cells infected are part of the lung parenchyma or cells within the pulmonary lymphoid aggregates. It is very unlikely that the peripheral blood cells contribute significantly to the load of vaccine DNA detected in this study, as the load of vaccine DNA in blood within the first week of age is very low. Sharma (Citation1987b) reported that the target cell of herpesvirus of turkey infection in the lung was not lymphoid in nature as they were not adherent, and did not react with antisera against chicken thymocytes, bursal cell, or Ia antigen. Pulmonary infection with oncogenic serotype 1 MDV has been studied. Purchase (Citation1970) detected MDV antigens in epithelial cells lining the air capillaries of lungs 5 days after intra-abdominal inoculation with various oncogenic MDV strains. Addinger and Calnek (Citation1973) infected chickens via inhalation and demonstrated the presence of MDV antigens in lungs during the first week of infection using immunofluorescent technique. Recently, St Hill et al. (Citation2004) detected the genome of an oncogenic MDV in lungs of chickens 3, 5, 7, and 10 days post inoculation. The nature of cells supporting infection with oncogenic and attenuated serotype 1 MDV is still unknown, and further studies using double-staining techniques like immunofluorescence/imunohistochemistry or in situ hybridization will be needed.
Results of this work show that serotype 1 MDV vaccine replication in the lung within the first few days of life is influenced by various factors. In particular, efficacy of the vaccines (HP replicates better than LP vaccines) and age/route of inoculation (dynamics of infection is very different when vaccine is administered i.o. or when it is administered s.c.) seemed to play a major role. The relevance of lung in the pathogenesis of MD cannot be overemphasized as it is the natural route of infection. A better understanding of MD vaccine pathogenesis in lung and MD vaccine-induced pulmonary immune response might aid in developing better methods of control against MD.
Acknowledgement
The authors thank Dr Arun Pandiri for helpful discussion and review of this manuscript.
References
- Abdul-Careem , M.F. , Haq , K. , Shanmuganathan , S. , Read , L.R. , Schat , K.A. , Heidari , M. and Sharif , S. 2009 . Induction of innate host responses in the lungs of chickens following infection with a very virulent strain of Marek's disease virus . Virology , 393 : 250 – 257 .
- Addinger , H.K. and Calnek , B.W. 1973 . Pathogenesis of Marek's disease: early distribution of virus and viral antigens in infected chickens . Journal of National Cancer Institute , 50 : 1287 – 1298 .
- Beasley , J.N. , Patterson , L.T. and McWade , D.H. 1970 . Transmission of Marek's disease by poultry house dust and chicken dander . American Journal of Veterinary Research , 31 : 339 – 344 .
- Butter , C. , Staines , K. , Baaten , B. , Smith , L.G. and Davison , T.F. 2007 . Route of challenge is critical in determining the clinical outcome of infection with a very virulent oncogenic herpesvirus, Marek's disease virus . Avian Pathology , 36 : 93 – 99 .
- Calnek , B.W. , Alexander , A.M. and Kahn , D.E. 1970 . Feather follicle epithelium: a source of enveloped and infectious cell-free herpesvirus from Marek's disease . Avian Diseases , 14 : 219 – 233 .
- Cho , K.O. , Endoh , D. , Kimura , T. , Ochiai , K. and Itakura , C. 1998 . Significance of Marek's disease virus serotype 1-specific phosphorylated proteins in Marek's disease skin lesions . Avian Pathology , 26 : 707 – 720 .
- Churchill , A.E. , Payne , L.N. and Chubb , R.C. 1969 . Immunization against Marek's disease using a live attenuated virus . Nature , 221 : 744 – 747 .
- Cortes A.L. Witter R.L. Gimeno I.M. 2009a Characterization of the immune responses elicited by double vaccination against Marek's disease In 146th Annual Convention of the American Veterinary Medical Association Seattle WA USA
- Cortes R.A. Cortes A.L. Fletcher O.J. Gimeno I.M. 2009b Effect of route of vaccination with serotype 1 Marek's disease vaccines in the recruitment of lymphocytes and macrophages in the lung In 146th Annual Convention of the American Veterinary Medical Association Seattle WA USA
- de Boer , G.F. , Groenendal , J.E. , Boerrigter , H.M. , Kok , G.L. and Pol , J.M.A. 1986 . Protective efficacy of Marek's disease virus (MDV) CVI-988 CEF65 clone C against challenge infection with three very virulent MDV strains . Avian Diseases , 30 : 276 – 283 .
- Dudnikov , L.A. and Witter , R.L. 2001 . “ A comparison of autologous and heterologous vaccination against Marek's disease ” . In Proceedings of the 6th International Symposium on Marek's Disease , Edited by: Schat , K.A. , Morgan , R.W. , Parcells , M.S. and Spencer , J.L. 249 – 255 . Kennett Square , PA : American Association of Avian Pathologists .
- Gandon , S. , Mackinnon , M.J. , Nee , S. and Read , A.F. 2001 . Imperfect vaccines and the evolution of pathogen virulence . Nature , 414 : 751 – 756 .
- Gimeno , I.M. , Cortes , A.L. and Silva , R.F. 2008a . Load of challenge Marek's disease virus DNA in blood as a criterion for early diagnosis of Marek's disease tumors . Avian Diseases , 52 : 203 – 208 .
- Gimeno I.M. Cortes A.L. Witter R.L. 2008b Optimization of revaccination procedure to improve protection against Marek's disease In 145th Annual Convention of the American Veterinary Medical Association New Orleans LA USA
- Gimeno I.M. Cortes A.L. Witter R.L. 2009 Role of the pulmonary immune response on the efficacy of serotype 1 Marek's disease virus vaccines In 146th Annual Convention of the American Veterinary Medical Association Seattle WA USA
- Gimeno , I.M. , Witter , R.L. , Fadly , A.M. and Silva , R.F. 2005 . Novel criteria for the diagnosis of Marek's disease virus-induced lymphomas . Avian Pathology , 34 : 332 – 340 .
- Gimeno , I.M. , Witter , R.L. , Hunt , H.D. , Lee , L.F. , Reddy , S.M. and Neumann , U. 2001 . Marek's disease virus infection in the brain: virus replication, cellular infiltration and major histocompatibility complex antigen expression . Veterinary Pathology , 38 : 491 – 503 .
- Gimeno , I.M. , Witter , R.L. , Hunt , H.D. , Reddy , S.M. and Reed , W.M. 2004 . Biocharacteristics shared by highly protective vaccines against Marek's disease . Avian Pathology , 33 : 59 – 68 .
- Kaiser , P. , Underwood , G. and Davison , F. 2003 . Differential cytokine responses following Marek's disease virus infection of chickens differing in resistance to Marek's disease . Journal of Virology , 2003 : 762 – 768 .
- Kross , I. 1996 . “ Isolation of highly lytic serotype 1 Marek's disease viruses from recent field outbreaks in Europe ” . In Current Research on Marek's Disease , Edited by: Silva , R.F. , Cheng , H.H. , Coussens , P.M. , Lee , L.F. and Velicer , L.F. 113 – 118 . Kennett Square , PA : American Association of Avian Pathologists .
- Levy , A.M. , Burgess , S.C. , Davidson , I. , Underwood , G. , Leitner , G. and Heller , E.D. 2003 . Interferon-containing supernatants increase Marek's disease herpesvirus genomes and gene transcription levels, but not virion replication in vitro . Viral Immunology , 16 : 501 – 509 .
- Morrow , C.J. and Fehler , F. 2004 . “ Marek's disease: a worldwide problem ” . In Marek's Disease: an Evolving Problem , Edited by: Davison , F. and Nair , V. 49 – 61 . London : Elsevier .
- Pandiri , A.K. , Cortes , A.L. , Lee , L.F. and Gimeno , I.M. 2008 . Marek's disease virus infection in the eye: chronological study of the lesions, virus replication, and vaccine-induced protection . Avian Diseases , 52 : 572 – 580 .
- Purchase , H.G. 1970 . Virus-specific immunofluorescent and precipitin antigens and cell-free virus in the tissues of birds infected with Marek's disease . Cancer Research , 30 : 1898 – 1908 .
- Rispens , B.H. , Van Vloten , J. , Mastenbroek , N. , Maas , H.J.L. and Schat , K.A. 1972 . Control of Marek's disease in the Netherlands. I. Isolation of an avirulent Marek's disease virus (strain CVI 988) and its use in laboratory vaccination trials . Avian Diseases , 16 : 108 – 125 .
- Sharma , J.M. 1987a . Delayed replication of Marek's disease virus following in ovo inoculation during late stages of embryonal development . Avian Diseases , 31 : 570 – 576 .
- Sharma , J.M. 1987b . Embryo vaccination of chickens with turkey herpesvirus: characteristics of the target cell of early viral replication in embryonic lung . Avian Pathology , 16 : 567 – 579 .
- Sharma , J.M. and Burmester , B.R. 1982 . Resistance to Marek's disease at hatching in chickens vaccinated as embryos with the turkey herpesvirus . Avian Diseases , 26 : 134 – 149 .
- Sharma , J.M. , Lee , L.F. and Wakenell , P.S. 1984 . Comparative viral, immunologic and pathologic responses of chickens inoculated with herpesvirus of turkeys as embryos or at hatch . American Journal of Veterinary Research , 45 : 1619 – 1623 .
- St Hill , C.A. and Sharma , J.M. 2000 . Viral pathogenesis in chicken embryos and tumor induction in chickens after in ovo exposure to serotype 1 Marek's disease virus . Avian Diseases , 44 : 842 – 852 .
- St Hill , C.A. , Silva , R.F. and Sharma , J.M. 2004 . Detection and localization of avian alphaherpesviruses in embryonic tissues following in ovo exposure . Virus Research , 100 : 243 – 248 .
- Swaggerty , C.L. , Pevzner , I.Y. , Kaiser , P. and Kogut , M.H. 2008 . Profiling pro-inflammatory cytokine and chemokine mRNA expression levels as a novel method for selection of increased innate immune responsiveness . Veterinary Immunology and Immunopathology , 126 : 35 – 42 .
- Venugopal , K. , Bland , A.P. , Ross , L.J.N. and Payne , L.N. 1996 . “ Pathogenicity of an unusual highly virulent Marek's disease virus isolated in the United Kingdom ” . In Current Research on Marek's Disease , Edited by: Silva , R.F. , Cheng , H.H. , Coussens , P.M. , Lee , L.F. and Velicer , L.F. 119 – 124 . Kennett Square , PA : American Association of Avian Pathologists .
- Witter , R.L. 1987 . New serotype 2 and attenuated serotype 1 Marek's disease vaccine viruses: comparative efficacy . Avian Diseases , 31 : 752 – 765 .
- Witter , R.L. 1991 . Attenuated revertant serotype 1 Marek's disease viruses: safety and protective efficacy . Avian Diseases , 35 : 877 – 891 .
- Witter , R.L. 1997 . Increased virulence of Marek's disease virus field isolates . Avian Diseases , 41 : 149 – 163 .
- Witter , R.L. 2001 . “ Protective efficacy of Marek's disease vaccines ” . In Current Topics in Microbiology and Immunology , Edited by: Hirai , K. 58 – 90 . Berlin : Springer-Verlag .
- Witter , R.L. 2002 . Induction of strong protection by vaccination with partially attenuated serotype 1 Marek's disease viruses . Avian Diseases , 46 : 925 – 937 .
- Witter , R.L. and Kreager , K.S. 2004 . Serotype 1 viruses modified by backpassage or insertional mutagenesis: approaching the threshold of vaccine efficacy in Marek's disease . Avian Diseases , 48 : 768 – 782 .
- Witter , R.L. , Lee , L.F. and Fadly , A.M. 1995 . Characteristics of CVI988/Rispens and R2/23, two prototype vaccine strains of serotype 1 Marek's disease virus . Avian Diseases , 39 : 269 – 284 .
- Xu , H. , Yao , Y. , Zhao , Y. , Smith , L.P. , Baigent , S.J. and Nair , V. 2008 . Analysis of the expression profiles of Marek's disease virus-encoded microRNAs by real-time quantitative PCR . Journal of Virological Methods , 149 : 201 – 208 .
- Zhang , Y. and Sharma , J.M. 2001 . Early posthatch protection against Marek's disease in chickens vaccinated in ovo with a CVI988 serotype 1 vaccine . Avian Diseases , 45 : 639 – 645 .