Abstract
Recently, the causal relationship between eggshell apex abnormalities (EAA) and Mycoplasma synoviae was described. This eggshell pathology has only been documented in table egg layers both spontaneously and experimentally infected with M. synoviae, suggesting that meat-type layers are less prone to this condition. In this study the susceptibility of specified pathogen free (SPF) broiler breeder hens to produce eggs with EAA after M. synoviae infection was assessed. Five groups of 12 hens each were made: a negative control group, a group inoculated intratracheally (i.t.) with a M. synoviae EAA strain at 19 weeks of age, a group inoculated i.t. with this strain at 19 and 26 weeks of age, a group inoculated with M. synoviae i.t. at 19 weeks of age and infected 5 days earlier with infectious bronchitis virus D1466 (IBV), and a fifth group similar to the former but inoculated i.t. twice with an M. synoviae EAA strain at 19 and 26 weeks of age. Eggs with EAA were only produced after a single i.t. inoculation with the M. synoviae EAA strain if preceded by an infection with IBV. The production of eggs with EAA started 6 weeks after M. synoviae EAA inoculation and the proportion of eggs with EAA during the experiment was 9/449 (2%), which was much lower than that in SPF layer hens (14–22%). The present results suggest that broiler breeder hens are less susceptible to producing eggs with EAA after an infection with a M. synoviae EAA strain preceded by an IBV infection, compared with table egg layers. Similar to SPF egg layers, the mean daily egg production per hen was significantly reduced by the M. synoviae EAA strain and there was a general negative effect on eggshell strength by this strain, suggesting it could also have a detrimental effect on hatching egg quality.
Introduction
Mycoplasma synoviae in turkeys and chickens has been associated mainly with joint disease as a cause of synovitis (Olson et al., Citation1956; Kleven et al., Citation1975; Morrow et al., Citation1990; Landman & Feberwee, Citation2001, Citation2004) but also with respiratory disease, although respiratory infections are largely regarded as subclinical in nature (Stipkovits & Kempf, Citation1996; Kleven, Citation2003). Recently, the pathogenic spectrum of M. synoviae increased further after the causal relationship between this mycoplasma species and eggshell apex abnormalities (EAA) was demonstrated (Feberwee et al., Citation2009a).
Eggs with EAA were increasingly found in table egg-producing layer flocks. The EAA observed were characterized by altered shell surface (roughed aspect), shell thinning, increased translucency and the occurrence of cracks and breaks.
Egg production losses including the loss of eggs due to breakage of soft-shell eggs, increased number of downgraded eggs and increased labour costs due to the selection of eggs with EAA and cleaning of the facilities due to broken eggs were the main contributors to the economic impact of this eggshell pathology. The estimated average economic loss of a flock with 5% eggs with EAA from 30 weeks up to 75 weeks of age is about 3% of egg price gross return (Feberwee et al., Citation2009a).
To date, all field reports on EAA eggs in the Netherlands are from layer-type stock; reports on EAA production in meat-type stock are lacking. A possible explanation for this may well be that M. synoviae strains with affinity for the oviduct have not yet spread from layers to broiler breeders as the production chain of both bird types runs separately. However, there could also be a difference in susceptibility between both bird types. In order to assess the susceptibility of broiler breeder hens for M. synoviae strains with oviduct tropism, an experiment aiming at the induction of EAA was conducted in broiler breeder hens.
Materials and Methods
Bird experiment design
The M. synoviae EAA isolate (chicken/NL/Dev/SP255/Tom/05) obtained from the oviduct of layers and known to induce eggs with EAA (Feberwee et al., 2009a) was used in this experiment. At the start of the experiment the specified pathogen free (SPF) breeder hens were 18 weeks of age (D0), and the experiment ended at 30 weeks of age (W12). Five experimental groups, each of 12 birds, were set up as follows. A negative control group (control) inoculated intratracheally (i.t.) with 1 ml Mycoplasma Experience (ME) broth (Mycoplasma Experience, Reigate, Surrey, UK). Two groups (Ms1 and IBVMs1) were inoculated i.t. with ME broth containing 106 to 107 colony-forming units (CFU) M. synoviae EAA strain/ml at 19 weeks of age (W1 of the experiment), of which one group (IBVMs1) was also inoculated with infectious bronchitis virus (IBV) D1466 (1 ml i.t. and 0.5 ml subcutaneously with allantoic fluid containing 106.6 median embryo infective dose (EID50)/ml) 5 days earlier. The other two groups (Ms2 and IBVMs2) were inoculated a second time with ME broth containing 106 to 107 CFU M. synoviae EAA strain/ml at 26 weeks of age (W8 of the experiment), and one group (IBVMs2) was also inoculated with IBV D1466 (1 ml i.t. and 0.5 ml subcutaneously with allantoic fluid containing 106.6 EID50/ml) 5 days prior to the first M. synoviae EAA challenge.
Control birds were not inoculated with IBV alone as in previous studies it was shown that IBV alone, in contrast to M. synoviae alone, does not induce EAA (Feberwee et al., Citation2009a, Citationb).
Birds and housing
The birds were placed in the isolators 3 weeks before the start of the experiment. They were free of M. gallisepticum, M. synoviae and IBV, and other common avian pathogens as described elsewhere (Feberwee et al., Citation2005). Before the start of the experiment the birds were weighed, divided into weight classes and proportionally allocated in each experimental group. Each group was housed in a different negative-pressure HEPA isolator (194 cm width, 95 cm height and 75 cm depth; Beyer & Eggelaar, Utrecht, the Netherlands) containing four laying nests. The housing temperature ranged from 18 to 20°C. Birds were daily provided with 14 h of light. Drinking water was given ad libitum, but feed was restricted. As egg picking occurred in one group, probably caused by restricted feeding, 2.5 g oats/chicken and 2.5 g grit/chicken were given daily in order to increase scratching behaviour and distract the birds.
Sampling and monitoring
At D0 and W12 the birds were weighed individually, and at W12 blood samples collected from individual chickens were used for M. synoviae and IBV D1466 serology. At W12 all experimental birds were stunned using CO2+O2 and were exsanguinated by incision of the jugular vein. Besides post mortem analysis, general bacteriology and mycoplasma culture of the oviduct were performed.
Lay was synchronized to 9 a.m. and eggs were collected twice a day. Onset of egg production and its monitoring commenced 2 weeks (W-1 and W-2) before M. synoviae EAA challenge. The daily egg production (including non-broken and broken eggs) was registered until W12 after mycoplasma inoculation. At the end of the experiment, eggshell strength measurement was performed on all collected eggs with EAA (that were not broken or cracked) and non-affected eggs per experimental group (n=20 to 28).
M. synoviae EAA and IBV inocula
Freeze-dried M. synoviae EAA culture was suspended in 1 ml distilled water and transferred to 50 ml ME broth and was incubated at 37°C until a change of colour was observed. Control of the bacterial concentrations in the inocula used was performed by means of bacterial counting following the International Organization for Standardization (Citation1983).
IBV D1466 (batch number 06500; Animal Health Service (GD), Deventer, the Netherlands) in freeze-dried vials (–20°C) containing 2.5×106.6 EID50/5 ml was dissolved in phosphate-buffered saline (PBS) aiming at a concentration of 106 to 107 EID50/ml.
Serology
At the end of the experiment (W12) M. synoviae serology was performed using an enzyme-linked immunosorbent assay (ELISA) test (IDEXX Corporation, Westbrook, Maine, USA). The test was performed according to the manufacturer's instructions. IBV D1466 antibodies were assessed with the haemagglutination inhibition test at D0 and W12 of the experiment (Alexander & Chettle, Citation1977; De Wit et al., Citation1997).
Bacteriology and mycoplasma culture
Bacteriology and mycoplasma culture of the oviduct was performed after sterilizing the outer surface of the oviduct with a hot scalpel blade. Subsequently, an incision was made with a sterile scalpel and two sterile cotton swabs were used to swab the isthmus and uterus. One swab was plated out on 5% sheep blood agar plate for general bacteriology and the other on a ME agar plate (Mycoplasma Experience) for mycoplasma culture. The ME agar was incubated at 37°C in a humid environment. The ME plates were examined for colonies every 2 to 3 days up to 28 days. One separate colony was plated out on a fresh ME agar and ME agar with positive clones, and approximately 2 x 0.5 cm2 was transferred to 5 ml ME broth and incubated at 37°C. Positive mycoplasma cultures were identified as M. synoviae by polymerase chain reaction (PCR) as described (Mekkes & Feberwee, Citation2005).
Assessment of EAA and eggshell strength
Twice a week, all eggs produced were examined for the presence of EAA by careful visual inspection of the apex (roughened aspect of the eggshell apex and by candling detection of a clear demarcation zone and increased translucency). Furthermore, at the end of the study, as a complementary test, the eggshell strength (in Newtons) of all eggs with EAA was measured using an eggshell compression device (Futura 3/A, OQT-II; Futura-Werner Fürste Gbr, Lohne, Germany).
Molecular identification
The tracheal swabs were eluted individually in 1 ml PBS and centrifuged for 10 min at 16,000 x g, and the pellet was then resuspended in 1 ml PBS and centrifuged at 16,000 x g. Subsequently the pellet was resuspended in 25 µl PBS and incubated at 110°C for 15 min, and after that was cooled on ice for 5 min. After a final centrifugation for 2 min at 16,000 x g, supernatants were cooled at 4°C and used directly for PCR.
Positive broth cultures were pelleted for 10 min at 16,000 x g and the pellets were resuspended in 200 µl sterile PBS. DNA was extracted using the protocol for Cultured Animal Cells of the QiaAmp DNA mini kit (Qiagen Benelux B.V., Venlo, the Netherlands).
The DNA extracts were tested by the M. synoviae quantitative PCR test as described earlier (Mekkes & Feberwee, Citation2005; Feberwee et al., 2009b). The forward primer 5′-GAGAAGCAAAATAGTGATATCA-3′ and the reverse primer 5′-CAGTCGTCTCCGAAGTTAACAA-3′ (GenBank) were used. These primers amplify a 211 bp sequence from the 16S ribosomal RNA gene of M. synoviae.
Statistical analysis
The proportions of sera that had detectable antibodies against M. synoviae, the proportions of cultures of oviducts that yielded mycoplasmas and the fraction of eggs with EAA on the total egg production were compared using the two-sample proportion test (Statistix®, Citation2005). IBV antibody titres, the eggshell strength measurements and the mean daily egg production per chicken were analysed using Kruskal–Wallis one-way analysis of variance. The Kruskal–Wallis all-pairwise comparisons test was performed as a post hoc analysis in order to compare all possible pairwise differences between the means of the different treatment groups (Statistix®, Citation2005). Means were considered significantly different if P < 0.05.
Results and Discussion
In the present study, birds were infected with the M. synoviae EAA strain by the i.t. inoculation route. This was based on the results of earlier work on EAA by Feberwee et al. (Citation2009a), who showed that induction of this condition by M. synoviae in white layers was inoculation route dependent, the i.t. route being more efficient than the intravenous route. Moreover, in those investigations, a synergistic effect between IBV and M. synoviae concerning the production of eggs with EAA was shown. Also, the production of eggs with EAA could never be induced with IBV alone in contrast to M. synoviae alone (Feberwee at al., 2009b). Therefore, a group challenged with only IBV 1466 was not included in this experiment.
Clinical signs were not observed in any of the M. synoviae-infected groups during the whole experimental period, which was in agreement with similar work with white commercial layer hens (Feberwee et al., Citation2009a). However, in contrast to the study with white layers, no significant reduction was observed in the increase of body weight (data not shown).
At the end of the experiment the M. synoviae serology was positive in all groups except for the control group and the IBV antibodies were only present in the Ms1IBV-treated and Ms2IBV-treated groups (). The results of M. synoviae ELISA and IBV HI test showed that IBV inoculation and M. synoviae EAA challenge were performed successfully and that the infection remained confined to the treated groups.
Table 1. Serology, PCR, mycoplasma culture of the oviduct, total egg production, production of eggs with EAA and eggshell strength of the experimental SPF breeder hens
At W12 the M. synoviae PCR performed on tracheal samples showed negative results for the control group, while M. synoviae DNA was detected in all other groups (four out of 12 in the Ms2 and Ms2IBV group, five out of 12 in the Ms1 group and two out of 12 in the Ms1IBV group), indicating that M. synoviae persists in the trachea for a long time after challenge, which is in accordance with what has been described in other studies (Kleven, Citation2003; Mekkes & Feberwee, Citation2005). The proportions of positive M. synoviae PCR results were not significantly different between these four groups ().
At post-mortem examination, no pathogenic agents were isolated from the oviduct except M. synoviae. The fraction of mycoplasma-positive cultures from oviducts of the Ms2, Ms1IBV and Ms2IBV groups was one out of 12 in each group. In one M. synoviae-positive oviduct (Ms1IBV), an egg with EAA was present. The two other hens with a M. synoviae-positive oviduct culture showed a negative M. synoviae PCR result of the tracheal swabs. The latter finding confirms the oviduct tropism of this particular M. synoviae strain. This is in accord with findings of Lockaby et al. (Citation1998), who showed that M. synoviae WVU1853, a strain isolated from the chicken joint, was isolated less frequently from the respiratory tract compared to that of joint tissues 4 weeks after infection.
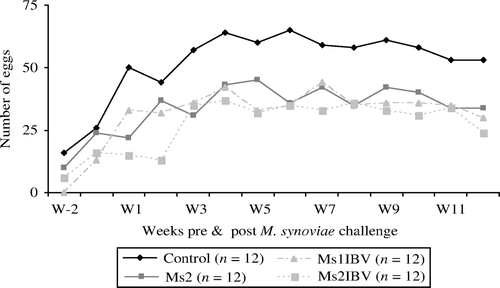
Unfortunately, egg picking occurred in the Ms1 group; therefore, results of egg production and the production of eggs with EAA could not be included in the analyses. The total egg production from W1 to W12 in the control group (n=712) was higher than in the other groups (Ms2, n=454; Ms1IBV, n=449; and Ms2IBV, n=368). The mean daily egg production per chicken was significantly higher (P < 0.05) in the control group (0.66±0.02 eggs) compared with that of the other groups (Ms2, 0.42±0.02 eggs; Ms1IBV, 0.42±0.02 eggs; and Ms2IBV, 0.34±0.02 eggs). From W1 to W12 after M. synoviae EAA challenge, there was no significant difference between the mean daily egg production per chicken of the treated groups, but it was significantly higher in the control group compared with that of the other groups ( and ). Pre-inoculation production data showed that the significant differences in mean daily egg production occurred after inoculation, indicating that mentioned differences were probably the consequence of the inoculation of the birds with the M. synoviae EAA strain and/or IBV 1466 (). However, a negative effect of the M. synoviae EAA strain alone on the total egg production has been described previously (Feberwee et al., Citation2009a).
Figure 1. Weekly egg production per experimental group was registered from 2 weeks (W-1 and W-2) before M. synoviae EAA challenge until W12 after mycoplasma inoculation. Ms1 is not included as results were influenced by egg picking. Control = not inoculated; Ms1 = single inoculation with M. synoviae EAA strain; Ms2 = double inoculation with M. synoviae EAA strain; Ms1IBV = IBV inoculation prior to the inoculation of the M. synoviae EAA strain; Ms2IBV = IBV inoculation prior to the first inoculation of the M. synoviae EAA strain.
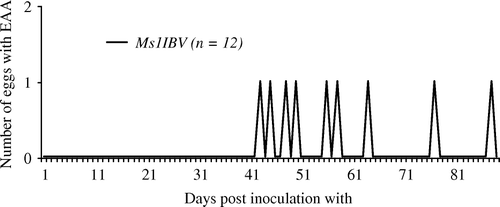
The production of eggs with EAA only occurred in the Ms1IBV group. In total, nine eggs with EAA were produced during the whole experimental period. The first egg with EAA was produced 6 weeks after M. synoviae challenge (), which is 3 weeks later compared with table egg layers (Feberwee et al., 2009a, b). The proportion of eggs with EAA induced in broiler hens (2%, 9/449) was much lower than in layer type of birds (14–22% in the M. synoviae EAA/IBV group). However, egg compression tests showed a significant decrease (P < 0.05) in average eggshell strength of eggs with EAA (11.5±1.8 N, n=6) compared with that of unaffected eggs of the control group (39.2±2.1 N, n=20) and the unaffected eggs of the Ms1 (37.5±2.1 N, n=22), Ms2 (36.1±2.1 N, n=24) and Ms1IBV group (35.7±2.3 N, n=24), but not with that of the Ms2IBV group (31.4±1.8, n=21). There was no significant difference in the eggshell strength of unaffected eggs between the different groups, although eggshell strength in the Ms2IBV group was lower. The latter finding suggests a possible negative effect of M. synoviae EAA strain on eggshell strength in general.
Figure 2. Eggs with EAA were only produced by the Ms1IBV group (IBV inoculation prior to the inoculation of the M. synoviae EAA strain).
The much lower production of eggs with EAA in the Ms1IBV group compared with that of commercial layers in previous experiments and the fact that eggs with EAA were not even produced in the groups challenged twice with M. synoviae EAA (Ms2IBV and Ms2 groups) during the whole experimental period indicates that broiler breeder hens are less susceptible to M. synoviae-induced EAA compared with table egg layers. This is also in agreement with field reports: to date, spontaneous cases of M. synoviae-induced EAA have only been reported in table egg layers in the Netherlands and elsewhere in the world. Similarly, M. synoviae-induced joint pathology has also mainly been reported in layer type flocks in our country (Landman & Feberwee, Citation2001; Landman & Bronnenberg, Citation2003) and elsewhere (Morrow et al., Citation1990; Kleven, Citation2003)—although an early field report (Morrow et al., Citation1990) documented the occurrence of M. synoviae synovitis in a broiler breeder flock.
Egg production losses in commercial poultry have been well documented for M. gallisepticum, with egg production drops varying between 5 and 50% (Evans et al., Citation1992; Stipkovits & Kempf, Citation1996; Kleven, Citation2003). In contrast, reports on egg production losses due to M. synoviae are scarce, and papers describing a detrimental effect of M. synoviae on egg production (Lott et al., Citation1978; Morrow et al., Citation1990) and describing no effect can both be found (Van Eck et al., Citation1980; Mohammed et al., Citation1987). In the present and previous works in layers (Feberwee et al., Citation2009a, Citationb), it was shown that M. synoviae strains with oviduct tropism and ability to induce EAA can also significantly decrease egg production. Moreover, as in the studies in layers (Feberwee et al., Citation2009a, Citationb), a negative effect on eggshell strength in general was also encountered in broiler breeder hens, which could have a detrimental effect on hatchability (Roque & Soares, Citation1994). These findings underline the increasing importance of M. synoviae in commercial poultry despite the absence of clinical signs in infected birds.
Acknowledgements
This research was supported by a grant from the Dutch Commodity Board for Poultry and Eggs (PPE). The authors thank Dr J. J. De Wit for his technical advice.
References
- Alexander , D.J. and Chettle , N.J. 1977 . Procedures for the haemagglutination and haemagglutination inhibition tests for avian infectious bronchitis virus . Avian Pathology , 6 : 9 – 17 .
- De Wit , J.J. , Mekkes , D.R. , Kouwenhoven , B. and Verheyden , J.H.M. 1997 . Sensitivity and specificity of serological tests for detection of infectious bronchitis virus-induced antibodies in broilers . Avian Pathology , 26 : 105 – 118 .
- Evans , R.D. , Hafez , Y.S. and Orthel , F.W. 1992 . Mycoplasma gallisepticum vaccination-challenge: an egg-production model . Avian Diseases , 36 : 956 – 963 .
- Feberwee , A. , Mekkes , D.R. , De Wit , J.J. , Hartman , E.G. and Pijpers , A. 2005 . Comparison of culture, PCR and different serological tests for detection of Mycoplasma gallisepticum and M. synoviae infections . Avian Diseases , 49 : 260 – 268 .
- Feberwee , A. , De Wit , J.J. and Landman , W.J.M. 2009a . Induction of eggshell apex abnormalities by Mycoplasma synoviae: field and experimental studies . Avian Pathology , 38 : 77 – 85 .
- Feberwee , A. , Morrow , C.J. , Ghorashi , S.A. , Noormohammadi , A.H. and Landman , W.J.M. 2009b . Effect of a live Mycoplasma synoviae vaccine on the production of eggshell apex abnormalities induced by a Mycoplasma synoviae infection preceded by an infection with infectious bronchitis virus D1466 . Avian Pathology , 38 : 333 – 343 .
- International Organization for Standardization 1983 Microbiology—General Guidance for Preparation of Dilutions for Microbiological Examination ISO 6887 , 1st edn Geneva International Standard Organization
- Kleven , S.H. 2003 . “ Mycoplasma synoviae infection ” . In Diseases of Poultry , 11th edn , Edited by: Saif , Y.M. , Barnes , H.J. , Glisson , J.R. , Fadly , A.M. , McDougald , L.R. and Swayne , D.E. 756 – 766 . Ames : Iowa State Press .
- Kleven , S.H. , Fletcher , O.J. and Davis , R.B. 1975 . Influence of strain of Mycoplasma synoviae and route of infection on development of synovitis or airsacculitis in broilers . Avian Diseases , 19 : 126 – 135 .
- Landman , W.J.M. and Bronnenberg , R.G.C. 2003 . Mycoplasma synoviae-associated amyloid arthopathy in white leghorns: case report . Tijdschrift voor Diergeneeskunde , 2 : 36 – 40 .
- Landman , W.J.M. and Feberwee , A. 2001 . Field-studies on the association between amyloid arthropathy and Mycoplasma synoviae infection, and experimental reproduction of the condition in brown layers . Avian Pathology , 30 : 629 – 639 .
- Landman , W.J.M. and Feberwee , A. 2004 . Aerosol-induced Mycoplasma synoviae arthritis: the synergistic effect of infectious bronchitis virus infection . Avian Pathology , 33 : 591 – 598 .
- Lockaby , S.B. , Hoerr , F.J. , Lauerman , L.H. and Kleven , S.H. 1998 . Pathogenicity of Mycoplasma synoviae in broiler chickens . Veterinary Pathology , 35 : 178 – 190 .
- Lott , B.D. , Drott , T.H. , Vardaman , T.H. and Reece , F.N. 1978 . Effect of M. synoviae on egg quality and egg production of broiler breeders . Poultry Science , 57 : 309 – 311 .
- Mekkes , D.R. and Feberwee , A. 2005 . Real-time PCR for the qualitative and quantitative detection of Mycoplasma gallisepticum . Avian Pathology , 34 : 348 – 354 .
- Mohammed , H.O. , Carpenter , T.E. and Yamamoto , R. 1987 . Economic impact of Mycoplasma gallisepticum and M. synoviae in commercial layer flocks . Avian Diseases , 31 : 477 – 482 .
- Morrow , C.J. , Bell , I.G. , Walker , S.B. , Markham , P.F. , Thorp , B.H. and Whithear , K.G. 1990 . Isolation of Mycoplasma synoviae from infectious synovitis of chickens . Australian Veterinary Journal , 67 : 121 – 124 .
- Olson , N.O. , Shelton , D.C. , Bletner , J.K. , Munro , D.A. and Anderson , G.C. 1956 . Studies of infectious synovitis in chickens . American Journal of Veterinary Research , 17 : 747 – 754 .
- Roque , L. and Soares , M.C. 1994 . Effects of eggshell quality and broiler breeder age on hatchability . Poultry Science , 73 : 1838 – 1845 .
- Statistix® 2005 User's Manual Analytical software version 8.0 Tallahassee, FL , , USA Analytical Software®
- Stipkovits , L. and Kempf , I. 1996 . Mycoplasmosis in Poultry . Revue Scientifique et Technique (International Office of Epizootics) , 15 : 1495 – 1525 .
- Van Eck , J.H. , Van Kol , N. and Kouwenhoven , B. 1980 . Egg production in relation to the results of a long term serological survey of 73 flocks of fowl . Tijdschrift voor Diergeneeskunde , 15 : 15 – 24 .