Abstract
Experiments were first conducted to compare and evaluate different methods of Ascaridia galli larval recovery from the chicken intestine. The number of larvae recovered from the intestinal wall of chickens infected with 1000 embryonated A. galli eggs and killed 15 days post infection (p.i.) by three methods (ethylenediamine tetraacetic acid [EDTA], pepsin digestion and scraping) were compared. The EDTA and pepsin digestion were found to be the most efficient methods with no significant difference (P > 0.05) in the number of recovered larvae between the two. Subsequently, three different A. galli cohorts were established using the polymerase chain reaction-linked restriction fragment length polymorphism (PCR-RFLP) technique. A 533-bp long region of the cytochrome c oxidase subunit 1 gene of the mitochondrial DNA was targeted and 22 A. galli females were allocated to three different haplotypes. The four females with the highest embryonation rate from each haplotype group (total 12 females) were selected and used to inoculate each of 12 chickens with a dose of 1000 embryonated eggs. The chickens were killed 15 days p.i. and A. galli larvae were recovered from the small intestinal wall by the EDTA method and by sieving the lumen content on a 90 µm sieve. DNA of 40 larvae from each of the three different haplotypes was extracted using a worm lysis buffer, and PCR-RFLP analysis of these larvae revealed the same haplotype as that of their maternal parent. The identification of distinguishable cohorts may be a powerful tool in population studies of parasite turnover within the animal host.
Introduction
Ascaridia galli (Schrank, 1788) is a nematode parasite that infects domestic as well as wild birds (Soulsby, Citation1982). Studies have suggested that A. galli is the most common nematode in all types of production systems and has a worldwide distribution (Permin et al., Citation1997; Ashenafi & Eshetu, Citation2004; Martin-Pacho et al., Citation2005; Rabbi et al., Citation2006; Abdelqader et al., Citation2008). The adult stage inhabits the lumen of the small intestine of the host, feeding mostly on ingesta (Ackert et al., Citation1940; Soulsby, Citation1982). A. galli infections have been associated with reductions in egg production in laying hens and in overall growth in chickens (Soulsby, Citation1982; Ramadan & Abou Znada, Citation1991). A. galli may also play a role in transmission of Salmonella infections (Chadfield et al., Citation2001; Eigaard et al., Citation2006) and avian reoviruses (Calnek, 1997) resulting in disease and economic losses.
The lifecycle of A. galli is direct and the infective stage is eggs harbouring third-stage larvae. After ingestion, eggs reach the duodenum and hatch within 24 h (Ackert, Citation1923), and larvae are released into the lumen of the intestine. The larvae then enter the mucosa of the small intestine and most larvae initiate a histotrophic phase between days 8 and 17 post infection (p.i.) (Tugwell & Ackert, Citation1952; Herd & McNaught, Citation1975). Both the onset and length of this phase depend on infection dose (Herd & McNaught, Citation1975).
New regulations in the European Union, setting standards for the protection and welfare of laying hens (Anon., Citation1999), will be implemented from 1 January 2012 and prescribe substitution of traditional cage systems with floor systems and enriched cage systems (Willer & Yussefi, Citation2004). In floor and free-range systems, the risk of A. galli infection is known to be very high (Permin et al., Citation1997). In addition, the increased demand for more natural or ethically responsible products in recent years is making organic farming systems more common. As a consequence, the overall prevalence of A. galli is expected to increase in coming years, and alternative control strategies are warranted in order to avoid increasing dependence on repeated use of anthelmintics.
Understanding the host–parasite relationships is a necessary prerequisite to develop such control strategies. For this purpose, tools for recovery of the larval stages and identification of subpopulations will be very useful. The present study was aimed at developing appropriate research tools for understanding the population dynamics of A. galli. A standard larval recovery technique for postmortem examination and a method to establish genetically different A. galli cohorts were therefore developed and evaluated.
Materials and Methods
Preparation of egg batches
Thirty Hy-line breed chickens collected at an organic farm (no use of anthelmintics for 10 years) were killed by cervical disarticulation, were eviscerated and a total of 30 adult A. galli female worms were collected from 20 hosts. A. galli eggs were isolated from the uterus of these worms separately and incubated for 6 weeks in 0.1 N sulphuric acid (Permin et al., Citation1997). Twenty-two egg batches were selected based on the highest embryonation rates (above 50%).
Experiment 1: larval recovery
Infection of chickens
Twenty-eight 4-week-old Lohmann Silver chickens were obtained from a conventional indoor pullet-raising farm, supposedly uninfected premises, and randomly allocated to three groups of 10, nine and nine chickens and housed in separate sheds. After 2 days of acclimatization, seven chickens in each shed were orally infected with a dose of 1000 embryonated eggs and the rest kept as uninfected controls. The egg batch was made as a mixture of eggs originating from the 22 females mentioned above in equal proportions.
Processing the intestinal contents
Fifteen days p.i., 27 chickens including six controls were killed by cervical disarticulation and randomly allocated to three larval recovery methods (see below) after stratification for shed. The pepsin digestion method was applied for controls. The gastrointestinal tract was removed from the gizzard to the cloaca. The intestines were cut open longitudinally and washed gently. All of the luminal contents including the washings were collected on a 90 µm sieve and washed thoroughly with tap water. The retained material was transferred to a 50 ml tube and stored in 70% ethanol at 5°C. The intestinal wall was subsequently subjected to one of three different methods: ethylenediamine tetraacetic acid (EDTA), pepsin digestion, and scraping. Furthermore, after initial processing by EDTA or scraping, intestines were pepsin digested.
EDTA method
A 10 mM EDTA in 0.9% saline solution was prepared (Kringel et al., Citation2002). The small intestine was cut into four pieces longitudinally and hung in 10 mM EDTA in 0.9% NaCl for 6 h in a 500 ml conical glass. After removing the intestinal wall, the contents were allowed to settle for 30 min. The supernatant was discarded and the sediment was collected and stored in 70% ethanol at 5°C.
Pepsin digestion method
The digestion fluid of 1 litre was prepared freshly (Kapel & Gamble, Citation2000). The small intestine was cut into 2 to 3 cm pieces and digested in a beaker with 100 ml digestion fluid under moderate stirring (125 r.p.m.) at 38°C for 45 min. The undigested intestinal pieces were rinsed on a metal sieve of 2 to 3 mm size with water and digested material was transferred to a conical glass, filled with water and left for 20 min. The supernatant was poured off and again filled with water and allowed to sediment for 20 min. This procedure was repeated five or six times. The sediment was collected and stored in 70% ethanol at 5°C.
Scraping method
Using a glass slide, the mucosal and sub-mucosal layers were carefully scraped off the small intestine and collected on a 90 µm sieve and washed thoroughly with water. The retained material was collected and stored in 70% ethanol at 5°C.
Measurement of the length of A. galli larvae
With the help of tweezers, the length of each larva was measured manually using a measuring scale.
Comparison of quality of larvae DNA following recovery
DNA from two times 20 larvae recovered by the EDTA and pepsin digestion methods, respectively, was extracted as described below (see next section). Polymerase chain reaction (PCR) was conducted and bands were visualized after gel electrophoresis.
Experiment 2: cohort study
Extraction of DNA
DNA was extracted from the 22 selected adult worms using the MasterPure™ DNA purification kit (Epicentre Biotechnologies) according to the manufacturer's protocol. DNA from single larvae was extracted using a worm lysis buffer (Maafi et al. Citation2003), carried out according to Nejsum et al. (Citation2008).
Selection of the target region (cox1 gene) in mitochondrial DNA
Sequence information of the cytochrome c oxidase subunit 1 (cox1) gene of the mitochondrial DNA was obtained from Ascaris suum, Caenorhabditis elegans and Necator americanus using BLAST (http://www.ncbi.nlm.nih.gov/blast/Blast.cgi). Conserved regions were subsequently identified by aligning the sequences using TCoffee (http://igs-server.cnrsmrs.fr/Tcoffee/tcoffee_cgi/index.cgi) and were used as target regions for primers. The forward (GCox18F, 5′-TTGTGGTCTGGTATGGTTGG) and reverse (GCox18R, 5′-TGATGAGCTCAAACAACACAAC) primers were designed using Primer3 (http://frodo.wi.mit.edu/) and flanked a region of ~800 bp.
PCR and sequencing
The PCR was performed in a TECHNE® TC-512 thermal cycler. The thermal profile for the PCR reaction was 15 min at 95°C for initial activation of Taq polymerase, followed by 35 cycles consisting of 30 sec at 95°C (denaturation), 40 sec at 55°C (annealing), 1 min at 72°C (extension) and a final elongation of 10 min at 72°C. Negative H2O controls were included in all runs. The amplified fragment was purified by Qiaquick PCR-purification kit (Qiagen, Germany) and sequenced in both directions using a BigDye terminator sequencing system according to the manufacturer's instructions (Applied Biosystems, USA). After ethanol precipitation, the sequencing products were run on an ABI3130XL (Applied Biosystems).
Design of new primers
The sequence information was used to design new primers (GCox1F4F, 5′-ATTATTACTGCTCATGCTATTTTGATG; and GCox14R, 5′-CAAAACAAATGTTGATAAATCAAA GG) in conserved regions of the A. galli sequence giving an amplicon with a length of 533 bp.
Selection of restriction enzymes
After obtaining the sequence information, single nucleotide polymorphisms were identified manually and NEB cutter V2.0 (http://tools.neb.com/NEBcutter2/index.php) was used to identify two restriction enzymes (NlaIII and SfcI) that could allocate the 22 samples into three haplotypic groups and also had the property of sharing similar buffers and reaction conditions. The PCR products of all 22 A. galli samples were digested overnight at 37°C using 3 units of both NlaIII and SfcI (New England Biolabs, USA) and the digested products were subjected to gel electrophoresis on a 1.5% agarose gel (100 V, 90 min).
Infection of chickens
Twenty-five 9-week-old chickens of Lohmann Sandy hybrid were obtained from another farm than in the former experiment. Ten chickens were immediately killed and all were found to be A. galli-negative. The remaining 15 chickens were randomly allocated into three groups of five birds and housed in three different sheds. Four chickens in each group were inoculated with 1000 embryonated A. galli eggs of the three different haplotypes and one chicken in each shed was kept as uninfected control. Fifteen days p.i., all chickens were killed and larvae recovered from the intestines by the EDTA method.
PCR-linked restriction fragment length polymorphism on larvae
The DNA of both the selected adult worms (positive controls) and larvae was subjected to PCR-linked restriction fragment length polymorphism (RFLP) as described above.
Data analysis
The recoveries of larvae from the intestinal lumen and wall by different methods were compared by a generalized linear model (Proc Genmod, SAS v 9.1) by specifying a negative binomial distribution of larval counts. The difference in the length of the larvae recovered by different methods from the intestinal wall was analysed in a mixed-effect variance model with the method as the fixed effect and the chicken as a random effect. The difference in the length of larvae between intestinal content and intestinal wall was analysed in a mixed-effect variance model with location (lumen or wall) as the fixed effect and the chicken as the random effect.
One-way analysis of variance was used to compare the recovery of larvae in the intestinal lumen, wall and total, respectively, of three different haplotypes in the cohort experiment.
Animal ethics
The study was carried out in accordance with the requirements of The Danish Animal Ethics Committee (permit number 2005/561-1060).
Results
Experiment 1: larval recovery
The percentage of total larvae recovered by different methods ranged from 0.83 to 2.56% of the infection dose (). The recoveries from the intestinal wall were overall significantly different (P = 0.018), and subsequent pair-wise analysis showed significant higher recovery by the EDTA method compared with scraping (P=0.006) and higher recovery by pepsin digestion than scraping (P=0.003). Pepsin digestion of the intestines after EDTA incubation yielded no more larvae, while digestion after scraping yielded a single larva.
Table 1. Number of A. galli larvae recovered from the intestinal wall and lumen contents of chickens experimentally infected with 1000 embryonated eggs and euthanized 15 days p.i.
The recovered larvae had an overall mean length of 3.1 mm. There was no statistical difference in the mean length of larvae recovered from the intestinal wall by either the EDTA method or the pepsin digestion method (P = 0.66). Similarly there was no significant difference in the mean length of larvae recovered from the lumen and those recovered from the intestinal wall (P=0.90).
Three chickens harboured nine adult worms indicating previous exposure to A. galli.
For all larvae (n=40) recovered by EDTA and pepsin digestion, it was possible to amplify the cox1 gene by PCR.
Experiment 2: cohort study
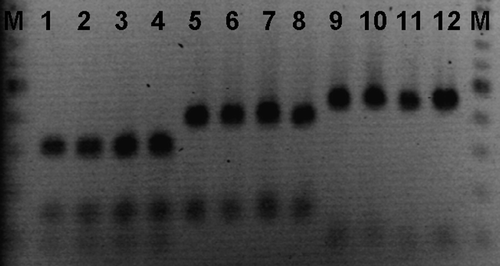
In the three groups infected with eggs of haplotypes I, II and III, respectively, a mean percentage of 2.2%, 2.6% and 1.2% of the infection dose with a mean number of 21.5 (individual numbers 1, 28, 43 and 14) larvae, 25.7 (individual numbers 3, 7, 85 and 8) larvae and 12.0 (individual numbers 8, 4, 21 and 15) larvae were recovered. There was no significant difference in larval recovery between different haplotype groups. Forty larvae randomly selected from all four chickens of each of three maternal-haplotype groups (total 120) recovered from the chickens were PCR-RFLP typed and all were found to hold the same band pattern as their female of origin. The band pattern of some of the larvae is shown in . The nucleotide sequences for the three haplotypes have been submitted to the GenBank under the accession numbers GU138668 to GU138670.
Figure 1. PCR-RFLP on mitochondrial DNA. Lane M, 100 bp size marker; lanes 1 and 2 to 4, band patterns of adult female and larvae of haplotype I; lanes 5 and 6 to 8, band patterns of adult female and larvae of haplotype II; lanes 9 and 10 to 12, band patterns of adult female and larvae of haplotype III, respectively.
Discussion
Previous studies reported that the histotrophic phase is a normal part of the A. galli lifecycle (Herd & McNaught, Citation1975) and that most histotrophic larvae are in the mucosal layer and only rarely penetrate deeper into the intestinal wall (Ackert, Citation1923). A commonly used method for isolation of histotrophic larvae has been pepsin digestion; for example, as used by Herd & McNaught (Citation1975), who incubated the intestinal wall for 4 to 12 h at 37°C. However, Irvine & Dallas (Citation2002) reported that digestion for a prolonged period (more than 2 h) may be associated with reduced PCR efficiency. We therefore compared a pepsin digestion technique developed for the release of Trichinella spp. larvae (Kapel & Gamble Citation2000), but with a reduced digestion period (45 min), with the EDTA method that has been developed for the recovery of small immature Trichuris suis larvae from the large intestine of pigs (Kringel et al., Citation2002). These methods were compared with manual scraping. Our study showed no difference in larval recovery by the EDTA and pepsin digestion methods and that both were superior compared with scraping. Following scraping it was very difficult to find the larvae embedded in the clumps of mucosa. EDTA presumably loosens the connections between mucosal cells whereby embedded larvae are released, whereas digestion with acid pepsin dissolves the mucosa and releases the larvae. The fact that A. galli larvae rarely penetrate deep in to intestinal tissue (Ackert, Citation1923) might be the reason for not finding any additional larvae when the intestines were pepsin digested after EDTA incubation. Based on this, and the fact that these samples were the easiest samples to read, the EDTA method is recommended for quantitative recovery.
We found no difference with respect to length either between the lumen and the wall or between the EDTA and the pepsin digestion methods. It might have been suspected that a given larval recovery method would be better to recover larvae of a certain size, but we did not find any indication for such a selection in our study although the larvae had a relatively large variation in size.
Applying PCR-RFLP on the mitochondrial DNA, it was possible to establish three different types of cohorts. We haplotyped 120 larvae and all showed a similar band pattern on the gel to that of their maternal parent. This supports the underlying hypothesis that mitochondrial DNA is maternally inherited in A. galli, as is the case for A. suum (Nejsum et al., Citation2008), for example, and mammals in general (Hutchison et al., Citation1974).
The possibility to identify different cohorts makes it possible to follow different groups of A. galli, their location in the host and their relative establishment rates. Dobson et al. (Citation1990) made a cohort study using levamisole resistant and susceptible strains of Trichostrongylus colubriformis, whereas Roepstorff et al. (Citation1996) used pyrantel resistant and susceptible strains of Oesophagostomum dentatum in their study. But this approach to cohort studies results only in two different cohorts and depends on availability of solidly resistant strains. The latter is not the case for a range of nematodes. In contrast, Sørensen et al. (Citation1999) used the PCR-RFLP technique to distinguish between two isolates of Schistosoma japonicum whereas Nejsum et al. (Citation2008) used the present PCR-RFLP method on the mitochondrial DNA and were able to establish four different cohorts of A. suum. The utilization of the natural genetic variation in the mitochondrial DNA to identify different cohorts of parasites therefore not only makes it possible to identify several different cohorts, but also provides an ability to work with naturally occurring worms in contrast to isolates or specific strains. However, similar establishment rates need to be confirmed before embarking on comparisons.
The development of reliable quantitative recovery methods in combination with genetically marked cohorts provides powerful tools for the study of population dynamics of nematodes in poultry and other livestock.
Acknowledgements
The Danish Centre for Experimental Parasitology is acknowledged for this project. Thanks to Minna Jakobsen for technical assistance on DNA sequencing and Lise-Lotte Christiansen for assistance with the larval recovery experiment.
References
- Abdelqader , A. , Gauly , M. , Wollny , B.A. and Abo-Shehada , M.N. 2008 . Prevalence and burden of gastrointestinal helminthes among local chickens, in northern Jordan . Preventive Veterinary Medicine , 85 : 17 – 22 .
- Ackert , J.E. 1923 . On the habitat of Ascaridia perspicillum (Rud) . Anatomical Records , 26 : 101 – 104 .
- Ackert , J.E. , Whitlock , J.H. and Freeman , A.E. Jr . 1940 . The food of the fowl nematode, Ascaridia lineata (Schneider) . The Journal of Parasitology , 1 : 17 – 32 .
- Anonymous 1999 Council Regulation (EC) No 1804/1999 of 19 July 1999, European Commission, Brussels
- Ashenafi , H. and Eshetu , Y. 2004 . Study on gastrointestinal helminths of local chickens in central Ethiopia . Revue de Médecine Vétérinaire , 10 : 504 – 507 .
- Chadfield , M. , Permin , A. , Nansen , P. and Bisgaard , M. 2001 . Investigation of the parasitic nematode Ascaridia galli (Shrank 1788) as a potential vector for Salmonella enterica dissemination in poultry . Parasitology Research , 87 : 317 – 325 .
- Dobson , R.J. , Waller , P.J. and Donald , A.D. 1990 . Population-dynamics of Trichostrongylus colubriformis in sheep—the effect of infection rate on the establishment of infective larvae and parasite fecundity . International Journal for Parasitology , 20 : 347 – 352 .
- Eigaard , N.M. , Schou , T.W. , Permin , A. , Christensen , J.P. , Ekstrom , C.T. Ambrosini , F. 2006 . Infection and excretion of Salmonella enteritidis in two different chicken lines with concurrent Ascaridia galli infection . Avian Pathology , 35 : 487 – 493 .
- Herd , R.P. and McNaught , D.J. 1975 . Arrested development and the histotropic phase of Ascaridia galli in the chicken . International Journal for Parasitology , 5 : 401 – 406 .
- Hutchison , C.A. 3rd , Newbold , J.E. , Potter , S.S. and Edgell , M.H. 1974 . Maternal inheritance of mammalian mitochondrial DNA . Nature , 251 : 536 – 538 .
- Irvine , R.J. and Dallas , J.F. 2002 . Efficient polymerase chain reaction detection of the second internal transcribed spacer of mucosa-derived larvae is dependent on the larval extraction method . The Journal of Parasitology , 88 : 807 – 809 .
- Kapel , C.M.O. and Gamble , H.R. 2000 . Infectivity, persistence, and antibody response to domestic and sylvatic Trichinella spp. in experimentally infected pigs . International Journal for Parasitology , 30 : 215 – 221 .
- Kringel , H. , Roepstorff , A. and Murrell , K.D. 2002 . A method for the recovery of immature Trichuris suis from pig intestine . Acta Veterinaria Scandinavica , 43 : 185 – 189 .
- Maafi , Z.T. , Subbotin , S.A. and Moens , M. 2003 . Molecular identification of cyst-forming nematodes (Heteroderidae) from Iran and a phylogeny based on ITS-rDNA sequences . Nematology , 5 : 99 – 111 .
- Martín-Pacho , J.R. , Montoya , M.N. , Arangüena , T. , Toro , C. , Morchón , R. , Atxutegi , C.M. and Simon , F. 2005 . A coprological and serological survey for the prevalence of Ascaridia spp. in laying hens . Journal of Veterinary Medicine , 52 : 238 – 242 .
- Nejsum , P , Thamsborg , T.M. , Jørgensen , C. , Fredholm , M. and Roepstorff , A. 2008 . A novel technique for identification of Ascaris suum cohorts in pigs . Veterinary Parasitology , 154 : 171 – 174 .
- Permin , A. , Pearman , M. , Wansen , P. , Bisgaard , M.F. and Frandsen , F. 1997 . On investigation in different media for embryonation of Ascaridia galli eggs . Helminthologia , 34 : 75 – 79 .
- Rabbi , A.K.M.A. , Islam , A. , Majumder , S. , Anisuzzaman , A. and Rahman , M.H. 2006 . Gastrointestinal helminths infection in different types of poultry . Bangladesh Journal of Veterinary Medicine , 4 : 13 – 18 .
- Ramadan , H.H. and Abou Znada , N.Y. 1991 . Some pathological and biochemical studies on experimental ascaridiasis in chickens . Nahrung , 35 : 71 – 84 .
- Roepstorff , A. , Bjørn , H. , Nansen , P. , Barnes , E.H. and Christensen , C.M. 1996 . Experimental Oesophagostomum dentatum infections in the pig: worm populations resulting from trickle infections with three dose levels of larvae . International Journal for Parasitology , 26 : 399 – 408 .
- Ruff , M.D. and Norton , R.A. 1997 . “ Nematodes and Acanthocephalans ” . In Diseases of Poultry , Edited by: Calnek , B.W. 815 – 849 . Ames : Iowa State University Press .
- Sørensen , E. , Johansen , M.V. , Wilson , S. and Bøgh , H.O. 1999 . Elucidation of Schistosoma japonicum population dynamics in pigs using PCR-based identification of individuals representing distinct cohorts . International Journal for Parasitology , 29 : 1907 – 1915 .
- Soulsby E.J.L. 1982 Helminths, Arthropods and Protozoa of Domesticated Animals , 7th edn Balliére Tindall East Sussex London, UK
- Tugwell , R.L. and Ackert , J.E. 1952 . On the tissue phase of the life cycle of the fowl nematode Ascaridia galli (Schrank) . The Journal of Parasitology , 4 : 277 – 288 .
- Willer , H. and Yussefi , M. 2004 . The World of Organic Agriculture: Statistics and Emerging Trends , Bonn : International Federation of Organic Agriculture Movements & Frick: Research Institute of Organic Agriculture FiBL .