Abstract
A total of 122 dead broiler breeders randomly selected from a flock showing normal production parameters and covering the age from 44 to 61 weeks were subjected to a comprehensive routine post-mortem examination including examination for lesions of endocarditis. Forty-two hens (34%) showed valvular endocarditis caused by Avibacterium endocarditidis (43%), Enterococcus faecalis (31%), Staphylococcus aureus (5%) and Streptococcus pluranimalium (5%), while growth was not obtained from 17% with the methods used for isolation. Gross lesions associated with the different bacterial pathogens did not allow separation according to pathogens involved. Port of entry and pathogenesis associated with the high prevalence of valvular endocarditis remained speculative. The present findings demonstrated the newly described species of Pasteurellaceae, Avibacterium endocarditidis associated with endocarditis in chickens and confirm previous observations on the prevalence of endocarditis in chickens, partly explaining the slightly increased mortality normally observed in broiler breeders during the last weeks of production.
Introduction
Bacterial endocarditis is rarely reported in the literature and systematic investigations have not been reported to the knowledge of the authors. Povar & Brownstein (Citation1947) reported on many cases of valvular endocarditis observed during routine post-mortem examination of chickens from a large breeding farm. Some 15% of the chickens older than 40 weeks of age had valvular endocarditis, while only 3% of the investigated birds between 10 and 40 weeks of age were affected. Chickens with chronic infections showed a significantly greater incidence of valvular endocarditis than did chickens dying from all other causes. Staphylococci or streptococci were isolated from the valvular lesions.
According to Gross (1978) cited by Peckham (Citation1978), sporadic mortality due to endocarditis totalling less than 0.5%, occurring over a period of many months, is the usual history for infected flocks. However, a higher incidence would probably be reported if all birds are subjected to a comprehensive post-mortem examination that includes opening and careful examination of the heart. Previous investigations on the aetiology of valvular endocarditis have underlined the importance of Staphylococcus aureus and Enterococcus faecalis (Povar & Brownstein, Citation1947; Gross & Domermuth, Citation1962) and, less frequently, Streptococcus zooepidemicus (Peckham, Citation1966). However, a clonal outbreak of Streptococcus gallinaceus associated with septicaemia and valvular endocarditis in broiler breeders aged 26 to 56 weeks was recently reported by Chadfield et al. (Citation2004).Valvular endocarditis due to Streptococcus pluranimalium has also been observed in broiler breeders (Hedegaard et al., Citation2009).
Although field cases of valvular endocarditis seem to be limited to Gram-positive bacteria, Gross & Domermuth (Citation1962) reported on experimental production of endocarditis in chickens and turkeys by intravenous injection of Pasteurella multocida obtained from naturally infected birds. Experimental reproduction of endocarditis with field isolates of Avibacterium gallinarum has also been reported (Tjahjowati et al., Citation1995).
In broiler breeders a slightly increased mortality is normally observed from week 40 until the end of production at an average age of 60 weeks. According to Povar & Brownstein (Citation1947), part of this mortality might be due to endocarditis. For the same reason, the aims of the present study were to investigate the incidence and causes of endocarditis in a flock of broiler parents showing normal production parameters including mortality, and to examine whether infections due to S. gallinaceus recently demonstrated on the farm (Chadfield et al., Citation2004) might persist on previously infected farms.
Materials and Methods
Flock data
A broiler breeder flock totalling 42,570 hens and 3400 males kept on a farm previously affected by endocarditis (Chadfield et al., Citation2004) was investigated. Production parameters including body weight, egg production and egg weight, number of hatching eggs, hatchability and number of chicks/hen followed standards for Ross 308 (Parent Stock Performance Objectives, June 2007, Aviagen, Huntsville, Alabama, USA) (www.aviagen.com). Hen mortalities observed from the age of 20 to 41 weeks were 3.1%, 3.4%, 4.1% and 3.7% for Houses 1, 2, 3 and 4, respectively. The weekly mortality for the single houses covering the rest of the production period is shown in .
Figure 1. Weekly mortality (%) in Houses 1 to 4 covering ages from 41 to 61 weeks. Dead birds were received for investigation from week 44 until the end of production.
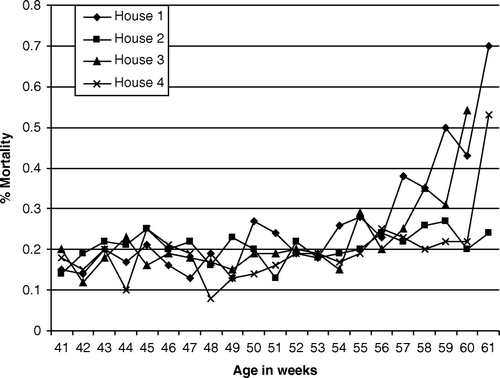
Dead hens randomly selected from all four houses were received weekly from the age of 44 weeks until slaughtering at the age of 61 weeks, with the exceptions of weeks 47, 52, 53, 54, 58 and 59 where dead animals were not delivered for post-mortem inspection by the farmer. A total of 122 hens including 31, 25, 34 and 32 birds from Houses 1, 2, 3 and 4, respectively, were received and subjected to a comprehensive post-mortem examination.
Bacteriology
Standard methods were used for isolation and identification of bacterial pathogens associated with lesions observed (Swayne et al., Citation1998). When present, endocarditis lesions on the valves were swabbed with a sterile cotton swab and subsequently plated onto blood agar (Blood Agar Base, CM55; Oxoid, Basingstoke, Hampshire, UK) containing 5% sterile bovine blood. In addition, samples from liver and/or spleen were plated on blood agar. In cases of other lesions, samples for bacteriology were taken upon indication. A total of 27 isolates that could not be classified with known species of bacteria were classified as Avibacterium endocarditidis (Bisgaard et al., Citation2007).
The type strain of A. endocarditidis is 20186H4H1 and has been deposited with the Culture Collection, University of Göteborg (CCUG) under CCUG 52860. For comparison, three strains isolated from salpingitis, peritonitis and septicaemia of chickens in Denmark (CCUG 18396=10607/4; CCUG 18397=10607/5; CCUG 18551=30084L) and one isolate from a chicken heart in Australia (CCUG 18732) were included. These isolates have previously been demonstrated to cluster with the type strains of A. gallinarum and Avibacterium volantium by ribotyping, using HindIII and HpaII for digestion of DNA (Petersen et al., Citation1998).
Ribotyping
Ribotyping, using HpaII for digestion of DNA, and cluster analysis were carried out as reported previously (Petersen et al., Citation1998) for the type strain of A. endocarditidis and the four reference strains.
rpoB and 16S rRNA sequencing and analysis
The partial rpoB sequence was determined according to Mollet et al. (Citation1997) and covered the region 509 to 680 (positions refer to Escherichia coli K-12, accession number U00096) of the deduced protein sequence as reported previously (Angen et al., Citation2003; Korczak et al., Citation2004). In addition to published primers, the forward polymerase chain reaction (PCR) primer rpobfPm (5′-GCAGTGAAAGAATTCTTTGGTTC-3′) was used for PCR amplification and DNA sequencing. The rpoB gene sequences of strains 20186H4H1T (Bisgaard et al., Citation2007), CCUG 18732, CCUG 18396, CCUG 18397 and CCUG 18551 (Petersen et al., Citation1998) were generated. The sequences of strains 20186H4H1T, CCUG 18732, CCUG 18396 and CCUG 18397 were identical and only the sequence of strain 20186H4H1T was deposited, with accession number GU270585. Strain CCUG 18551 was deposited with accession number GU270584. 16S rRNA gene sequences were determined for CCUG 18396 and CCUG 18732 and were deposited with accession numbers GU270583 and GU270582, respectively. Comparison of these sequences with the 16S rRNA gene sequences of 20186H4H1T was reported previously (Bisgaard et al., Citation2007). A BLAST search (Altschul et al., Citation1997) was performed in GenBank (Benson et al., Citation2006). Pairwise comparisons for similarity were performed by the program WATER included in EMBOSS (Rice et al., Citation2000). Multiple alignment was performed by ClustalX (Thompson et al., Citation1997). Phylogenetic analysis of the five rpoB gene sequences determined in this investigation, the closest related sequences belonging to Avibacterium and representatives of the avian genera Gallibacterium and Volucribacter as well as P. multocida included 488 positions, and were carried out by neighbour joining included with PHYLIP (Felsenstein, Citation1995).
Results and Discussion
A slightly increased mortality was observed in Houses 1, 2, 3 and 4 from weeks 54, 56, 55 and 56, respectively, to week 61 (), resulting in a total mortality of 8.2%—which is approximately 1% above the standard observed for Ross 308 (Parent Stock Performance Objectives, June 2007, Aviagen).
Post-mortem findings including causes of mortality are presented in . A total of 76 hens (62%) died as a result of septicaemic conditions, of which 42 (34%) suffered from valvular endocarditis. The most frequent causes of septicaemia included E. faecalis, E. coli and the newly described species of Pasteurellaceae, A. endocarditidis, constituting 22 (29%), 19 (25%) and 19 (25%) cases, respectively ().
Table 1. Post-mortem findings observed for 122 dead hens received for investigation including identification of bacterial agents.
Valvular endocarditis observed in the 42 hens was caused by A. endocarditidis (43%), E. faecalis (31%), S. aureus (5%), and S. pluranimalium (5%), while growth was not obtained from seven (17%) out of 42 hens investigated. A. endocarditidis was isolated from valvular endocarditis for the first time at the age of 50 weeks. Before week 50, only six out of 33 hens investigated (18%) showed valvular endocarditis—confirming previous observations by Povar & Brownstein (Citation1947). Subsequently, the frequency of valvular endocarditis increased to 40% (36 out of 89 birds). The increased frequency of valvular endocarditis during the last 10 weeks of production was associated with a slightly increased mortality, which might be explained as a result of infections with A. endocarditidis spreading through all four houses (). Increased mortality due to E. coli and E. faecalis infections, however, also contributed to the increased mortality.
Organisms identified as A. endocarditidis (Bisgaard et al., Citation2007) are phenotypically related with other species of Avibacterium as defined by Blackall et al. (Citation2005). Only differences in d(−)-mannitol, d(−)-sorbitol and l(−)-fucose separate A. endocarditidis from A. gallinarum, while more than three phenotypical characters separate A. endocarditidis from Avibacterium paragallinarum ().
Table 2. Phenotypical characters separating Avibacterium endocarditidis from other species of Avibacterium.
According to the original publication on A. gallinarum (Hall et al., Citation1955), acid production from mannitol, sorbitol and inositol was irregular. For the same reason it might be speculated whether some of their isolates in fact represent A. endocarditidis.
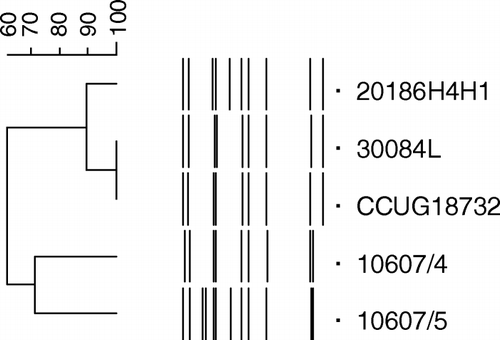
The three Danish isolates from salpingitis, (CCUG 18396), salpingitis and peritonitis (CCUG 18397) or septicaemia in chickens (CCUG 18551) and the single Australian isolate from the heart of a chicken, previously demonstrated to cluster with Avibacterium by ribotyping (Petersen et al., Citation1998), shared the phenotypical characters of A. endocarditidis.
Ribotyping of the type strain of A. endocarditidis, representing the 27 strains previously reported (Bisgaard et al., Citation2007), and four strains from different lesions in chickens showed that these isolates were highly related genetically ().
Figure 2. Genotypic relationship between the type strain of A. endocarditis and four unclassified strains from other lesions in chickens as demonstrated by ribotyping.
The rpoB gene, encoding the β-subunit of DNA-dependent RNA polymerase, has been recommended for DNA sequence-based identification of Pasteurellaceae by Christensen et al. (Citation2007). The rpoB sequences of all four strains stated above were identical or with high similarity to the type strain of A. endocarditidisrepresenting the present outbreak, underlining the existence of this organism on at least two continents. Similarities of 98.8%, 97.9% and 96.5% were demonstrated with the type strains of A. gallinarum, Avibacterium avium and A. volantium, respectively.
Phylogenetic analysis of the four rpoB gene sequences determined in this investigation confirmed the monophyly of Avibacterium and their relationship with other avian genera (data not shown). 16S rRNA sequencing demonstrated 99.9% and 98.9% similarity between the type strain of A. endocarditidis and CCUG 18396 and CCUG 18732, respectively, underlining that these organisms belong to Avibacterium. However, as previously discussed, 16S rRNA cannot be used for separation of species of Avibacterium (Blackall et al., Citation2005).
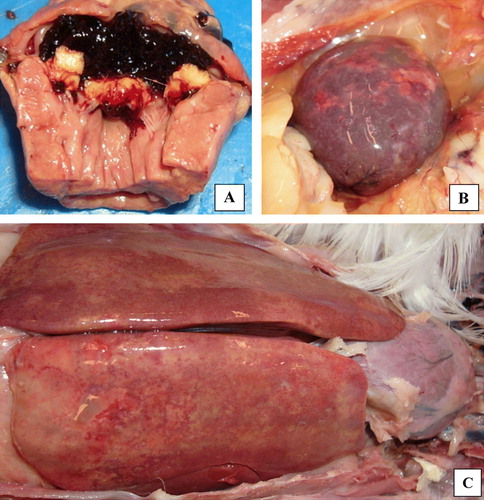
Post-mortem lesions recorded as septicaemic included general hyperaemia, subserosal discoloration of fat tissue and hepatomegaly and splenomegaly, in addition to a regressive ovary. Gross lesions of valvular endocarditis included yellow–white or greyish verruciform, vegetative endocarditis associated with the mitral valve (a). In addition, dry necroses varying from pin-point to larger irregular dry lesions demarcated by a haemorrhagic zone were also observed in the liver and/or spleen in addition to amyloidosis (b,c). Similar lesions were also observed in cases not associated with endocarditis. Septicaemic conditions caused by E. coli resulted in complete liver necroses, but endocarditis and amyloidosis were never observed in E. coli-infected birds. Valvular endocarditis caused by A. endocarditidis, E. faecalis, S. aureus and S. pluranimalium could not be separated based upon gross lesions. However, major differences were observed as to isolation of these taxa from associated lesions in the liver and spleen. While abundant growth of E. faecalis and S. aureus could always be obtained from these organs, A. endocarditidis was only isolated from affected mitral valves and the liver in six out of 18 cases (33%), from mitral valves and the spleen in five out of 18 cases (28%), and from mitral valves, the liver and spleen in only three out of 18 cases (17%), the mitral valves constantly showing abundant growth. In addition, only moderate growth or a few colonies were obtained from the liver and spleen, indicating that the infection in these organs seems to be eradicated while it persists on the mitral valves. This means that infections due to A. endocarditidis might be overlooked if comprehensive post-mortem examinations are not carried out, including culturing from the affected mitral valves.
Figure 3. (3a) Vegetative, valvular endocarditis, accompanied by multiple necroses and amyloidosis in (3b) the spleen and (3c) the liver in all cases associated with the isolation of A. endocarditis.
The port of entry and the pathogenesis of valvular endocarditis reported remain unclear and speculative. In a previous longitudinal study, an increased prevalence of pododermatitis was observed with increased age (Bisgaard et al., unpublished data). This might explain an increased prevalence of endocarditis with increasing age due to staphylococci, enterococci and streptococci, but not Pasteurellaceae, the habitat of which are mucosal membranes of the upper respiratory and lower genital tracts (Bisgaard, Citation1993). With an increased age, the possibility of adherence of potential pathogenic bacteria to open wear lesions on the valves combined with a weakened immune competence might add to an increased risk of endocarditis. Subsequent spreading from the valves to other organs was similar for A. endocarditidis and species of Gram-positive cocci, but A. endocarditidis seems less pathogenic and more easily controlled by the body defence mechanisms in these organs than Gram-positive cocci, for reasons that remain to be investigated. Increased mortality from A. endocarditidis in all four houses was due to the same clone (Bisgaard et al., Citation2007), the source of which remained unknown. However, a single isolate of a bacterium isolated from a carrion crow in Scotland, characterized phenotypically by one of the authors, shared the phenotypical characters of A. endocarditidis—indicating that certain wild birds might represent a reservoir for these organisms (Bisgaard, unpublished data).
Only two cases of valvular endocarditis due to S. pluranimalium were demonstrated. Valvular endocarditis due to S. gallinaceus, previously reported on the same farm (Chadfield et al., Citation2004), was not demonstrated, however, underlining that these organisms do not persist if proper cleaning and disinfection are carried out between flocks.
References
- Altschul , S.F. , Madden , T.L. , Schaffer , A.A. , Zhang , J. , Zhang , Z. , Miller , W. and Lipman , D.J. 1997 . Gapped BLAST and PSI-BLAST: a new generation of protein database search programs . Nucleic Acids Research , 25 : 3389 – 3402 .
- Angen , Ø. , Ahrens , P. , Kuhnert , P. , Christensen , H. and Mutters , R. 2003 . Proposal of Histophilus somni gen. nov., sp. nov., for the three species incertae sedis “Haemophilus somnus”, “Haemophilus agni” and “Histophilus ovis” . International Journal of Systematic and Evolutionary Microbiology , 53 : 1449 – 1456 .
- Benson , D.A. , Karsch-Mizrachi , I. , Lipman , D.J. , Ostell , J. and Wheeler , D.L. 2006 . GenBank . Nucleic Acids Research , 34 : D16 – D20 .
- Bisgaard , M. 1993 . Ecology and significance of Pasteurellaceae in animals . Zentralblatt für Bakteriologie , 279 : 7 – 26 .
- Bisgaard , M. , Christensen , J.P. , Bojesen , A.M. and Christensen , H. 2007 . Avibacterium endocarditidis sp. nov., isolated from valvular endocarditis in chickens . International Journal of Systematic and Evolutionary Microbiology , 57 : 1729 – 1734 .
- Blackall , P.J. , Christensen , H. , Beckenham , T. , Blackall , L.L. and Bisgaard , M. 2005 . Reclassification of Pasteurella gallinarum, [Haemophilus] paragallinarum, Pasteurella avium and Pasteurella volantium as Avibacterium gallinarum gen. nov., comb. nov., Avibacterium paragallinarum comb. nov., Avibacterium avium comb. nov. and Avibacterium volantium comb. nov . International Journal of Systematic and Evolutionary Microbiology , 55 : 353 – 362 .
- Chadfield , M.S. , Christensen , J.P. , Christensen , H. and Bisgaard , M. 2004 . Characterization of streptococci and enterococci associated with septicaemia in broiler parents with a high prevalence of endocarditis . Avian Pathology , 33 : 610 – 617 .
- Christensen , H. , Kuhnert , P. , Busse , H.-J. , Frederiksen , W. and Bisgaard , M. 2007 . Proposed minimal standards for the description of genera, species and subspecies of the Pasteurellaceae . International Journal of Systematic and Evolutionary Microbiology , 57 : 166 – 178 .
- Felsenstein , J. 1995 . PHYLIP (Phylogeny Inference Package) version 3.5c , Seattle , WA : Department of Genetics, University of Washington .
- Gross , W.B. and Domermuth , C.H. 1962 . Bacterial endocarditis of poultry . American Journal of Veterinary Research , 23 : 320 – 329 .
- Hall , W.J. , Heddleston , K.L. , Legenhausen , D.H. and Hughes , R.W. 1955 . Studies on Pasteurellosis. I. A new species of Pasteurella encountered in chronic fowl cholera . American Journal of Veterinary Research , 16 : 598 – 604 .
- Hedegaard , L. , Christensen , H. , Chadfield , M.S. , Christensen , J.P. and Bisgaard , M. 2009 . Association of Streptococcus pluranimalium with valvular endocarditis and septicaemia in adult broiler parents . Avian Pathology , 38 : 155 – 160 .
- Korczak , B. , Christensen , H. , Emler , S. , Frey , J. and Kuhnert , P. 2004 . Phylogeney of the familiy Pasteurellaceae based on rpoB sequences . International Journal of Systematic and Evolutionary Microbiology , 54 : 1393 – 1399 .
- Mollet , C. , Drancourt , M. and Raoult , D. 1997 . rpoB sequence analysis as a novel basis for bacterial identification . Molecular Microbiology , 26 : 1005 – 1011 .
- Peckham , M.C. 1966 . An outbreak of streptococcosis (apoplectiform septicemia) in White Rock chickens . Avian Diseases , 10 : 413 – 421 .
- Peckham , M.C. 1978 . “ Vices and miscellaneous diseases ” . In Diseases of Poultry , 7th edn , Edited by: Hofstad , M.S. , Calnek , B.W. , Helmboldt , C.F. , Reid , W.M. and Yoder , H.W. Jr . 847 – 893 . Ames : Iowa State University Press .
- Petersen , K.D. , Christensen , J.P. and Bisgaard , M. 1998 . Phenotypic and genotypic diversity of organisms previously classified as maltose positive Pasteurella multocida . Zentralblatt für Bakteriologie , 288 : 1 – 12 .
- Povar , M.L. and Brownstein , B. 1947 . Valvular endocarditis in the fowl . Cornell Veterinarian , 37 : 49 – 54 .
- Rice , P. , Longden , I. and Bleasby , A. 2000 . EMBOSS: The European Molecular Biology Open Software Suite . Trends in Genetics , 16 : 276 – 277 .
- Swayne , D.E. , Glisson , J.E. , Jackwood , M.W. , Pearson , J.E. and Reed , W.M. 1998 . A Laboratory Manual for the Isolation and Identification of Avian Pathogens , 4th edn , American Association of Avian Pathologists: University of Pennsylvania .
- Thompson , J.D. , Gibsen , T.J. , Plewniak , F. , Jeanmougin , F and Higgins , D.G. 1997 . The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools . Nucleic Acids Research , 25 : 4876 – 4882 .
- Tjahjowati , G. , Orr , J.P. , Chirino-Trejo , M. and Mills , H.L. 1995 . Experimental reproduction of endocarditis with Pasteurella gallinarum in mature leghorn chickens . Avian Diseases , 39 : 489 – 498 .