Abstract
The recent epidemic caused by H5N1 highly pathogenic avian influenza (HPAI) viruses has spread over many parts of Asia, Europe and Africa. Wild birds, particularly waterfowl, are considered to play a role in viral dissemination. However, detailed information on whether wild terrestrial birds act as carriers is currently unavailable. To investigate the susceptibility of terrestrial birds to HPAI viruses, two species of wild bird (great reed warbler and pale thrush) that are common in East Asia were infected with H5N1 HPAI virus. The results showed that both species were highly susceptible to the virus. The great reed warbler showed fatal infection with 100% mortality, but the pale thrush survived for longer periods (>8 days) with viral shedding. These findings suggest that there is variation in clinical outcome after infection of wild terrestrial birds, and that some bird species could become subclinical excretors of the H5N1 virus.
Introduction
Avian influenza viruses are divided into subtypes based on virion surface proteins, haemagglutinin (H1 to H16) and neuraminidase (N1 to N9). All virus subtypes, other than strains found exclusively in shorebirds such as gulls (H13 and H16), have been isolated from waterfowl (Webster et al., Citation1992; Fouchier et al., Citation2005). Therefore, wild waterfowl are thought to be a natural reservoir that plays a role in the prevalence of viruses (Webster et al., Citation1992). If poultry such as chickens and turkeys are infected with avian influenza viruses, they exhibit various disease signs ranging from mild respiratory signs caused by low-pathogenic avian influenza viruses to high mortality induced by highly pathogenic avian influenza (HPAI) viruses. On the other hand, clinical signs are rarely seen in infected wild waterfowl, although the viruses replicate efficiently in their bodies (Webster et al., Citation1992); an exception was the recent fatal H5N1 viruses that caused systemic infection, neurological dysfunction and death in ducks (Sturm-Ramirez et al., Citation2004; Chen et al., Citation2006).
Since 2003, HPAI outbreaks in Southeast Asia have continued to smoulder and spread, and H5N1 virus has been seen in many parts of Asia, Europe, Africa and the Middle East. Some migratory waterfowl appear to carry and disseminate the highly pathogenic H5N1 strains currently circulating. Whether these birds can migrate long distances after being infected, however, remains controversial (World Organisation for Animal Health, Citation2009).
In the beginning of 2007, H5N1 HPAI outbreaks occurred at three chicken farms in the Miyazaki Prefecture and one chicken farm in the Okayama Prefecture, Japan. Genetically, the causative viruses were closely related to viruses isolated in Korea at the end of 2006 and their haemagglutinin genes were classified into clade 2.2, represented by viruses isolated from dead wild birds at Lake Qinghai, China in 2005 (Ministry of Agriculture, Forestry & Fisheries of Japan, Citation2007; Ito, Citation2008). In addition, the results of epidemiological investigations conducted in Japan suggested that H5N1 HPAI viruses had not been present in Japan until the 2007 outbreaks; therefore, the 2007 isolates were thought to have been newly introduced from elsewhere.
One possible explanation is that the H5N1 viruses might have been introduced through imported poultry, birds, eggs and meat products or illegally imported exotic birds. However, according to the report on epidemiological surveillance and quarantine measures in outbreak countries, direct evidence of connection between the outbreak farms and foreign countries—for example, farm-related persons who had a history of contact with the infected sources or travel to outbreak countries or import of feed and medicines from such countries—have not been reported (Ministry of Agriculture, Forestry & Fisheries of Japan, Citation2007). The other conceivable explanation is that the H5N1 viruses might have been carried by infected wild birds, as many species of migratory bird travel from Korea to Japan in winter from October to March. The reports of HPAI viruses being isolated from live wild birds support this hypothesis (Li et al., Citation2004; Kou et al., Citation2005; Yee et al., Citation2009).
Wild waterfowl obviously favour rivers, ponds and lakes; however, such sites did not exist around the farms in Japan affected by HPAI in 2007. Therefore, it is possible that terrestrial birds, rather than waterfowl, were involved in viral dissemination and introduction to the farms. However, there have been few reports on the susceptibility of terrestrial birds to H5N1 HPAI viruses. In this study, in order to obtain more information, we performed experimental infections of terrestrial birds that migrate among HPAI-prevalent countries with the recent H5N1 virus, and we discuss the possibility that they may act as viral carriers.
Materials and Methods
Virus
A HPAI virus, A/mountain hawk-eagle/Kumamoto/1/07 (MHE/Kumamoto/07; H5N1), was the first isolated from a wild terrestrial bird in Japan. Genetically, all Japanese isolates in 2007 including MHE/Kumamoto/07 were closely related and classified as clade 2.2 Qinghai Lake-related strains (Ministry of Agriculture, Forestry & Fisheries of Japan, Citation2007). MHE/Kumamoto/07 was confirmed to be of the highly pathogenic phenotype by experimental infection of chickens, in which 100% mortality within 24 to 45 h post inoculation was recorded. The stock virus used in this study was deposited at the Avian Zoonosis Research Centre, Faculty of Agriculture, Tottori University.
Birds
Pale thrushes (Turdus pallidus) and great reed warblers (Acrocephalus arundinaceus) used for experimental infection were captured using mist nets inside the Tottori Prefecture with permission from the Japanese Ministry of the Environment. Birds were quarantined for 3 to 5 days before the experiment.
Experimental infection
Ten pale thrushes and eight great reed warblers were inoculated intranasally with 50 µl containing 106.3 median embryo infective dose (EID50) and 30 µl containing 106.4 EID50 stock virus, respectively. Infected birds were observed twice a day for clinical signs and mortality for an 8-day period. At 5 days post inoculation (d.p.i.), two randomly selected pale thrushes were killed humanely in order to determine virus titres in each organ, as described below. Control groups consisted of seven pale thrushes and six great reed warblers, which were given phosphate buffered saline (PBS) intranasally and were observed for the same period as inoculated birds of each species. Control and infected birds were housed separately in negative-pressure isolator cages.
In order to determine the virus titre in organs, the brain, trachea, lung, heart, liver, colon and kidney were collected from two great reed warblers (selected randomly among the dead birds at 3 d.p.i.), two sacrificed pale thrushes without any clinical signs at 5 d.p.i. and one pale thrush that died at 8 d.p.i. Collected organs were homogenized into 10% w/v homogenates with PBS and were centrifuged (2200 x g, 5 min). One hundred microlitres of serial 10-fold dilutions of supernatants were inoculated into the allantoic cavities of three 10-day-old embryonated hens' eggs (Kida et al., Citation1980). Virus titres were calculated by the Reed and Muench (Citation1938) method and were expressed as the EID50 per gram of tissue.
When the inoculated great reed warblers died, tracheal and cloacal swabs were collected for virus recovery. In the case of pale thrushes, because it was difficult to take swabs from surviving birds without use of anaesthesia, collection was not conducted. Control birds were swabbed after being killed humanely. These experiments were performed in a biosafety level 3 facility at Tottori University Avian Zoonosis Research Centre, Japan.
Histopathological and immunohistological tests
Tissues including the brain, trachea, lung, heart, liver, colon and kidney were taken from one bird showing typical signs in each group (i.e. dead great reed warbler at 2 d.p.i. and sacrificed pale thrush at 8 d.p.i.). Tissues from control birds were also collected on the same days as inoculated birds of each species. Collected tissues were fixed in 10% v/v formalin in PBS and then dehydrated, embedded in paraffin and cut into 5-µm sections that were stained with standard haematoxylin and eosin. Immunohistological analysis for viral antigen detection was also performed as described previously (Shinya et al., Citation2004) using anti-A/Vietnam/1203/04 (H5N1) prepared in rabbits as a primary antibody.
Results
In order to investigate the susceptibility of terrestrial wild birds to H5N1 HPAI virus, two bird species (great reed warbler and pale thrush) were inoculated with A/mountain hawk-eagle/Kumamoto/1/07 (MHE/Kumamoto/07) virus. Within 3 d.p.i., all eight inoculated great reed warblers had died after exhibiting depression (). In contrast, only three of the eight pale thrushes died (one died suddenly at 2 d.p.i. and two died suddenly at 8 d.p.i.), while the others showed no clinical signs during the observation period. In the case of control birds, all great reed warblers and pale thrushes survived. These results indicate that pale thrushes infected with the H5N1 virus are capable of survival for a longer period than great reed warblers. These findings suggest that there is a significant difference in the mortality rate of H5N1 viral infection among wild terrestrial bird species.
Table 1. Mortality of wild terrestrial birds infected with H5N1 HPAI virus, A/mountain hawk-eagle/Kumamoto/1/07.
Virus titres in the brain, trachea, lung, heart, liver, colon and kidney of infected birds revealed that the virus was able to replicate efficiently in all of the tested organs from great reed warblers (). The virus titres at 3 d.p.i. in different organs ranged from 103.7 to 109.5 EID50/g. Virus titres in pale thrushes at 5 d.p.i. were below the detection limit (<101.5 EID50/g) in the brain and from 101.5 to 105.5 EID50/g in other organs. Virus titres in pale thrushes that died at 8 d.p.i. were only obtained from the brain and trachea (105.6 and 101.7 EID50/g, respectively).
Table 2. Virus titres in organs of wild terrestrial birds infected with H5N1 HPAI virus, A/mountain hawk-eagle/Kumamoto/1/07.
The results of virus recovery from swabs of great reed warblers showed that seven out of eight tracheal samples and four out of eight cloacal samples were positive for the virus. The virus recovery rate from tracheal swabs was higher than from cloacal swabs, thus suggesting this species may shed larger amounts of virus in the trachea than the cloaca. No virus was isolated from swabs of any control birds.
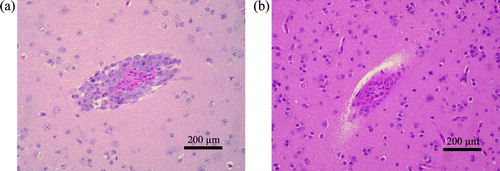
On histological examination of infected birds, noteworthy alterations were not seen in any tissues from the great reed warbler. In contrast, the brains of pale thrushes showed apparent perivascular inflammation of mononuclear cells and non-suppurative meningitis (a). In control birds, no histopathological changes were observed in any organs (b).
Figure 1. Histological examination of tissues from (1a) wild terrestrial birds experimentally infected with H5N1 HPAI virus, A/mountain hawk-eagle/Kumamoto/1/07 and (1b) control birds given PBS. 1a: Perivascular inflammation with mononuclear cells was observed in brain sections of infected pale thrush sacrificed at 8 d.p.i. Haematoxylin and eosin stain. 1b: No inflammation was observed in brain sections of control pale thrush sacrificed at 8 d.p.i. Haematoxylin and eosin stain.
Discussion
In the present study, we investigated the susceptibility of two species of wild terrestrial bird (great reed warbler and pale thrush) belonging to the order Passeriformes by performing experimental infections using a recently isolated H5N1 HPAI virus. The tested species are widely distributed in Asian countries where HPAI outbreaks have frequently occurred. Every spring, the great reed warbler migrates from Southeast Asia into Japan. On the other hand, in autumn, the pale thrush arrives from Northeast Asia through the Korean Peninsula. In addition, both species were seen around the farms affected in Japan by the HPAI in 2007. In order to examine the possibility that these terrestrial birds may become infected and excrete virus without showing clinical signs, and thus have the potential to spread the virus over significant distances, MHE/Kumamoto/07, which was isolated from wild birds and showed high pathogenicity in chickens, was used in this study. Our results showed that both species examined have high susceptibility to infection with the H5N1 virus and have the potential to spread the virus. However, this potential was greater for pale thrushes as these birds showed greater resistance and survived infection longer.
Boon et al. (Citation2007) demonstrated that the mortality rate of house sparrows was 66 to 100%, but no deaths were seen in starlings infected with H5N1 HPAI viruses, which were classified as clade 2.3.4 and isolated from dead wild terrestrial birds. In addition, Perkins & Swayne (Citation2003) reported that zebra finches and house finches inoculated with H5N1 HPAI virus, A/chicken/Hong Kong/220/97, showed high mortality and morbidity rates, while all of the inoculated sparrows and starlings survived, and only some of the sparrows experienced mild disease signs. In the present study, the great reed warbler showed high fatality rates within 3 d.p.i., while many of the inoculated pale thrushes survived for 8 days, despite showing high virus titres at 5 d.p.i. ( and ). These results indicate that there are different survival rates among terrestrial wild bird species in the order Passeriformes following H5N1 viral infection.
As shown by the virus recovery from swab samples, more virus appear to be excreted from the upper respiratory tract than from the intestinal tract of great reed warblers infected with the recent H5N1 virus. Similar findings were observed in waterfowl (Sturm-Ramirez et al., Citation2004; Brown et al., Citation2006). It is important for more efficient viral detection to collect specimen materials likely to contain larger amounts of virus. Therefore, virus isolation from both tracheal and cloacal swabs is necessary for surveillance in wild terrestrial birds.
In the present study, we demonstrated that two species of migratory wild terrestrial birds have high susceptibility to infection with clade 2.2 HPAI H5N1 virus and that these birds have the potential to spread this virus. It has been considered that the 2007 HPAI isolates entered Japan and were disseminated over large areas by infected wild birds. In addition, the habitats of terrestrial birds, such as those used in this study, are in close proximity to those of poultry and humans. Therefore, the possibility that these terrestrial birds can contribute to the spread of H5N1 HPAI viruses cannot be ignored, although direct evidence of viral dissemination is lacking (Ministry of Agriculture, Forestry & Fisheries of Japan, Citation2007; Ito, Citation2008). Hence, to establish an early warning system for HPAI outbreaks, it is vitally important to perform surveillance of both wild waterfowl and terrestrial birds.
Acknowledgements
The authors would like to thank Jyunya Nakamori for providing and keeping the birds used in this study.
References
- Boon , A.C. , Sandbulte , M.R. , Seiler , P. , Webby , R.J. , Songserm , T. , Guan , Y. and Webster , R.G. 2007 . Role of terrestrial wild birds in ecology of influenza A virus (H5N1) . Emerging Infectious Diseases , 13 : 1720 – 1724 .
- Brown , J.D. , Stallknecht , D.E. , Beck , J.R. , Suarez , D.L. and Swayne , D.E. 2006 . Susceptibility of North American ducks and gulls to H5N1 highly pathogenic avian influenza viruses . Emerging Infectious Diseases , 12 : 1663 – 1670 .
- Chen , H. , Li , Y. , Li , Z. , Shi , J. , Shinya , K. , Deng , G. , Qi , Q. , Tian , G. , Fan , S. , Zhao , H. , Sun , Y. and Kawaoka , Y. 2006 . Properties and dissemination of H5N1 viruses isolated during an influenza outbreak in migratory waterfowl in western China . Journal of Virology , 80 : 5976 – 5983 .
- Fouchier , R.A.M. , Munster , V. , Wallensten , A. , Bestebroer , T.M. , Herfst , S. , Smith , D. , Rimmelzwaan , G.F. , Olsen , B. and Osterhaus , A.D.M.E. 2005 . Characterization of a novel influenza A virus hemagglutinin subtype (H16) obtained from black-headed gulls . Journal of Virology , 79 : 2814 – 2822 .
- Ito , T. 2008 . Outbreaks of highly pathogenic avian influenza in Japan . Global Environmental Research , 12 : 15 – 20 .
- Kida , H. , Yanagawa , R. and &Matsuoka , Y. 1980 . Duck influenza lacking evidence of disease signs and immune response . Infection and Immunity , 30 : 547 – 553 .
- Kou , Z. , Lei , F.M. , Yu , J. , Fan , Z.J. , Yin , Z.H. , Jia , C.X. , Xiong , K.J. , Sun , Y.H. , Zhang , X.W. , Wu , X.M. , Gao , X.B. and Li , T.X. 2005 . New genotype of avian influenza H5N1 viruses isolated from tree sparrows in China . Journal of Virology , 79 : 15460 – 15466 .
- Li , K.S. , Guan , Y. , Wang , J. , Smith , G.J.D. , Xu , K.M. , Duan , L. , Rahardjo , A.P. , Puthavathana , P. , Buranathai , C. , Nguyen , T.D. , Estoepangestie , A.T.S. , Chaisingh , A. , Auewarakul , P. , Long , H.T. , Hanh , N.T.H. , Webby , R.J. , Poon , L.L.M. , Chen , H. , Shortridge , K.F. , Yuen , K.Y. , Webster , R.G. and Peiris , J.S.M. 2004 . Genesis of a highly pathogenic and potentially pandemic H5N1 influenza virus in eastern Asia . Nature , 430 : 209 – 213 .
- Ministry of Agriculture, Forestry & Fisheries of Japan 2007 Infection route of the 2007 outbreak of highly pathogenic avian influenza in Japan Available online at: http://www.maff.go.jp/j/syouan/douei/tori/pdf/report2007e.pdf (accessed 30 September 2009)
- Perkins , L.E. and Swayne , D.E. 2003 . Varied pathogenicity of a Hong Kong-origin H5N1 avian influenza virus in four passerine species and budgerigars . Veterinary Pathology , 40 : 14 – 24 .
- Reed , L.J. and Muench , H. 1938 . A simple method for estimating fifty percent endpoints . American Journal of Hygiene , 27 : 493 – 497 .
- Shinya , K. , Hamm , S. , Hatta , M. , Ito , H. , Ito , T. and Kawaoka , Y. 2004 . PB2 amino acid at position 627 affects replicative efficiency, but not cell tropism, of Hong Kong H5N1 influenza A viruses in mice . Virology , 320 : 258 – 266 .
- Sturm-Ramirez , K.M. , Ellis , T. , Bousfield , B. , Bissett , L. , Dyrting , K. Rehg , J.E. 2004 . Reemerging H5N1 influenza viruses in Hong Kong in 2002 are highly pathogenic to ducks . Journal of Virology , 78 : 4892 – 4901 .
- Webster , R.G. , Bean , W.J. , Gorman , O.T. , Chambers , T.M. and Kawaoka , Y. 1992 . Evolution and ecology of influenza A viruses . Microbiological Reviews , 56 : 152 – 179 .
- World Organisation for Animal Health 2009 Highly Pathogenic Avian Influenza Paris; World Organisation for Animal Health. Available online at: http://www.cfsph.iastate.edu/FactSheets/pdfs/highly_pathogenic_avian_influenza.pdf (accessed 29 June 2009)
- Yee , K.S. , Carpenter , T.E. and Cardona , C.J. 2009 . Epidemiology of H5N1 avian influenza. Comparative Immunology . Microbiology and Infectious Diseases , 32 : 325 – 340 .