Abstract
Opportunistic observations of and necropsies from selected commercial (meat) turkey flocks revealed skeletal lesions consistent with chondrodystrophy, characterized by leg and vertebral deformities, occurring at very low incidences in turkeys from two primary breeds and various multiplier breeder flocks. Mycoplasma organisms were cultured and identified as Mycoplasma iowae by immunofluorescence and polymerase chain reaction from some of the vertebral lesions but not from leg joints. This is the first detailed description of the gross and microscopic lesions of vertebral chondrodystrophy associated with M. iowae, which should now be considered in the differential diagnosis of turkeys with these lesions.
Introduction
Husbandry practices, metabolic and infectious diseases, and rapid growth may all play a role in the development of skeletal diseases in poultry (Morrow et al., Citation1997). Bone abnormalities occur in all ages of growing poultry with incidences ranging from 0.5 to 25% (Morrow et al., Citation1997). An estimated annual cost due to skeletal problems in the US turkey industry was $32 million to $40 million (Sullivan, Citation1994). Skeletal problems described in poultry include: dyschondroplasia, spondylolisthesis, chondrodystrophy, osteomyelitis and synovitis, Mycoplasma synoviae infection, and others (Sullivan, Citation1994; Crespo & Shivaprasad, Citation2008).
Mycoplasma iowae infection in turkey breeder hens causes late embryo mortality, reduced hatchability (2 to 5%), and leg abnormalities in progeny (Bradbury et al., Citation1988; Trampel & Goll, Citation1994; Bradbury & Kleven, Citation2008). In experimentally infected chickens and turkeys, M. iowae also induced airsacculitis, tenosynovitis and arthritis, rupture of digital flexor tendons, rotated tibia, and cartilage erosion (Trampel & Goll, Citation1994; Bradbury & Kleven, Citation2008). Following experimental infections of 1-day-old poults (Bradbury et al., Citation1988), M. iowae caused stunting, poor feathering, and leg abnormalities including chondrodystrophy. Infection in ovo caused severe generalized disease in hatched poults with high mortality, and the only two birds that survived into the third week developed chondrodystrophy (Bradbury et al., Citation1988). M. iowae was most widely disseminated in tissues following in ovo infection, and least disseminated after oral infection. Isolations became less frequent with age and no organisms were recovered in birds sampled at 12 weeks (Bradbury et al., Citation1988).
Trampel & Goll (Citation1994) described 17-day-old turkey poults with leg weakness, dehydration, chondrodystrophy of the hock joints, clear fluid in hock joint spaces, valgus deformities, shortening of the tarsometatarsal bones, and curled toes associated with M. iowae. M. iowae was recovered from hock joints of 1-day-old poults and older turkeys (up to 8 weeks of age) with leg deformities, and from intervertebral spaces of older turkeys with wry neck and back deformities (H. J. Barnes & D. H. Ley, personal communication reported in Al-Ankari & Bradbury, Citation1996). More recently, Dunn et al. (Citation2005) reported several cases of commercial turkeys from 3 days to 3 weeks of age with leg problems, uneven growth, increased culls, and histories suggesting that infection originated from certain multiplier breeder flocks. Leg lesions were suggestive of chondrodystrophy, and M. iowae was identified from joint swabs. The presence of vertebral lesions was not noted.
Control of M. iowae by eradication from some primary turkey breeder flocks has been accomplished (Al-Ankari & Bradbury, Citation1996; Bradbury & Kleven, Citation2008), but M. iowae is not currently included in the US National Poultry Improvement Plan.
Herein, we report the association of M. iowae with a range of skeletal lesions consistent with chondrodystrophy in commercial turkeys of two primary breeds from various multiplier breeder flocks. We describe in detail the gross and microscopic lesions, and include a discussion of the relevant terminology and description of lesions.
Materials and Methods
2006 turkey flocks
In 2006, a North Carolina commercial turkey company obtained breeding stock (eggs) of the same primary breed (Nicholas 700; Aviagen Turkeys Inc., East Lewisburg, West Virginia, USA) from two different multiplier breeder sources (A and B). Company A multiplier hens were 43 weeks of age, and company B multiplier hens were 48 weeks of age. Poults from each multiplier breeder company were separated according to sex at the hatchery and females were placed at production facilities in two locations. One production facility was a typical commercial (COM1) farm that received 8000 turkeys from each multiplier breeder source (16,000 in total). The other production facility was the Teaching Animal Unit (TAU) at the North Carolina State University, College of Veterinary Medicine (NCSU CVM), which received 1000 turkeys from each breeder source (2000 in total). Thus, each location/production facility had female poults from both breeder sources A and B.
At the COM1 farm, poults A and B were brooded in rings on different sides of the same house, separated (according to breeder flock source) by a heavy wire fence. This was a typical commercial turkey production house for the southeastern US, with curtain side-walls, cross-ventilation, end doors, and automatic watering and feeding systems. After they were released from the brooder rings, poults A and B remained separated in the house by the wire fence, which allowed them to be distinguished in the brooder house. The house contained 8000 A poults and 8000 B poults. At 6 weeks of age, poults from each breeder source (A and B) were moved to two separate grow-out houses, each containing approximately 8000 turkeys. These turkeys were to be processed as “heavy hens” with a target weight of 23 lbs (10.43 kg) at an approximate age of 18.5 weeks. The COM1 flock was examined at weeks 1, 2, 4, 6, 8, 10, and 12 of production.
The TAU production facility is a commercial-style house constructed on a smaller scale (40×140 sq ft; 12.19×42.67 m2) with curtain side-walls, cross-ventilation, end doors, automatic watering and feeding systems, and is used for both brooding and growing. This facility produces one flock of meat turkeys and one flock of broiler chickens each year. Poults from each breeder source intended for the TAU were distinguished by different trimming of the first toe at the hatchery. At 1 day post-hatch, poults from breeder sources A and B were transported to the TAU. On arrival, 1000 A and B poults (2000 in total) were commingled and placed in brooder rings for 2 weeks. Poults placed in the TAU were to be raised as “light hens” with a target weight of 15 lbs (6.8 kg) at approximate age of 13.5 weeks.
2008 turkey flocks
In 2008, Hybrid Converter turkeys (Hybrid; Hendrix Genetics, Kitchener, Ontario, Canada) from a single multiplier breeder source were grown at the same TAU facility but a different commercial turkey farm (COM2) from that described above. Otherwise, the production facilities and management were similar to those described above. Two thousand female poults were placed at the TAU and 7000 poults at COM2. Various production and sampling data were collected at the same intervals from TAU and COM2 flocks. Necropsies and sampling were done on all TAU and selected COM2 culls and mortalities.
Necropsy and histopathology
Among the reasons for culling were musculoskeletal disorders that prevented turkeys from accessing feed or water and/or if they were severely stunted. Turkeys were killed by cervical dislocation. Carcasses (culls and daily mortalities) from the commercial turkey farms were transported on ice to the NCSU CVM for necropsy and sample collection. TAU turkeys were observed two or more times each day throughout production. Each turkey that was found dead or culled was necropsied. TAU turkeys were individually identified by numbered wing bands and necropsies were performed, usually on the day of death, but occasionally after overnight refrigeration at 4°C. A record of each necropsy was kept and any samples taken were labelled to match the wing band number. Samples of tissues taken at necropsy were placed into 10% neutral buffered formalin. Fixed tissues were trimmed, and spinal columns decalcified, processed by standard paraffin embedding procedures, sectioned, and stained with haematoxylin and eosin (HE) for histopathologic examination.
At the TAU, daily observations were made and recorded from placement to processing. Turkeys were culled based primarily on stunting and lameness. Culled birds were killed by cervical dislocation, individually identified with a numbered wing band, and taken to the necropsy room for a complete external and internal examination and sample collection.
Routine bacteriology, mycoplasma culture and identification
At necropsy, enlarged hock joints and vertebral lesions were sampled after surface disinfection with 70% alcohol and incision of the affected area with a sterile blade. Joint fluid and exudate from the lesions were sampled using two sterile swabs, one of which was used immediately to inoculate blood and MacConkey's agar plates and the other to inoculate 2 ml Frey's mycoplasma broth with 15% swine serum (FMS) (Kleven, Citation2008). From each inoculated FMS broth, 1 ml was used for mycoplasma culture and 1 ml was removed and used for molecular identification of M. iowae by polymerase chain reaction (PCR) as described by Boyle et al. (Citation1995) and Lauerman (Citation1998), or Laigret et al. (Citation1996). Inoculated FMS broths were incubated aerobically at 37°C with periodic transfer of aliquots to FMS agar. Mycoplasmal colonies on agar were identified as M. iowae by direct immunofluorescence (Kleven, Citation2008) using fluorescein-conjugated rabbit antiserum (Leiting & Kleven, Citation2000) provided by S. H. Kleven (College of Veterinary Medicine, University of Georgia, Athens, Georgia, USA), and some isolates in FMS broth cultures were identified as M. iowae by PCR.
Results
2006 turkey flocks
Eight turkeys (four TAU and four COM1) were identified at necropsy with swollen hocks or vertebral lesions. Swollen hocks were noted at 12 and 15 days of age in three turkeys: two TAU breeder source A, and one COM1 breeder source B. M. iowae was not identified by culture or PCR of joint swabs from any of these three turkeys. However isolations of Escherichia coli plus Enterobacter spp., and of Staphylococcus spp. were made from two of these turkeys. Vertebral lesions were noted in five turkeys necropsied between 5 and 10 weeks of age: two TAU breeder source A, two COM1 breeder source A, and one COM1 breeder source B. M. iowae was identified by culture or PCR from spinal lesions of three turkeys (two TAU breeder source A, and one COM1 breeder source B). In two of three M. iowae-positive cases, the initial PCR tests from spinal lesion swabs were negative, although the organism was isolated in culture and identified by immunofluorescence and repeat PCR. In another case, initial PCR from the lesion was not done, but the organism was isolated in culture and the follow-up PCR was positive for M. iowae. In one case the initial M. iowae PCR was negative and the mycoplasma culture was contaminated, thus not permitting isolation or follow-up PCR. Therefore, the M. iowae status of this case is considered uncertain. In the final vertebral lesion case, M. iowae PCR and mycoplasma culture were both negative.
2008 turkey flocks
A variety of skeletal lesions occurred at a low estimated rate in the TAU (0.25 to 0.5%) and COM2 turkey flocks. The most common gross lesion was chondrodystrophy characterized by short, thick, deformed legs and enlarged joints. Other gross lesions consisted of vertebral malformations involving the free thoracic vertebra and adjacent ribs, with wry neck an uncommon finding. Splenomegaly was common, but arthritis and tenosynovitis (swollen joints, feet, or tendon sheaths) was not a finding in chondrodystrophic turkeys. The diagnosis of chondrodystrophy was based on gross lesions, not histopathology, as discussed below.
Chondrodystrophy was found in turkeys at 3 to 7 weeks of age, and accounted for 17.3% of the mortalities and culls in the TAU flock. Of 21 samples from chondrodystrophic leg joints (foot, hock, or stifle), none were positive for M. iowae by culture or PCR. M. iowae was detected in only five (from one neck and four back lesions) of 33 samples (15.2% positive rate) submitted from chondrodystrophic TAU and COM2 turkeys. Four of 11 samples from backs with vertebral lesions were positive for M. iowae, and five samples submitted from normal backs were negative. Several turkeys were noted with wry neck, but only one was sampled from the cervical spine and it was positive for M. iowae. Mycoplasmas were not detected in two samples from air sacs or two samples from spleens.
Pathology
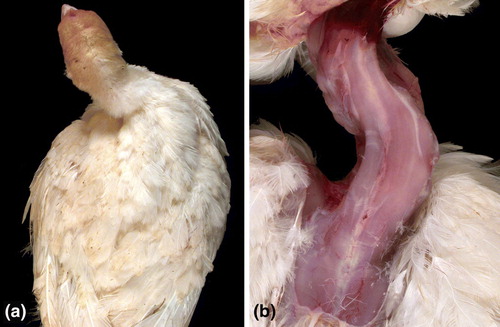
Clinical signs and gross lesions compatible with chondrodystrophy were characterized by lameness, stunting, and legs that were short, bowed and thickened, with enlarged hock joints (). Cut sections of affected tibiotarsi showed enlargement of the ends with thickening of the bone and growth plate, hypertrophy of cartilage, and curvature of the bone (). Typical examples of spinal lesions noted at necropsy were vertebral columns markedly shorter in length than normal () with severely deformed caudal thoracic vertebrae because of lesions in the caudal notarium and free thoracic vertebra, with resulting deformity of adjacent ribs (). Wry neck () was the least common skeletal change seen in the turkeys. The neck was permanently deviated laterally and actually rotated, not simply held to the side.
Figure 1. (1a) Normal legs of a 28-day-old turkey and (1b) legs of a 42-day-old turkey showing bilateral chondrodystrophy; although weights were comparable, the chondrodystrophic turkey has short, thick shanks and toes compared with the unaffected turkey. The affected legs also have enlarged hocks, and there is marked medial bowing of both, which has resulted in bilateral varus deformity. The unaffected turkey died of round heart disease while the chondrodystrophic turkey was a cull.
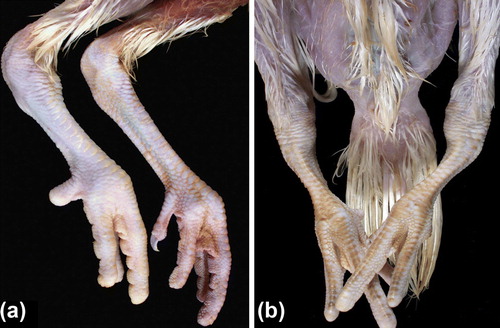
Figure 2. Proximal tibiotarsi of unaffected turkey (left) and chondrodystrophic turkey (right) from the birds in . Thickening of the bone and physis, and curvature of the tibiotarsus are evident in the bones from the affected bird.
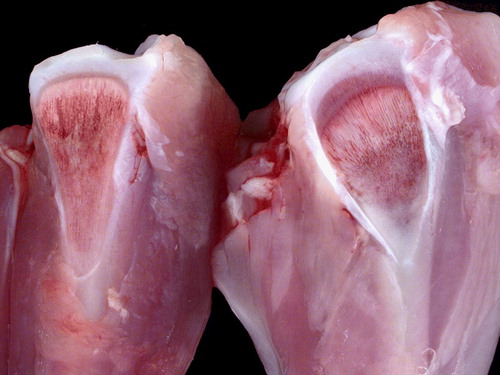
Figure 3. Normal and chondrodystrophic spines from 43-day-old turkeys. Spines are from two similarly sized birds. The upper chondrodystrophic spine is much shorter than that of the bird with the normal back; the vertebral column is distorted and thickened, and the ribs are unevenly spaced.
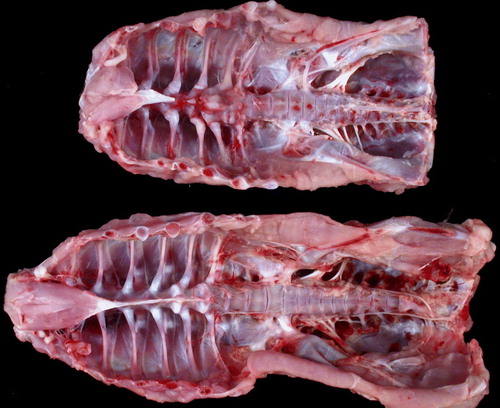
Figure 4. Spine of a 39-day-old female turkey that was culled due to stunting and lameness with vertebral chondrodystrophy. The caudal thoracic vertebrae are severely deformed because of lesions in the caudal notarium (N) and free thoracic vertebra (FTV). Mycoplasma iowae was isolated from the intervertebral space within the lesion.
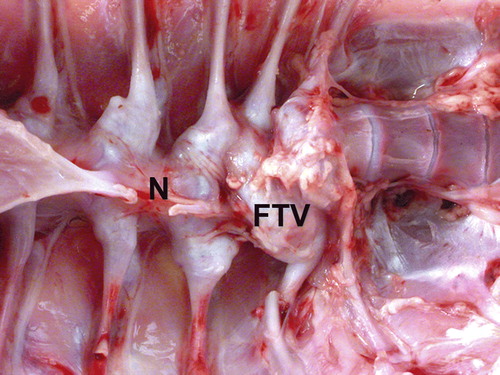
Figure 5. Wry neck in a 30-day-old turkey before (5a) and after (5b) removal of the skin. The neck is permanently deviated to the right and is actually rotated, not simply held to the side. Inflammation of cervical air sacs that spreads to the intervertebral joints is believed to cause the lesion. M. iowae was detected in a sample from the intervertebral space of this turkey.
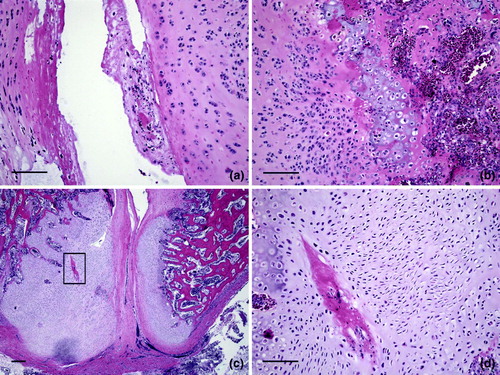
Representative microscopic lesions in the vertebral column are shown in to . shows a distorted free thoracic vertebra that compressed the spinal cord causing mild demyelination. Longitudinal bands of cartilage represented persisting lateral growth plates. Joint surfaces were irregular due to degeneration and necrosis. There was excess cartilage matrix and disorganization of chondrocytes, features of osteochondrosis, also shown at higher magnification in . Chondrocytes were haphazardly arranged in a matrix with irregular, acellular areas of degeneration. Larger areas of acellular matrix were in some regions of persisting cartilage. Degeneration of cartilage was characterized by accumulation of eosinophilic material with loss of chondrocytes, and there were focal areas of chondrocyte necrosis. Articular cartilage of the free thoracic vertebra was necrotic (). Cartilage persisted in the ventral portions of the vertebra adjacent to the articular surface, which showed degeneration and necrosis of cartilage with an irregular surface. Osteomyelitis was in the bone beneath the cartilage. a, b present a higher magnification of d, showing a tag of degenerated and necrotic cartilage in the expanded articular space of the thoracic vertebra. Necrotic cartilage was also on the left side of this space. Chondrocytes were disorganized and necrotic, and osteomyelitis was in bone beneath the articular surface of the vertebra. Higher magnification of a (c, d) showed the distorted articular surface and prominent persisting cartilage. An area of degeneration of cartilage was characterized by loss of cells and accumulation of eosinophilic material. Chondrocytes were variable in size and arranged in an irregular pattern.
Figure 6. Vertebral lesions. 6a: The free thoracic vertebra (arrow) is distorted and compressing the spinal cord. Longitudinal bands of cartilage (*) represent persisting longitudinal growth plates. Joint surfaces are irregular due to degeneration and necrosis. Bar = 20 mm. HE. 6b: A longitudinal band of cartilage (*) is prominent and the free thoracic vertebra (arrow) is distorted on the ventral surface. Compression of the spinal cord is mild, but some demyelination is present. Bar = 20 mm. HE. 6c: Excess cartilage matrix and disorganization of chondrocytes, features of osteochondrosis, are shown. This is a higher magnification of area * in 6b. Bar = 20 µm. HE. 6d: Higher magnification of 6c shows the disorganization of chondrocytes within an expanded matrix. Bar = 20 µm. HE.
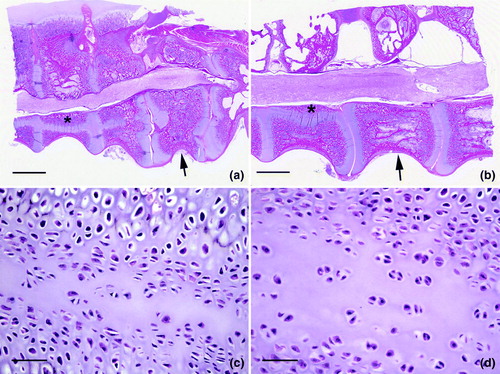
Figure 7. Cartilage lesions in osteochondrosis. This series of images are higher magnification of areas * in Figure b. 7a: Chondrocytes are haphazardly arranged in a matrix with irregular, acellular areas of degeneration. Bar = 50 µm. HE. 7b: Larger areas of acellular matrix are in some regions of persisting cartilage. Bar = 50 µm. HE. 7c: Degeneration of cartilage is characterized by accumulation of eosinophilic material with loss of chondrocytes. Bar = 50 µm. HE. 7d: A focal area of chondrocyte necrosis. Bar = 50 µm. HE.
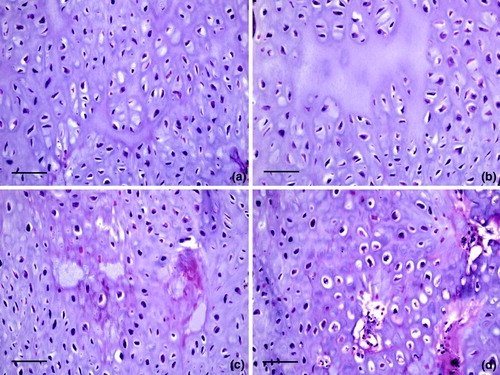
Figure 8. Lesions in vertebral articular cartilage. 8a: Articular cartilage of the free thoracic vertebra is necrotic (box). Cartilage persists (*) in the ventral portions of the vertebra adjacent to the articular surface. Note the compression of the spinal cord. Bar = 20 mm. HE. 8b: A higher magnification of 8a from the region at the bottom and just below the box. The articular surface is irregular and a large mass of cartilage persists in this ventral portion of the free thoracic vertebra. Bar = 20 µm. HE. 8c: A higher magnification of the region within the box in 8a shows degeneration and necrosis of cartilage with an irregular surface. Osteomyelitis is in the bone beneath the cartilage on the right. Bar = 20 µm. HE. 8d: A higher magnification of 8c shows irregularity of the articular surface and streaks in the articular cartilage indicative of degeneration and necrosis. Bar = 200 µm. HE.
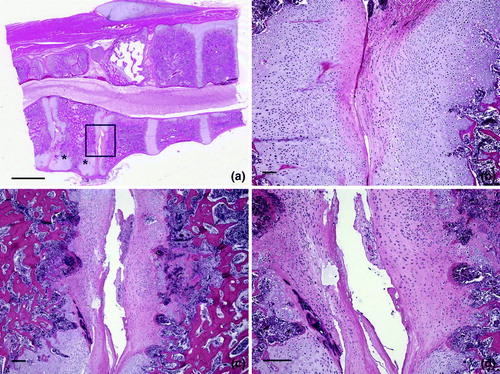
Figure 9. Osteochondrosis in vertebral cartilage. 9a: A higher magnification of d shows a tag of degenerated and necrotic cartilage in the expanded articular space of the thoracic vertebra. Necrotic cartilage also is on the left side of this space. Bar = 100 µm. HE. 9b: Chondrocytes are disorganized and necrotic. Osteomyelitis is in bone beneath the articular surface of the vertebra. Bar = 100 µm. HE. 9c: A higher magnification of a (* below the box) and shows the distorted articular surface and prominent persisting cartilage. The area within the box is shown at higher magnification in 8d. Bar = 200 µm. HE. 8d: An area of degeneration of cartilage is characterized by loss of cells and accumulation of eosinophilic material. Chondrocytes are variable in size and arranged in an irregular pattern. Bar = 100 µm. HE.
Discussion
The TAU at NCSU CVM includes an on-site poultry house that is used to provide our veterinary students and Poultry Health Management residents with production experience. For the 2006 and 2008 TAU turkey flocks, plans were in place to compare TAU flocks and commercial turkey flocks for breeder source effects and time-of-placement effects, respectively. However, in the course of observing these flocks for clinical signs and conducting necropsies on cull and dead turkeys, we identified an association between chondrodystrophy and M. iowae.
In 2006 we found M. iowae variably associated with low incidences of stunting, lameness, swollen hocks/arthritis, and vertebral lesions in turkeys of the same primary breed from two multiplier breeder companies in two production locations/systems. Possible sources of M. iowae infections could have originated with the primary breeder, or both multiplier breeder flocks could have been infected without regard to the primary breeder. Swollen hocks and arthritis were noted in a few turkeys between 12 and 15 days of age, with no evidence of M. iowae but with other bacteria present. This does not completely rule out the possibility that M. iowae was involved in the development of these leg lesions, but does show that other bacteria may be involved as primary or possibly secondary aetiologies. Dunn et al. (Citation2005) found that M. iowae was associated with swollen joints in turkeys from 3 days to 3 weeks of age, and that PCR was more sensitive than culture with pooled joint swabs. The evidence from experimental and natural infections with M. iowae suggests that the organism may be associated with lesions in low numbers and relatively early in the development of pathology, properties that add to the challenges of determining aetiology and making a definitive diagnosis.
In our study, vertebral lesions commonly described as chondrodystrophy were noted in turkeys between 39 and 65 days of age, and M. iowae was identified in samples of these lesions. The incidence of vertebral lesions was apparently very low and their occurrence could easily be missed without a careful examination of the spine. This was a small dataset, but the results suggested that mycoplasma culture of spinal lesions was a more sensitive diagnostic method than M. iowae PCR. We found instances where viable M. iowae was present in lesions but below the detection threshold of PCR on initial testing, but was detected by PCR following enrichment in culture. Recently we compared the sensitivities of M. iowae PCR using two different primer sets for conventional PCR (Boyle et al., Citation1995; Laigret et al., Citation1996) and real-time PCR (Raviv & Kleven, Citation2009), and found real-time PCR the most sensitive and superior to culture that relies on the presence of viable organisms (data not shown).
In 2008 similar clinical signs and pathology were found in turkeys from another primary breeder. Gross lesions were consistent with chondrodystrophy, and microscopic lesions consisted of osteochondrosis, with dyschondroplasia and osteomyelitis also present. Based on these findings, M. iowae should be considered in the differential diagnosis of turkeys with chondrodystrophy.
There is no universal acceptance of the terminology describing skeletal lesions. One problem is that terms are sometimes used to name a disease condition or to describe gross changes, while in other instances the terms are used to describe microscopic lesions (morphologic diagnosis). Words with the root “chondro” or “osteo” refer to cartilage or bone, respectively. When both cartilage and bone are involved, then the term should include both roots (e.g. osteochondrosis). The root “osis” indicates a degenerative change while “itis” indicates an inflammatory change in otherwise normal tissue. The roots “plasia” and “trophy” refer to growth, and “dys” to abnormal. This means that chondrodystrophy and dyschondroplasia are synonymous with respect to morphologic changes, both meaning some abnormality in the growth and development of cartilage. However, both also have disease connotations that are quite different from each other—chondrodystrophy is the basis for a type of dwarfism (more commonly called “achondroplasia” medically) that is characterized by normal appositional growth of bones coupled with impaired linear growth, which makes the bones short and thick. Affected individuals are short but more or less normal in width. A number of different types of animals are affected. Affected turkeys have short, thick legs, which is why the term “chondrodystrophy” has been used to describe them. Other causes include nutrient deficiencies (biotin, zinc, others) and genetics as in other species, although this may not have been thoroughly investigated.
Dyschondroplasia has been used to describe a specific disease characterized by the retention of cartilage in the growth plate of the proximal tibiotarsus. So, using the term “chondrodystrophy” for these turkeys is describing their clinical appearance and not any microscopic changes. For chondrodystrophy to be used to describe the microscopic changes there would need to be evidence that the cartilage was growing in an abnormal way. If the cartilage appears normal but shows degeneration, or if there is involvement of the underlying bone (subchondral bone), the microscopic lesion would be osteochondrosis. As a further complication, chondrodystrophy and osteochondrosis could occur together, because the chondrodystrophy could produce abnormalities that would predispose to osteochondrosis. The gross description may not match the morphologic description and still be correct because terms are being used for both disease or gross changes, and morphologic descriptions. Microscopically, the questions to be asked are “Is only cartilage involved, or only bone, or both?” and “Has the lesion resulted from abnormal growth, or was normal tissue subsequently affected with either degeneration or inflammation?”
Evidence from experimental infections, and recent clinical cases in commercial turkeys, suggests that M. iowae may be associated with the development of chondrodystrophy in all of its manifestations even though it may be difficult to demonstrate the organism at affected sites. However, the origin (primary and/or multiplier breeder flocks) and the precise role (primary, secondary, predisposing, synergistic, other) of M. iowae in the pathophysiology of the disease, and its prevalence, extent and economic importance, are presently undetermined. This information is necessary if M. iowae is to be considered for inclusion in monitoring and control programmes such as the US National Poultry Improvement Plan. Key epidemiologic and economic data, especially disease prevalence correlated with presence or absence of the organism, need to be developed using the most sensitive and specific tests available.
Acknowledgements
This work was supported, in part, by the NCSU College of Veterinary Medicine and the State of North Carolina. The authors thank Paula Jay, Sile Huyan and Judith McLaren for valuable technical assistance.
Additional information
Notes on contributors
Eduardo J. Vivas
Current address: Western Institute for Food Safety and Security, University of California, Davis, One Shields Avenue, Davis, CA 95616, USAReferences
- Al-Ankari , A.R. and Bradbury , J.M. 1996 . Mycoplasma iowae: a review . Avian Pathology , 25 : 205 – 229 .
- Boyle , J.S. , Good , R.T. and Morrow , C.J. 1995 . Detection of the turkey pathogens Mycoplasma meleagridis and M. iowae by amplification of genes coding for rRNA . Journal of Clinical Microbiology , 33 : 1335 – 1338 .
- Bradbury , J.M. , Ideris , A. and Oo , T.T. 1988 . Mycoplasma iowae infection in young turkeys . Avian Pathology , 17 : 149 – 171 .
- Bradbury , J.M. and Kleven , S.H. 2008 . “ Mycoplasma iowae infection ” . In Diseases of Poultry , 12th edn , Edited by: Saif , Y.M. , Fadly , A.M. , Glisson , J.R. , McDougald , L.R. , Nolan , L.K. and Swayne , D.E. 856 – 862 . Ames , IA : Blackwell Publishing .
- Crespo , R. and Shivaprasad , H.L. 2008 . “ Diseases of the skeleton ” . In Diseases of Poultry , 12th edn , Edited by: Saif , Y.M. , Fadly , A.M. , Glisson , J.R. , McDougald , L.R. , Nolan , L.K. and Swayne , D.E. 1154 – 1162 . Ames , IA : Blackwell Publishing .
- Dunn P. Wallner-Pendleton E. Love B. 2005 Case report: Mycoplasma iowae infection in commercial turkeys Paper presented at the 77th Northeastern Conference on Avian Diseases Ithaca NY June 15–17
- Kleven , S.H. 2008 . “ Mycoplasmosis ” . In A Laboratory Manual for the Isolation, Identification and Characterization of Avian Pathogens , 5th edn , Edited by: Dufour-Zavala , L. , Glisson , J.R. , Jackwood , M.W. , Pearson , J.E. , Reed , W.M. , Swayne , D.E. and Woolcock , P.R. 59 – 64 . Athens , GA : American Association of Avian Pathologists .
- Laigret , F. , Deaville , J. , Bove , J.M. and Bradbury , J.M. 1996 . Specific detection of Mycoplasma iowae using polymerase chain reaction . Molecular and Cellular Probes , 10 : 23 – 29 .
- Lauerman , L.H. 1998 . “ Mycoplasma PCR assays ” . In Nucleic Acid Amplification Assays for Diagnosis of Animal Diseases , Edited by: Lauerman , L.H. 41 – 42 . Turlock , CA : American Association of Veterinary Laboratory Diagnosticians .
- Leiting , V.A. and Kleven , S.H. 2000 . Preparation of a heterogeneous conjugate to detect Mycoplasma iowae by immunofluorescence . Avian Diseases , 44 : 697 – 700 .
- Morrow , C.J. , Bradbury , J.M. , Gentle , M.J. and Thorp , B.H. 1997 . The development of lameness and bone deformity in the broiler following experimental infection with Mycoplasma gallisepticum or Mycoplasma synoviae . Avian Pathology , 26 : 169 – 187 .
- Raviv , Z. and Kleven , S.H. 2009 . The development of diagnostic real-time TaqMan PCRs for the four pathogenic avian mycoplasmas . Avian Diseases , 53 : 103 – 107 .
- Sullivan , T.W. 1994 . Skeletal problems in poultry: estimated annual cost and descriptions . Poultry Science , 73 : 879 – 882 .
- Trampel , D.W. and Goll , F. Jr . 1994 . Outbreak of Mycoplasma iowae infection in commercial turkey poults . Avian Diseases , 38 : 905 – 909 .