Abstract
The lymphoid tissue that is associated with the intestinal tract, the so-called gut-associated lymphoid tissue (GALT), is well developed in the chicken. Depending on the location, it is present as aggregations of lymphoid cells, or organized in lymphoid follicles and tonsils. From proximal to distal, the intestinal tract contains a pharyngeal tonsil, diffuse lymphoid tissue and lymphoid follicles in the cervical and thoracic parts of the oesophagus, an oesophageal tonsil, diffuse lymphoid tissue in the proventriculus, a pyloric tonsil, Peyer's patches, Meckel's diverticulum, two caecal tonsils, diffuse lymphoid tissue in the rectum, the bursa of Fabricius, and diffuse lymphoid tissue in the wall of the proctodeum. The lymphoid tissues are frequently covered by a lympho-epithelium that is infiltrated by lymphoid cells. Such an epithelium often contains M or microfold cells, which are specialized in antigen sampling and transport antigens to the underlying lymphoid tissue. A solid knowledge of the avian GALT could contribute to the development of vaccines to be administered orally. Additionally, immune stimulation via pre- and probiotics is based on the presence of a well-developed intestinal immune system.
Introduction
While in mammals a large number of lymph nodes form a key feature of a well developed and rather complex lymphoid system, in birds typical lymph nodes are only present in some aquatic species such as ducks, geese and swans (Barone, Citation1996). In other birds, only small lymphoid nodules that are associated with the walls of the lymph vessels are present (Hodges, Citation1974). The mucosa-associated lymphoid tissue (MALT) of most birds is well developed (Schummer, Citation1973; Matsumoto & Hashimoto, Citation2000). This avian MALT consists of lymphoid cells that are mainly located in the lamina propria mucosae and the tela submucosa of the intestinal and respiratory tracts (Schummer, Citation1973). As such, it forms a first line of defence against harmful antigens that enter the body during feeding and breathing (Brandtzaeg, Citation1984). The lymphoid tissue is composed of either scattered or aggregated lymphoid cells, or is well organized into primary and secondary lymphoid follicles that are separated by interfollicular aggregations of lymphoid cells (Ogra, Citation2000). At some strategic anatomical locations, the lymphoid tissue is organized into tonsils (Oláh et al., Citation2003; Anonymous, Citation2005). The latter are complex lymphoid organs that often contain crypts which are lined by a lympho-epithelium (Oláh et al., Citation2003).
This review paper presents an overview of the MALT that is located in the avian intestinal tract, the so-called gut-associated lymphoid tissue (GALT) (Lillehoj & Trout, Citation1996; Liebler-Tenorio & Pabst, Citation2006). From proximal to distal, the avian intestinal tract contains a pharyngeal tonsil, diffuse lymphoid tissue and lymphoid follicles in the cervical and thoracic parts of the oesophagus, an oesophageal tonsil, diffuse lymphoid tissue in the proventriculus, a pyloric tonsil, Peyer's patches, Meckel's diverticulum, two caecal tonsils, diffuse lymphoid tissue in the rectum, the bursa of Fabricius and diffuse lymphoid tissue in the wall of the proctodeum. A schematic representation of the locations of these lymphoid structures is given in . With the exception of the bursa of Fabricius, which is a primary lymphoid organ, all these lymphoid tissues are secondary lymphoid organs (Pabst, Citation2007). The bursa of Fabricius, however, is also discussed in the present paper since it is closely associated with the intestinal tract. Most of the avian GALTs, such as the pharyngeal, oesophageal and caecal tonsils, were discovered many decades ago (Grau-Karlsbad, Citation1943; Payne, Citation1979). In contrast, the proventricular lymphoid tissue has been described more recently (Arai et al., Citation1988).
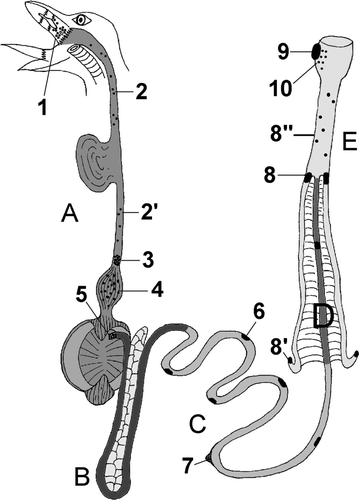
Figure 1. Schematic drawing of the chicken intestinal tract indicating the locations of GALT: 1, pharyngeal tonsil; 2 and 2′, lymphoid tissue in the cervical and thoracic parts of the oesophagus, respectively; 3, oesophageal tonsil; 4, lymphoid tissue in the proventriculus; 5, pyloric tonsil; 6, Peyer's patch; 7, vitelline diverticulum (Meckel); 8, caecal tonsils; 8′, lymphoid tissue in the apical wall of the caecum; 8′′, lymphoid tissue in the rectum; 9, cloacal bursa (Fabricius); 10, lymphoid tissue in the proctodeum. Segment A, oesophagus with ingluvies, proventriculus and ventriculus; segment B, duodenal loop with pancreas; segment C, jejunum; segment D, ileum; segment E, caeca, rectum and cloaca.
Interest in avian GALT is currently increasing. The oesophageal and pyloric tonsils have recently been studied in detail by means of state-of-the-art techniques (Oláh et al., Citation2003; Nagy et al., Citation2005; Nagy & Oláh, Citation2007). This increasing interest is probably due to the need for solid knowledge about the avian intestinal immune system in the framework of the development of vaccines that have to be administered orally (Nagy et al., Citation2005). Moreover, since the ban on growth-promoting antibiotics, research on the avian GALT is impelled by the search for suitable pre-and probiotics that stimulate the intestinal immune system (Yurong et al., Citation2005; Haghighi et al., Citation2008; Khan et al., Citation2008; Janardhana et al., Citation2009). The effects of these feed additives are local as well as systemic since they can also stimulate immune responses in other organs via the common mucosal immune system (Cesta, Citation2006).
To illustrate the various gut-associated lymphoid structures discussed in this review, routine histological sections were made of each structure and stained with haematoxylin and eosin. All tissues were obtained from a 3-month-old Sussex hen. Since the development of MALT is very dependent upon antigen stimulation (Campos & Godson, Citation2003), the presented figures might vary slightly from literature descriptions. Furthermore, the terminology used in this paper is based on the official anatomical and histological nomenclature (King, Citation1979; McLelland, Citation1979; Payne, Citation1979; Anonymous, Citation1992, Citation2005) and may also vary from nomenclature used in other texts.
Pharyngeal tonsil
The lamina propria mucosae of the avian pharyngeal wall contains diffusely disseminated or more compactly aggregated lymphoid cells (often named lamina propria lymphocytes; Casteleyn et al., Citation2009), and sometimes a few small lymphoid follicles (<1 mm) that may comprise a germinal centre (Schummer, Citation1973; Tudor & Woodward, Citation1975; Arai et al., Citation1988). Most of the lymphoid tissue is present around the choanal and infundibular clefts just rostral to the pharyngeal papillae (Grau-Karlsbad, Citation1943; Schummer, Citation1973; King & McLelland, Citation1975; König et al., Citation2001). Although this lymphoid tissue is not well organized and is devoid of crypts, it is often designated as the pharyngeal tonsil (Grau-Karlsbad, Citation1943; Payne, Citation1979; Botte & Pelagalli, Citation1982; König et al., Citation2001). Illustrations of this pharyngeal tonsil are, however, very scarce.
The clinical importance of the avian pharyngeal tonsil lies in the fact that it is prone to the formation of microabscesses after infection. This pathological condition results in creamy-white spots that can be observed macroscopically during clinical examination of the oral and pharyngeal cavities (Tudor & Woodward, Citation1975).
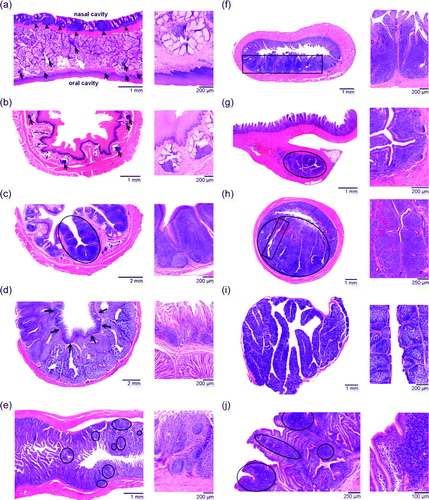
a shows disseminated and aggregated lymphoid cells present in the propria-sub mucosa just underneath the stratified squamous epithelium lining the oral side of the palate. Aggregations of lymphoid cells are also present in between the mucous glands that are located in the propria-submucosa. A lymphoid tissue consisting of aggregated lymphoid cells and lymphoid follicles is present at the nasal side of the palate. This lymphoid tissue resembles the tonsil of the ovine soft palate and could be designated nasal cavity-associated lymphoid tissue (Casteleyn et al., Citation2007, Citation2009).
Figure 2. Histological sections of the chicken GALT. For each part of the GALT, a smaller and a larger magnification are provided. 2a: Pharyngeal lymphoid tissue consisting of small aggregations of lymphoid cells (arrows). Notice the secondary lymphoid follicles at the nasal side (arrowheads). 2b: Lymphoid tissue composed of small aggregations of lymphoid cells (arrows) in the cervical part of the oesophagus. 2c: Oesophageal tonsil, of which a typical tonsillar unit is encircled. 2d: Lymphoid tissue in the lamina propria (arrows) of the proventriculus. 2e: The pyloric tonsil contains small aggregations of lymphoid cells (encircled). 2f: Peyer's patch (boxed area) present at the anti-mesenterial side of the jejunum. 2g: Lymphoid tissue in Meckel's diverticulum (encircled). 2h: The caecal tonsil lies at the medial side of the caecum (encircled) and consists of several tonsillar units (boxed area). 2i: Bursa of Fabricius containing numerous secondary lymphoid follicles. 2j: Large aggregations of lymphoid cells (encircled) are present in the lamina propria and tela submucosa of the proctodeum.
Oesophageal lymphoid tissue
The lymphoid tissue that is present in the wall of the oesophagus consists of scattered aggregations of lymphoid cells, and the oesophageal tonsil. Aggregated lymphoid cells can be present along the entire oesophagus, but they are mainly located in the cervical part of the oesophagus (Arai et al., Citation1988). Intra-epithelial monocytes and macrophages that transform the stratified squamous epithelium of the oesophagus into a lympho-epithelium have been observed by Vervelde & Jeurissen (Citation1993).
A histological image of this disseminated lymphoid tissue is presented in b. A few small aggregations of lymphoid cells can be seen in the tunica mucosa in close apposition to the mucous glands.
The oesophageal tonsil is located at the transition between the thoracic part of the oesophagus and the proventriculus (King & McLelland, Citation1975; Payne, Citation1979; Botte & Pelagalli, Citation1982; Sağsöz & Liman, Citation2009). It causes thickening of the oesophageal wall, which is far more obvious in the duck compared with the chicken (Grau-Karlsbad, Citation1943; Schummer, Citation1973). According to the number of longitudinal folds in the oesophageal wall, the tonsil is divided into several (up to eight) tonsillar units that are all located in the lamina propria mucosae between two folds (Oláh et al., Citation2003; Sağsöz & Liman, Citation2009). Consequently, each tonsillar unit surrounds a crypt (Oláh et al., Citation2003). The crypts are lined by a stratified squamous epithelium that is infiltrated by lymphocytes, macrophages and plasma cells, resulting in the formation of a lympho-epithelium that also lines the excretory ducts of the oesophageal mucous glands (Arai et al., Citation1988; Oláh et al., Citation2003, Nagy et al., Citation2005). The function of the lympho-epithelium is, however, not yet known (Oláh et al., Citation2003). The lymphoid tissue itself is composed of many large secondary B cell follicles (1 to 2 mm in diameter) separated by interfollicular regions that mainly contain T lymphocytes (Botte & Pelagalli, Citation1982; Arai et al., Citation1988; Oláh et al., Citation2003; Nagy et al., Citation2005). The presence of high endothelial venules (HEV) within these regions is suggestive of a close immunological association of the oesophageal tonsil with other lymphoid organs (Nagy et al., Citation2005). Consequently, the avian oesophageal tonsil is part of the common mucosal immune system. This statement is also supported by Arai et al. (Citation1988), who drew attention to the fact that the avian oesophageal tonsil not only produces IgA for mucosal immunity, but also IgG for systemic immunity. As a result, the avian oesophageal tonsil resembles the palatine tonsils of domestic mammals (Arai et al., Citation1988).
As the oesophageal and pharyngeal lymphoid tissues are located proximal to the stomach, these lymphoid tissues are exposed to undigested antigens (Botte & Pelagalli, Citation1982). As a result, they might be of major importance for the induction of immunity after per-oral vaccination (Oláh et al., Citation2003). However, this unique location of GALT makes the immune system also vulnerable since some pathogens, such as the infectious bursal disease virus, can colonize the pharyngeal and oesophageal tonsils very easily and can contribute to the pathogenesis of infectious bursal disease (Nagy et al., Citation2005).
A typical oesophageal tonsil of the chicken is illustrated in c. It shows two tonsillar units composed of secondary lymphoid follicles and interfollicular T cell regions that are each deeply indented by a central crypt. In these crypts, patches of lympho-epithelium alternate with the stratified squamous epithelium lining the oesophageal lumen.
Proventricular lymphoid tissue
The presence of lymphoid tissue in the avian proventriculus was described for the first time by Arai et al. (Citation1988). More recently, it was thoroughly studied by Matsumoto & Hashimoto (Citation2000) who discovered that lymphoid aggregations, mainly consisting of T lymphocytes, are present in the lamina propria mucosae just underneath the epithelium. Interestingly, a unique distribution pattern of lymphoid cells is present in the proventriculus since, in some aggregations, T lymphocytes are located in the centre and B lymphocytes at the periphery (Matsumoto & Hashimoto, Citation2000). Typical B cell follicles, however, can be found at the level of the deep proventricular glands (Jeurissen et al., Citation1989; Matsumoto & Hashimoto, Citation2000; Ogunkoya & Cook, Citation2009). Aggregations of T lymphocytes are also present at the junctions of the glandular ducts with the proventricular lumen. Additionally, the epithelium of the glandular ducts is often infiltrated by many T lymphocytes (Vervelde & Jeurissen, Citation1993; Matsumoto & Hashimoto, Citation2000). M cells are, however, not present in this lympho-epithelium (Matsumoto & Hashimoto, Citation2000).
d shows a histological section of the avian proventriculus. Large aggregations of lymphocytes and lymphoid follicles are located just underneath the epithelium.
Pyloric tonsil
Small secondary lymphoid follicles (<1 mm) and interfollicular T cell regions are present in the wall of the proximal part of the duodenum near the pyloric sphincter (Arai et al., Citation1988; Nagy & Oláh, Citation2007). When this ring of lymphoid tissue is well developed, the term pyloric tonsil can be used to designate this GALT. According to Nagy & Oláh (Citation2007) this tonsil consists of 15 to 20 tonsillar units that are encapsulated by connective tissue. The lymphoid tissue present in each unit is organized around a central crypt, which is a transformed crypt of Lieberkühn that is lined by an epithelium infiltrated mainly by T lymphocytes. Some M cells have also been observed in this lymphoid tissue (Nagy & Oláh, Citation2007).
Although an exact function has not yet been assigned to the pyloric tonsil, it could potentially compensate for the absence of mesenteric lymph nodes in birds. In mammals, these lymph nodes form a secondary barrier against the dissemination of antigens from the GALT. Together with the oesophageal tonsil, the avian pyloric tonsil controls the spread of antigens to the blood via the mesenteric lymphatics (Nagy & Oláh, Citation2007).
e shows a longitudinal section through the transition between the ventriculus and the duodenum at the level of the pyloric sphincter. The lamina propria of the proximal part of the duodenum contains some primary and secondary lymphoid follicles and interfollicular regions.
Peyer's patches
Up to six scattered Peyer's patches (lymphonoduli aggregati intestinales; Anonymous, Citation2005) are present at the anti-mesenterial side of the chicken jejunum (Grau-Karlsbad, Citation1943; Befus et al., Citation1980). These lymphoid structures are also referred to as intestinal tonsils (Grau-Karlsbad, Citation1943). One of the Peyer's patches is consistently located in the ileum, 5 to 10 cm proximal to the ileocaecal transition (Befus et al., Citation1980).
Avian Peyer's patches are very similar to those of mammals (Befus et al., Citation1980). Their lymphoid tissue is not only located in the lamina propria, but also penetrates the tela submucosa; it consists of primary and secondary lymphoid follicles that mainly contain B lymphocytes and are separated by interfollicular regions rich in T lymphocytes (Befus et al., Citation1980; Burns & Maxwell, Citation1986; Oláh et al., Citation2003). The epithelium overlying the Peyer's patches is formed by undifferentiated enterocytes and is heavily infiltrated by lymphoid cells. This lympho-epithelium is responsible for the intimate contact between the chyme and the intestinal immune system (Owen, Citation1977). Just like in mammals, M cells with small blunt apical microvilli are the major antigen sampling cells present in the avian intestinal lympho-epithelium (Burns & Maxwell, Citation1986).
Peyer's patches develop very quickly. In newborn chicks, Peyer's patches are not macroscopically visible, but lymphoid cell infiltrations are microscopically visible at some places. From the age of 10 days onwards, they can be seen macroscopically. Their volume increases significantly until the age of 3 months. Due to subsequent atrophy after 1 year, only a single Peyer's patch remains, and is located near the transition between the ileum and the caeca (Befus et al., Citation1980).
f presents a histological cross-section through the jejunum showing a large Peyer's patch at the anti-mesenterial side. The intestinal villi are thickened by the abundant lymphoid cells present within the lamina propria mucosae and the tela submucosa.
Meckel's diverticulum
Meckel's diverticulum, officially named diverticulum vitellinum, is the remnant of the yolk sac and stock that, during embryogenesis, connects the yolk sac with the intestinal lumen (Grau-Karlsbad, Citation1943; Schummer, Citation1973; King & McLelland, Citation1975; McLelland, Citation1979). It is macroscopically visible in 60% of adult chickens and pigeons as a dome-like or vermiform protrusion at the anti-mesenterial side of the jejunal wall, slightly distal to the middle region of this intestinal segment. It cannot be found consistently in adult birds, and is also absent in 20% of ducks and 10% of geese (König et al., Citation2001). In the four above-mentioned avian species, Meckel's diverticulum persists during life, whereas it disappears early in life of other birds (Schummer, Citation1973). The presence of Meckel's diverticulum was formerly used to differentiate the jejunum from the ileum. Nowadays, the ileum is defined as the terminal part of the small intestine, which is flanked by the caeca and attached to these structures by ileocaecal ligaments (König et al., Citation2001).
Immediately after hatching, Meckel's diverticulum is very large and filled with yolk (Grau-Karlsbad, Citation1943). According to Besoluk et al. (Citation2002), lymphoid tissue of Meckel's diverticulum starts to develop from 5 to 7 weeks of age in the chicken. However, small aggregations of macrophages and lymphocytes have been observed in 2-week-old broilers by Teirlynck (Citation2009). Fully mature lymphoid tissue is present from the age of 10 weeks onwards and remains active until the age of 21 months (Oláh et al., Citation1984). Granulocytes, monocytes and large numbers of plasma cells, but no erythrocytes or thrombocytes are found within Meckel's diverticulum (Oláh & Glick, Citation1984; Oláh et al., Citation1984). The function of Meckel's diverticulum is therefore double, and consists of nourishing the neonatal bird during the first days of life and acting as a myelopoietic organ in later stages.
A typical Meckel's diverticulum is presented in g. It resembles an appendix attached to the jejunal wall. In its central region, lymphoid tissue is organized into primary and secondary lymphoid follicles, and interfollicular lymphoid cell accumulations. The lymphoid tissue is located around the intestinal lumen, which in some places is lined by a lympho-epithelium.
Caecal tonsils
A large cluster of aggregated lymphoid tissue is located within the medial wall of both caeca at their transitions into the rectum (Schummer, Citation1973; King & McLelland, Citation1975; Payne, Citation1979; Botte & Pelagalli, Citation1982; Gómez del Moral et al., Citation1998; König et al., 2001). These so-called caecal tonsils are macroscopically visible in the chicken, duck and goose as wellvascularized ampullar dilatations of each caecal base (Schummer, Citation1973). The caecal tonsil is composed of several tonsillar units that consist of secondary lymphoid follicles with interspersed T cell regions located around a central fossula (Kitagawa et al., Citation1998). This fossula branches into several crypts that have direct connections with the caecal lumen (King & McLelland, Citation1975). The crypts are lined by a lympho-epithelium that contains some M cells scattered between the columnar epithelial cells (Kitagawa et al., Citation1998). The various tonsillar units are separated from their neighbouring units by septa of connective tissue (Kitagawa et al., Citation1998).
Lymphoid tissue is also located in the walls of the blind tips of the caeca (Grau-Karlsbad, Citation1943; Kitagawa et al., Citation1996). It is present as diffuse lymphoid tissue and secondary lymphoid follicles within the lamina propria mucosae and the tela submucosa. The lymphoid follicles are covered by a lympho-epithelium that is predominantly composed of columnar epithelial cells with occasional goblet cells and a few M cells (Kitagawa et al., Citation1996).
Although the caecal tonsils appear in the late embryonic stage, their major development takes place after hatching. On the first day after hatching both B and T lymphocytes are formed, but the number of T lymphocytes exceeds that of B lymphocytes. From 6 weeks onwards, the number of B lymphocytes increases significantly to exceed the number of T lymphocytes (Gómez del Moral et al., Citation1998). When fully mature, the caecal tonsils are of such a large size that they represent a major component of the avian GALT (Janardhana et al., Citation2009). As a result of their large size, the caecal tonsils are easy to investigate when immune modulation in birds is studied (Yurong et al., Citation2005; Haghighi et al., Citation2008; Janardhana et al., Citation2009).
The function of the caecal tonsils is not certain. Kitagawa et al. (Citation1998) suggest that they neutralize antigens that enter the caeca due to reflux of urates. They might also represent a bursal equivalent, since surgical extirpation or chemical destruction of the bursa of Fabricius (vide infra) does not result in a complete inability to produce antigen-specific antibodies (Lerner et al., Citation1971; Jankovic et al., Citation1976). The caecal tonsils and other GALTs, such as the cloacal lymphoid tissue, might therefore play a role in the differentiation of stem cells into B lymphocytes (Befus et al., Citation1980).
h shows a histological cross-section through one of the caeca. The lymphoid tissue of the caecal tonsils is present at the medial side of the gut. The caecal lumen and several crypts within the depth of the lymphoid tissue are visible. The crypts are surrounded by secondary lymphoid follicles with interspersed T cell regions.
Lymphoid tissue in the rectum
The avian rectum is the terminal straight part of the intestine between the ileum and the cloaca (Schummer, Citation1973; King & McLelland, Citation1975; McLelland, 1979). It is approximately 8 cm long in the chicken (Hodges, Citation1974). The lamina propria mucosae of the rectum is strongly infiltrated by lymphoid cells that are often organized into small lymphoid follicles (Schummer, Citation1973; Hodges, Citation1974). As such, it is very similar to the lymphoid tissue that is present in the wall of the proctodeum (Schummer, Citation1973) (j). This lymphoid tissue is, however, only seldom studied in birds.
Bursa of Fabricius
The bursa of Fabricius, officially called the bursa cloacalis (King, Citation1979), is a primary lymphoid organ located at the dorsal side of the proctodeum (Schummer, Citation1973). During embryogenesis, it develops initially as a protuberance on the dorsal wall of the cloaca (Schummer, Citation1973). With the formation of the bursal lumen around day 5 of embryonic development, it starts communicating with the proctodeum by means of a small stalk (collum bursae cloacalis) (Grau-Karlsbad, Citation1943; Schummer, Citation1973; Edwards et al., Citation1975; King & McLelland, Citation1975; King, Citation1979; Dolfi et al., 1988b; König et al., 2001). By day 9, the mucosal lining of the bursa forms several folds (plicae bursales) (Oláh et al., Citation1985).
Stem cells originating from the yolk sac and the liver migrate to the bursa of Fabricius from the seventh day of embryogenesis (Moore & Owen, Citation1966; Toivanen et al., Citation1972a). As a result of this process, a fully differentiated bursa colonized by numerous lymphoid cells and some stem cells is present in the 15-day-old embryo (Gasc & Stumpf, Citation1981). After hatching, the stem cells that are still present in the bursa migrate further to the bone marrow and the spleen where they will maturate into B lymphocytes (Toivanen et al., Citation1972b). It is thus clear that the whole lymphoid population of the bursa of Fabricius is derived from bloodborne stem cells (Moore & Owen, Citation1966; Le Douarin et al., Citation1975, Citation1976) and not from the bone marrow as sometimes claimed (Schummer, Citation1973; König et al., 2001).
From embryogenesis unto the age of approximately 2 months after hatching, the number of bursal lymphoid follicles increases in chickens and pigeons (Romppanen, Citation1982; Sanchez-Refusta et al., Citation1996). A fully mature bursa is present in these bird species until approximately 4 months. At this age, the bursa is 5 to 10 mm in diameter, presents over 10 longitudinal folds with smaller secondary folds, and contains up to 10,000 lymphoid follicles that are separated from each other by connective tissue (Schummer, Citation1973; Dolfi et al., Citation1988b; Bacha & Bacha, Citation2000). The bursa is ensheathed by a connective tissue capsule (Dolfi et al., Citation1988b) and is abundantly vascularized via branches of the pudendal artery and vein (Abbate et al., Citation2007). At the age of 5 months in chickens and 4 months in pigeons, bursal atrophy can be noticed (Naukkarinen & Sorvari, Citation1984; Bickford et al., Citation1985; Sanchez-Refusta et al., Citation1996). Bursal involution is faster in male chickens than in hens (Milićević et al., Citation1986). When involution is complete, at the age of 6 months, only a fibrotic residue without intact lymphoid structures remains (Naukkarinen & Sorvari, Citation1984; Bickford et al., Citation1985).
The lymphoid follicles that are not in contact with the bursal epithelium are completely encapsulated by connective tissue and contain a peripheral cortex that is separated from a central medulla by a capillary network and a basal membrane (Dasso et al., Citation2000; Nagy et al., Citation2001 Nagy et al., Citation2004). The cortex contains numerous densely packed B lymphocytes, along with some macrophages and cortical mesenchymal reticular cells (Nagy et al., Citation2004). In contrast, the medulla harbours a more heterogeneous cell population of B lymphocytes, macrophages, secretory dendritic cells and reticular epithelial cells (Nagy et al., Citation2004). The latter two cell types, however, regress immediately after hatching (Sanchez-Refusta et al., Citation1996; Nagy et al., Citation2001). The lymphoid follicles that are in direct contact with the pseudostratified columnar epithelium are described equivocally. According to Gülmez & Aslan (Citation1999) and Bacha & Bacha (Citation2000) they lack a cortex. In contrast, Nagy et al. (Citation2001) have demonstrated that a chalice-shaped cortex is present at their anti-epithelial side. However, what is important is that the medulla is directly covered with a lympho-epithelium in these follicles (Nagy et al., Citation2004). Since M cells are present in the lympho-epithelium, antigens can be transported from the lumen directly towards the medulla of the lymphoid follicles. This results in subsequent B cell proliferation and the production of antibodies that are the basis of humoral immunity (Sayegh & Ratcliffe, Citation2000). The role the bursa of Fabricius plays in humoral immunity can be demonstrated by the decreased resistance against infections after bursectomy (Schummer, Citation1973; Scott, Citation2004).
A histological view of a bursa of Fabricius is given in i. The bursa is encapsulated by connective tissue and contains many large mucosal folds. These folds are filled with groups of polyhedral lymphoid follicles, which are also encapsulated. The lymphoid follicles are characterized by a dark cortex surrounding a pale medulla.
Other lymphoid tissue in the proctodeum
Lymphoid tissues consisting of scattered and aggregated lymphoid cells are present in the dorsal wall of the proctodeum and in the stalk that connects the bursa of Fabricius with the proctodeum (Dolfi et al., Citation1988a; Budras & König, Citation2001). The presence of lymphoid tissues at this location was first described by Pintea & Rizkalla (Citation1967) by the term accessory bursa of Fabricius (bursa cloacalis accessoria). In contrast to the bursa, this region is rich in T cells (Dasso et al., Citation2000) and only contains few B cells in recently hatched birds, but the cell number will increase with age concomitant with an increase of the humoral immunity. According to Dolfi et al. (Citation1988a), the B cells are derived from the bursa of Fabricius. As such, this lymphoid tissue seems to compensate for the involution of the bursa. The lymphoid tissue of the proctodeum might also be important for local immunity as antigens present in the chyme and faeces have easy access to this lymphoid tissue (Dolfi et al., Citation1988a).
j shows heavy lymphoid cell infiltration into the lamina propria mucosae and the tela submucosa of the dorsal wall of the proctodeum. In some places, lymphoid cells are located within the epithelium that consists of tall columnar cells, resulting in a lympho-epithelium. Many tubular glands, which are modified crypts of Lieberkühn (Bacha & Bacha, Citation2000), are obvious in this part of the cloaca.
Acknowledgements
The authors thank L. De Bels and L. Standaert for their technical assistance. The scientific contribution of Dr S. Van der Heyden has also been appreciated.
References
- Abbate , F. , Pfarrer , C. , Jones , C.J.P. , Ciriaco , E. , Germanà , G. and Leiser , R. 2007 . Age-dependent changes in the pigeon bursa of Fabricius vasculature: a comparative study using light microscopy and scanning electron microscopy of vessel casts . Journal of Anatomy , 211 : 387 – 398 .
- Anonymous . 1992 . Histologia specialis . In Nomina Histologica , rev 2nd edn 13 34 . Ghent , , Belgium : International Committee on Veterinary Histological Nomenclature .
- Anonymous . 2005 . Nomina Anatomica Veterinaria , 5th edn . Hamburg : World Association of Veterinary Anatomists . Available online at http://www.wava-amav.org. (accessed 14 January 2010) .
- Arai , N. , Hashimoto , Y. , Kitagawa , H. , Kon , Y. and Kudo , N. 1988 . Immunohistochemical study on the distribution of lymphoid tissues in the upper alimentary and respiratory tracts of chickens . The Japanese Journal of Veterinary Science , 50 : 183 – 192 .
- Bacha , W.J. and Bacha , L.M. 2000 . Color Atlas of Veterinary Histology , 2nd edn , 71 London : Lippincott Williams and Wilkins .
- Barone R. 1996 . Angiologie . In R. Barone Anatomie Comparée des Mammifères Domestiques tome cinquième 687 . Paris : Editions Vigot .
- Befus , A.D. , Johnston , N. , Leslie , G.A. and Bienenstock , J. 1980 . Gut-associated lymphoid tissue in the chicken. I. Morphology, ontogeny, and some functional characteristics of Peyer's patches . Journal of Immunology , 125 : 2626 – 2632 .
- Besoluk , K. , Eken , E. , Boydak , M. and Tipirdamaz , S. 2002 . Morphological studies on Meckel's diverticulum in geese . Anatomia Histologia Embryologia , 31 : 290 – 292 .
- Bickford , A.A. , Kuney , D.R. , Zander , D.V. and McMartin , D.A. 1985 . Histologic characterization of the involuting bursa of Fabricius in single-comb white Leghorn chickens . Avian Diseases , 29 : 778 – 797 .
- Botte , V. and Pelagalli , G.V. 1982 . “ Splancnologia ” . In Anatomia Funzionale degli Uccelli Domestici , Edited by: Botte , V. and Pelagalli , G.V. 95 – 219 . Milan : Edi.ermes .
- Brandtzaeg , P. 1984 . “ Immune functions of human nasal mucosa and tonsils in health and disease ” . In Immunology of the Lung and Upper Respiratory Tract , Edited by: Bienenstock , J. 28 – 95 . New York : McGraw-Hill .
- Budras , K.-D. and König , H.E. 2001 . “ Immunsystem und lymphatische Organe (Organa lymphopoetica) ” . In Anatomie und Propädeutik des Geflügels , Edited by: König , H.E. and Liebich , H.-G. 161 – 168 . Stuttgart : Schattauer .
- Burns , R.B. and Maxwell , M.H. 1986 . Ultrastructure of Peyer's patches in the domestic fowl and turkey . Journal of Anatomy , 147 : 235 – 243 .
- Campos , M. and Godson , D.L. 2003 . The effectiveness and limitations of immune memory: understanding protective immune responses . International Journal of Parasitology , 33 : 655 – 661 .
- Casteleyn , C. , Broos , A. , Simoens , P. and Van den Broeck , W. 2009 . NALT (nasal cavity-associated lymphoid tissue) in the rabbit . Veterinary Immunology and Immunopathology , 133 : 212 – 218 .
- Casteleyn , C. , Van den Broeck , W. and Simoens , P. 2007 . Histological characteristics and stereological volume assessment of the ovine tonsils . Veterinary Immunology and Immunopathology , 120 : 124 – 135 .
- Cesta , M.F. 2006 . Normal structure, function, and histology of mucosa-associated lymphoid tissue . Toxicologic Pathology , 34 : 599 – 608 .
- Dasso , J.F. , Obiakor , H. , Bach , H. , Anderson , A.O. and Mage , R.G. 2000 . A morphological and immunohistological study of the human and rabbit appendix for comparison with the avian bursa . Developmental and Comparative Immunology , 24 : 797 – 814 .
- Dolfi , A. , Bianchi , F. and Lupetti , M. 1988a . Distribution of B-lymphocytes in the areas of bursal and cloacal lymphoid infiltration . Journal of Anatomy , 160 : 201 – 210 .
- Dolfi , A. , Lupetti , M. , Bianchi , F. and Michelucci , S. 1988b . Diffusely infiltrated lymphoid areas of the bursa of Fabricius and of the cloaca: an embryological study with morphological analogies . Journal of Anatomy , 156 : 17 – 26 .
- Edwards , J.L. , Murphy , R.C. and Cho , Y. 1975 . On the development of the lymphoid follicles of the bursa of Fabricius . The Anatomical Record , 181 : 735 – 754 .
- Gasc , J.M. and Stumpf , W.E. 1981 . The bursa of Fabricius of the chicken embryo: localization and ontogenic evolution of sex-steroid target cells . Journal of Embryology and Experimental Morphology , 63 : 225 – 231 .
- Gómez del Moral , M. , Fonfría , J. , Varas , A. , Jiménez , E. , Moreno , J. and Zapata , A.G. 1998 . Appearance and development of lymphoid cells in the chicken caecal tonsil . The Anatomical Record , 250 : 182 – 189 .
- Grau-Karlsbad , H. 1943 . “ Anatomie der Hausvögel ” . In Ellenberger-Baum—Handbuch der vergleichenden Anatomie der Haustiere 18 Auflage , Edited by: Zietzschmann , O. , Ackerknecht , E. and Grau , H. 1073 – 1124 . Berlin : Springer-Verlag .
- Gülmez , N. and Aslan , S. 1999 . Histological and histometrical investigations on bursa of Fabricius and thymus of native geese . Turkish Journal of Veterinary and Animal Sciences , 23 : 163 – 171 .
- Haghighi , H.R. , Abdul-Careem , M.F. , Dara , R.A. , Chambers , J.R. and Sharif , S. 2008 . Cytokine gene expression in chicken cecal tonsils following treatment with probiotics and Salmonella infection . Veterinary Microbiology , 126 : 225 – 233 .
- Hodges , R.D. 1974 . The Histology of the Fowl 84 180–187 . London : Academic Press .
- Janardhana , V. , Broadway , M.M. , Bruce , M.P. , Lowenthal , J.W. , Geier , M.S. , Hughes , R.J. and Bean , A.G. 2009 . Prebiotics modulate immune responses in the gut-associated lymphoid tissue of chickens . The Journal of Nutrition , 139 : 1404 – 1409 .
- Jankovic , B.D. , Isakovic , K. , Markovic , B.M. , Rajcevic , M. and Knezevic , Z. 1976 . Nonbursal origin of humoral immunity: immune capacity and cytomorphological changes in chickens bursectomized as 52- to 64-hour-old embryos . Experimental Hematology , 4 : 246 – 255 .
- Jeurissen , S.H. , Janse , E.M. , Koch , G. and De Boer , G.F. 1989 . Postnatal development of mucosa-associated lymphoid tissues in chickens . Cell and Tissue Research , 258 : 119 – 124 .
- Khan , M.Z.I. , Akter , S.H. , Islam , M.N. , Karim , M.R. , Islam , M.R. and Kon , Y. 2008 . The effects of selenium and vitamin E on the lymphocytes and immunoglobulin-containing plasma cells in the lymphoid organ and mucosa-associated lymphatic tissues of broiler chickens . Anatomia Histologia Embryologia , 37 : 52 – 59 .
- King , A.S. 1979 . “ Sytema urogenitale ” . In Nomina Anatomica Avium , Edited by: Baumel , J.J. 289 – 336 . London : Academic Press .
- King , A.S. and McLelland , J. 1975 . “ Lymphatic system ” . In Outlines of Avian Anatomy , Edited by: King , A.S. and McLelland , J. 103 – 105 . London : Baillière Tindall .
- Kitagawa , H. , Hiratsuka , Y. , Imagawa , T. and Uehara , M. 1998 . Distribution of lymphoid tissue in the caecal mucosa of chickens . Journal of Anatomy , 192 : 293 – 298 .
- Kitagawa , H. , Imagawa , T. and Uehara , M. 1996 . The apical caecal diverticulum of the chicken identified as a lymphoid organ . Journal of Anatomy , 189 : 667 – 672 .
- König , H.E. , Feder , F. and Liebich , H.-G. 2001 . “ Verdauungsapparat (Apparatus digestorius) ” . In Anatomie und Propädeutik des Geflügels , Edited by: König , H.E. and Liebich , H.-G. 81 – 104 . Stuttgart : Schattauer .
- Le Douarin , N.M. , Houssaint , E. , Jotereau , F.V. and Belo , M. 1975 . Origin of hemopoietic stem cells in embryonic bursa of Fabricius and bone marrow studied through interspecific chimeras . Proceedings of the National Academy of Sciences of the USA , 72 : 2701 – 2705 .
- Le Douarin , N.M. , Jotereau , F.V. , Houssaint , E. and Belo , M. 1976 . Ontogeny of the avian thymus and bursa of Fabricius studied in interspecific chimeras . Annals of Immunology , 127 : 849 – 856 .
- Lerner , K.G. , Glick , B. and McDuffie , F.C. 1971 . Role of the bursa of Fabricius in IgG and IgM production in the chicken: evidence for the role of a non-bursal site in the development of humoral immunity . Journal of Immunology , 107 : 493 – 503 .
- Liebler-Tenorio , E. and Pabst , R. 2006 . MALT structure and function in farm animals . Veterinary Research , 37 : 257 – 280 .
- Lillehoj , H.S. and Trout , J.M. 1996 . Avian gut-associated lymphoid tissues and intestinal immune responses to Eimeria parasites . Clinical Microbiology Reviews , 9 : 349 – 360 .
- Matsumoto , R. and Hashimoto , Y. 2000 . Distribution and developmental change of lymphoid tissues in the chicken proventriculus . The Journal of Veterinary Medical Science , 62 : 161 – 167 .
- McLelland , J. 1979 . “ Systema digestorium ” . In Nomina Anatomica Avium , Edited by: Baumel , J.J. , King , A.S. , Lucas , A.M. , Breazile , J.E. and Evans , H.E. 267 – 287 . London : Academic Press .
- Milićević , Z. , Vujić , C , Isaković , K. , Mićić , M. and Milićević , N.M. 1986 . Involution of bursa of Fabricius in male and female chickens: a light microscopic histoquantitative study . Poultry Science , 65 : 2318 – 2323 .
- Moore , M.A.S. and Owen , J.J.T. 1966 . Experimental studies on the development of the bursa of Fabricius . Developmental Biology , 14 : 40 – 51 .
- Nagy , N. and Oláh , I. 2007 . Pyloric tonsil as a novel gut-associated lymphoepithelial organ of the chicken . Journal of Anatomy , 211 : 407 – 411 .
- Nagy , N. , Igyártó , B. , Magyar , A. , Gazdag , E. , Palya , V. and Oláh , I. 2005 . Oesophageal tonsil of the chicken . Acta Veterinaria Hungarica , 53 : 173 – 188 .
- Nagy , N. , Magyar , A. , Dávid , C. , Gumati , M.K. and Oláh , I. 2001 . Development of the follicle-associated epithelium and the secretory dendritic cell in the bursa of Fabricius of the guinea fowl studied by novel monoclonal antibodies . The Anatomical Record , 262 : 279 – 292 .
- Nagy , N. , Magyar , A. , Tóth , M. and Oláh , I. 2004 . Quail as the chimeric counterpart of the chicken: morphology and ontogeny of the bursa of Fabricius . Journal of Morphology , 259 : 328 – 339 .
- Naukkarinen , A. and Sorvari , T.E. 1984 . Involution of the chicken bursa of Fabricius: a light microscopic study with special reference to transport of colloidal carbon in the involuting bursa . Journal of Leukocyte Biology , 35 : 281 – 290 .
- Ogra , P.L. 2000 . Mucosal immune response in the ear, nose and throat . The Pediatric Infectious Disease Journal , 19 : S4 – S8 .
- Ogunkoya , Y.O. and Cook , R.D. 2009 . Histomorphology of the proventriculus of three species of Australian Passerines: Lichmera indistincta, Zosterops lateralis and Poephila guttata . Anatomia Histologia Embryologia , 38 : 246 – 253 .
- Oláh , I. and Glick , B. 1984 . Meckel's diverticulum: extramedullary myelopoiesis in the yolk sac of hatched chickens . The Anatomical Record , 208 : 243 – 252 .
- Oláh , I. , Glick , B. and Taylor , R.L. 1984 . Meckel's diverticulum. II. A novel lymphoepithelial organ in the chicken . The Anatomical Record , 208 : 253 – 263 .
- Oláh , I. , Glick , B. and Törö , I . 1985 . Bursal development in normal and testosterone-treated chick embryos . Poultry Science , 65 : 574 – 588 .
- Oláh , I. , Nagy , N. , Magyar , A. and Palya , V. 2003 . Esophageal tonsil: a novel gut-associated lymphoid organ . Poultry Science , 82 : 767 – 770 .
- Owen , R.L. 1977 . Sequential uptake of horseradish peroxidase by lymphoid follicle epithelium of Peyer's patches in the normal unobstructed mouse intestine: an ultrastructural study . Gastroenterology , 72 : 440 – 451 .
- Pabst , P. 2007 . Plasticity and heterogeneity of lymphoid organs. What are the criteria to call a lymphoid organ primary, secondary or tertiary? . Immunology Letters , 112 : 1 – 8 .
- Payne , L.N. 1979 . “ Systema lymphaticum et splen ” . In Nomina Anatomica Avium , Edited by: Baumel , J.J. , King , A.S. , Lucas , A.M. , Breazile , J.E. and Evans , H.E. 409 – 415 . London : Academic Press .
- Pintea , V. and Rizkalla , W. 1967 . Lympho-epithelial and glomic structures in the upper wall of the cloaca in the hen . Acta Veterinaria Academiae Scientiarum Hungaricae , 17 : 249 – 255 .
- Romppanen , T. 1982 . Postembryonic development of the chicken bursa of Fabricius: a light microscopic histoquantitative study . Poultry Science , 61 : 2261 – 2270 .
- Sağsöz , H. and Liman , N. 2009 . Structure of the oesophagus and morphometric, histochemical-immunohistochemical profiles of the oesophageal gland during the post-hatching period of Japanese quails (Coturnix coturnix japonica) . Anatomia Histologia Embryologia , 38 : 330 – 340 .
- Sanchez-Refusta , F. , Ciriaco , E. , Germanà , A. , Germanà , G. and Vega , J.A. 1996 . Age-related changes in the medullary reticular epithelial cells of the pigeon bursa of Fabricius . The Anatomical Record , 246 : 473 – 480 .
- Sayegh , C.E. and Ratcliffe , M.J.H. 2000 . Perinatal deletion of B cells expressing surface Ig molecules that lack V(D)J-encoded determinants in the bursa of Fabricius is not due to intrafollicular competition . Journal of Immunology , 164 : 5041 – 5048 .
- Schummer , A. 1973 . “ Lymphgefäßsystem ” . In Lehrbuch der Anatomie der Haustiere Band V: Anatomie der Hausvögel , Edited by: Nickel , R. , Schummer , A. and Seiferle , E. 105 – 109 . Berlin : Verlag Paul Parey .
- Scott , T.R. 2004 . Our current understanding of humoral immunity of poultry . Poultry Science , 83 : 574 – 579 .
- Teirlynck , E. 2009 . Transformation of the yolk sac to the Meckel's diverticle in broiler chicks: vascularisation and histological aspects (p. 36) . Master's thesis, Laboratory Animal Science, Faculty of Veterinary Medicine, Ghent University .
- Toivanen , P. , Toivanen , A. and Good , R.A. 1972a . Ontogeny of bursal function in chicken. I. Embryonic stem cell for humoral immunity . Journal of Immunology , 109 : 1058 – 1070 .
- Toivanen , P. , Toivanen , A. , Linna , T.J. and Good , R.A. 1972b . Ontogeny of bursal function in chicken. II. Postembryonic stem cell for humoral immunity . Journal of Immunology , 109 : 1071 – 1080 .
- Tudor , D.C. and Woodward , H.L. 1975 . Tonsil abscesses of pigeons . Poultry Science , 54 : 412 – 414 .
- Vervelde , L. and Jeurissen , S.H. 1993 . Postnatal development of intra-epithelial leukocytes in the chicken digestive tract: phenotypical characterization in situ . Cell and Tissue Research , 274 : 295 – 301 .
- Yurong , Y. , Ruiping , S. , Shimin , Z. and Yibao , J. 2005 . Effects of probiotics on intestinal mucosal immunity and ultrastructure of caecal tonsils of chickens . Archives of Animal Nutrition , 59 : 237 – 246 .