Abstract
Reverse transcription-polymerase chain reaction (RT-PCR) was used to generate sequence data for recent Taiwanese strains of Newcastle disease virus (NDV) isolated from 1999 to 2003, covering the full length of the haemagglutinin-neuraminidase (HN) gene and protein. Nucleotide sequence analysis of the HN gene of these recent isolates revealed that the whole HN gene carries an open reading frame encoding 571 amino acids and possesses a shorter C-terminal extension. Six amino acid substitutions in epitopes on the HN glycoprotein of the recent Taiwanese NDV isolates were also found. All the recent Taiwanese NDV isolates have the amino acid sequence 112RRQKRF117 for the F protein. A phylogenetic tree analysis based on the nucleotide sequences of the F gene revealed that all recent Taiwanese isolates were related to genotype VII viruses. Since the recent Taiwanese NDV isolates exhibited a low level of haemagglutination (HA) activity, we generated two sets of mutants to elucidate whether mutations in the heptad repeat region of the HN protein could affect the HA activity. To demonstrate the presence of the viruses used in the HA test, a real-time RT-PCR was established to determine the copy number of NDV isolates. From sequence analysis, site-directed mutagenesis, and haemadsorption assays, it was found that the HN glycoprotein of recent Taiwanese NDV isolates carrying a substitution at the amino acid residue 81 (I to M) in the heptad repeat region in the stalk domain showed a dramatic decrease in the activity of HA. We infer from these results that a specific amino acid sequence within the heptad repeat region of the stalk is important for the HA activity of the HN glycoprotein.
Introduction
Newcastle disease is a devastating worldwide disease of poultry characterized by increased respiration, haemorrhagic enteritis, circulatory disturbances, and nervous signs. It is caused by Newcastle disease virus (NDV), belonging to the Avulavirus genus of the Paramyxoviridae family, and contains a single-stranded negative sense RNA of approximately 15 kb. The genome encodes for six major proteins: haemagglutinin-neuraminidase (HN) protein, fusion (F) protein, nucleoprotein, matrix protein, phosphoprotein, and RNA-dependent RNA polymerase (Millar et al., 1986). On the basis of pathogenicity, NDVs have been categorized into velogenic, mesogenic, and lentogenic pathotypes (Hodder et al., Citation1993; Seal et al., Citation1995). The F protein mediates the fusion of viral and cellular membranes during penetration and spread between infected and adjacent cells. The viral HN protein mediates attachment to sialic acid-containing receptor(s) and, via its neuraminidase (NA) activity, the apparently opposing activity of release of sialic acid from soluble and membrane-associated glycoconjugates (Scheid & Choppin, Citation1973). HN is a type II membrane glycoprotein, existing on the surface of virions and infected cells as a tetrameric spike (Ng et al., Citation1989; Collins & Mottet, Citation1991; Mirza et al., Citation1993). The ectodomain of the HN glycoprotein consists of a membrane-proximal, stalk-like segment supporting a terminal globular domain. The antigenic, receptor recognition, and NA active sites all reside in the latter. Previous studies suggested that there are at least five antigenic sites related to epitopes on the HN protein of NDV, including residues 193 to 201 (site 23), residues 345 to 355 (sites 1 and 14) and a C-terminal domain composed of residues 494, 513 to 521, and 569 (sites 12 and 2) (Iorio et al., Citation1991). The region of amino acid residues 341 to 355 of the HN glycoprotein was also defined as a linear epitope, and residues 352 to 355 are required for antibody recognition (Iorio et al., Citation1991).
Several outbreaks were reported in Taiwan in 1968, 1984, 1995, 1999, and 2003 following the first report in 1935. Although an extensive vaccination programme against Newcastle disease had been employed, recent outbreaks still caused damage to the poultry industry throughout Taiwan. These Taiwanese isolates were mainly classified into genotypes III, VI, and VII based on phylogenetic analysis of the F gene cleavage site (Ke et al., Citation2001, Ke et al., Citation2010). The recent Taiwanese NDV strains showed low cross-protection with vaccine strains based on neutralization tests and were also distinct from all vaccine strains with R values <70% (Lin et al., Citation2003).
In the present study, it was found that recent Taiwanese NDV isolates have lower haemagglutinin activity than published strains. We therefore explored the effect of substituting amino acid residue 81 (I to M) within the heptad repeat region A (HRA) of the stalk of the HN glycoprotein by site-directed mutagenesis on its activity. It was demonstrated that recent Taiwanese isolates carry a substitution at amino acid residue 81 (I to M) in the heptad repeat regions of the stalk of HN protein, which resulted in a marked reduction in activity. All Taiwanese NDV strains isolated from 1999 to 2003 were phylogenetically related to genotype VII viruses and had the same C-terminal extensions (571 amino acid residues) of HN glycoprotein.
Materials and Methods
Virus isolation
The Taiwanese NDV isolates TW/99-154, TW/99-156B, TW/99-158, TW/99-165, TW/99-167, TW/99-172, TW/99-173, TW/99-174, TW/99-175, and TW/99-178 were isolated in 1999, while isolates TW/02-301, TW/02-302, TW/02-303, TW/03-328, TW/03-329, TW/03-330, TW/03-332, and TW/03-333 were isolated in 2003. The recent NDV isolates and past Taiwanese isolates (TW84-1211 and TW/84-486) were further characterized in the present study (). The Newcastle disease-suspected field samples were collected and propagated in 9-day-old embryonated specific-pathogen free fowls' eggs (Alexander, Citation1989). Haemagglutination (HA) tests (Alexander, Citation1989) and reverse transcription-polymerase chain reaction (RT-PCR), were applied to aid in the identification of NDV isolates. Pathotyping of the isolates initially involved virus inoculation of 10-day-old embryonated specific-pathogen-free fowls' eggs to determine the mean death time and the intracerebral pathogenicity index.
Table 1. Characterization of NDV strains used in the present study.
Viral RNA preparation and RT-PCR
The NDV RNA was extracted directly from allantoic fluid using a viral RNA purification kit according to the manufacturer's instructions (Qiagen Co., Valencia, California, USA). The primers for RT-PCR and real-time quantitative PCR were designed by comparing multiple sequences of NDV F and HN genes using the DNASTAR software package (DNASTAR Inc., Madison, Wisconsin, USA). The conserved regions in the F and HN genes among the NDV isolates were selected for primer design. The primers were synthesized by MD Inc. (Taipei, Taiwan). A primer set of F47 and F2 for amplification of F gene has been reported by our laboratory (Ke et al., Citation2001). A portion of the F gene was amplified using the primers F47 (5′-ATGGG (C/T)CCAGA (C/T)CTTCTAC-3′; identical to nucleotides 4550 to 4569) and F2 (5′-CTGCCACTGCTAGTTGTGATAATCC-3′; complementary to nucleotides 5084 to 5060). A primer set of KH HNFu1 and KH HNFu2 was chosen on the basis of the NDV ZJ1/00 strain (GenBank accession number AF431744). The complete HN gene of each isolate was amplified using the primers KH HNFu1 (5′-TTCTATCACATCAC CACAACAAG-3′; identical to nucleotides 6386 to 6409) and KH HNFu2 (5′-GTGGG CGGGACTCAGAATAATCAT-3′; complementary to nucleotides 8307 to 8248) and used to amplify a fragment of 1922 base pairs (bp). In the RT-PCR test, 1 µg RNA was denatured in boiling water for 10 min, chilled on ice for 5 min, and used as a template. The RT-PCR reactions were carried out according to procedures provided by Perkin Elmer Co. (Branchburg, New Jersey, USA). Briefly, the RT-PCR reactions were performed in 50 µl containing 1x EZ buffer, 2.5 mM manganese acetate solution, 300 µM dNTPs, rTth DNA polymerase (5 units), 5 µM primer pair (KH HNFu1 and KH HNFu2), and 1 µg RNA. Reverse transcription was carried out at 50°C for 30 min. PCR reactions were subjected to 35 cycles consisting of denaturation for 1 min at 94°C, annealing for 1 min at 55°C, and extension for 2 min at 72°C and one final extension cycle at 72°C for 7 min.
After completion of the PCR, 5 µl reaction mixture was loaded onto a 1.5% (w/v) agarose gel, containing 0.5 µg/ml ethidium bromide, for electrophoresis and subsequent visualization by ultraviolet transillumination. Cycle sequencing was performed using the purified PCR products with the ABI Prism Ready Reaction Dideoxy Terminator cycle sequencing kit (Model 3730 version3.4; Applied Biosystems, Foster City, California, USA).
Real-time RT-PCR
A set of primers was designed according to the sequences of HN-encoding gene of NDV strain ZJ1/00 (Gen-Bank accession number AF431744) using the LC probe design software (Roche Molecular Biochemicals, Mannheim, Germany). The primer sequences were as follows: forward primer KeHN3 (5′-GCACTCGGA TACCCT CATTTGAC-3′; identical to nucleotides 6932 to 6954) and the reverse primer KeHN4 (5′-CCTTAGAGCACAGCATATCACAAC-3′; complementary to nucleotides 7177 to 7154). The amplified cDNA fragment using the primer pair KeHN3/ KeHN4 was expected to be 246 bp in length. PCR was performed in a Light Cycler (Roche Molecular Biochemicals). Each reaction was carried out in a 20 µl reaction of 1 µl purified RNA and 19 µl reaction mixtures, which were 0.4 units AMV reverse transcriptase, 0.2 units RNase inhibitor, 2 M MgCl2, 1 unit FastStart DNA Master Mix SYBR Green I (containing Taq DNA polymerase, SYBR Green I, and deoxynucleoside triphosphate mix), and 0.5 µM primers (forward and reverse). Reverse transcription was carried at 50°C for 10 min. PCR reaction mixtures were subjected to 10 min of 95°C hot-start enzyme activation, and 45 cycles of 95°C denaturation for 3 sec, 54°C annealing for 3 sec and 72°C elongation for 12 sec. To avoid cross-contamination and sample carryover, pre-PCR and post-PCR sample processing were performed in separate rooms. All fluid transfers were carried out with plugged pipette tips to eliminate aerosols. A negative control (diethylpyrocarbonote-treated water) and some avian pathogens—which included paramyxovirus APMV-2, infectious bronchitis virus, infectious bursal disease virus, avian influenza virus, avian reovirus, and Mycoplasma synoviae—were used to test the specificity of this technique. For analysis of the melting curves, the Light Cycler instrument's software automatically converts them into melting peaks. The melting temprature (Tm) values of the peaks were analysed using the best-fit analysis software provided by Roche Molecular Biochemicals.
A series of 10-fold dilutions were made with concentrations ranging from 101 to 108 copies per reaction. Water was used as diluent. To generate a standard curve, the threshold cycle (C t) of these standard dilutions was plotted against the number of plasmid copies used as input. The precision of real-time LC RT-PCR for detecting NDV was expressed by a coefficient of variation. The confidence interval level was set at 95% (P<0.05). The inter-assay and intra-assay reproducibility was obtained by employing 10 different experiments performed in 10 replicates of each run for all serial dilutions tested. Inter-assay variation was determined by performing real-time PCR on 10 consecutive days. Ten assays were used to determine the mean, standard deviation and coefficient of variation. The copy number was calculated using the following formula (Ke et al., Citation2006):
Cloning of PCR products
To better understand the degree of genetic variation and the phylogenetic relationship of NDV isolates, purified PCR products derived from the F and HN genes were subcloned into the SmaI site of deposphorylated plasmid pUC18. The recombinant plasmids were used to transform the DH5 strain of Escherichia coli competent cells. DNA minipreps using an alkaline lysis method were performed on white colonies suspected of containing an insert (blue/white selection). Colonies with the correct sizes were further cultured for preparation of recombinant plasmids. Recombinant plasmids were then purified using a purification kit (Qiagen Co. ) and sequenced with an Automated Laser Fluoresence DNA Sequencer (TAKARA Biotechnology Inc., Dalian, China).
Sequence and phylogenetic analysis of F and HN genes of NDV isolates
Nucleotide and deduced amino acid sequences of the F and HN genes of NDV were aligned using a J. Hein method with PAM250 residue weight table of DNASTAR software (DNASTAR Inc.). The deduced amino acid sequences of the HN protein among NDV isolates were aligned, corresponding to complete HN protein amino acid residues 1 to 571, 577, 580 or 616.
Nucleotide sequences from different genes or portions of the NDV genome were used to construct a phylogenetic tree for either epidemiological study or pathology prediction. In the present study, the F gene sequences corresponding to amino acid residues 1 to 125 were used to create a phylogenetic tree and to analyse genotypes of recent Taiwanese NDV isolates.
Site-directed mutagenesis and transfection of HN and mutated HN genes in Cos-7 cells
Site-directed mutagenesis was performed as described previously (Deng et al., Citation1994). Briefly, mutagenesis primers KH-HNm1 (5′-CAAGTCAAGAC GTGATAG ATAGGATATATA-3′; 6649 to 6673), KH-HNm (3′-CAAGTCAAGACGT GATGGA TAGGATATAT A-3′; 6649 to 6673), and KH-HNm2 (5′-ATCACGTCTTGA CTTGA ACTGAGTAAAGTA-3′; 6659 to 6630) were designed basing on the sequences of the NDV TW/02-302 strain (GeneBank accession number EU526303). The mutated HN genes were obtained and annealed to construct two HN mutants, TW/02-302 HN M81I and TW/84-1211 HN I81M.
To understand whether the amino acid residue at position 81 in the heptad repeat region (HRA) of the stalk domain is important for the HA of the HN glycoprotein, Cos-7 cells were transfected with mutant HN and HN genes, or its parental plasmid, pcDN3.1, without the HN gene, using a mediated transfection method. HN protein expression was confirmed by indirect immunofluorescent antibody using anti-NDV polyclonal antibodies. For validation of the test of haemadsorption activity, each set of transfected-cells from 10 fields of view was counted to determine the GFP expression rate 24 h after transfection. The expression of HN protein was also confirmed by western blot assay using anti-NDV polyclonal antibodies. The relative level of HN protein was normalized for actin.
Haemadsorption and NA assay
The haemadsorption activity of HN proteins was determined at 4°C by the ability of the expressed protein to adsorb chicken red blood cells. The HN-expressing monolayers were incubated for 30 min with a 2% suspension of red blood cells in phosphate-buffered saline supplemented with 1% (w/v) CaCl2 and MgCl2. After extensive washing, adsorbed red blood cells were lysed in 50 mM NH4Cl and the lysate was clarified by centrifugation. Activity was quantitated by measurement of the absorbance at 540 nm minus the background obtained with cells expressing the vector alone. In addition, NA was assayed as described elsewhere (Morrison & McGinnes, Citation1989). Values for NA activities were taken from three independent experiments.
Statistical analysis
All data were analysed using an independent-sample t-test and are expressed as averages of three independent experiments. P<0.05 was considered significant.
Results
Sequence and phylogenetic analysis of F and HN genes of recent Taiwanese NDV isolates
The deduced amino acid sequences of F and HN proteins of recent Taiwanese isolates were analysed and compared with published sequences. Based on the F gene genotype, all recent Taiwanese NDV isolates were phylogenetically related to genotype VII ( and ). Studies comparing the deduced amino acid sequences of the F0 precursor of recent Taiwanese NDV isolates showed that viruses that are virulent for chickens had the amino acid sequences 112R-R-Q-K-R116 at the C-terminus of the F2 protein and F (phenylalamine) at residue 117, the N-terminus of the F1 protein (). These viruses have an intracerebral pathogenicity index in 1-day-old chicks of 1.73 or greater, and a mean death time in 10-day-old embryonated specific-pathogen free fowls' eggs of about 44 to 48 h (). All of the data indicate that they are velogenic strains.
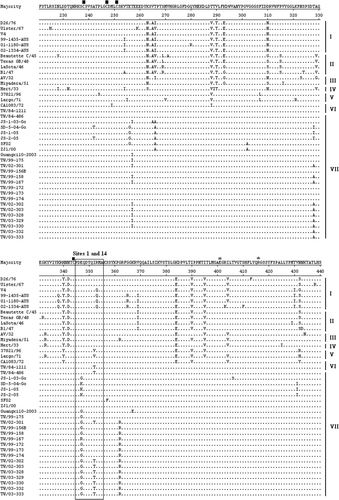
Figure 1. HN amino acid sequences were deduced from the HN nucleotide sequence of NDV. Alignment of HN amino acid sequences of recent Taiwanese isolates and previously published virus strains was performed using the DNASTAR software. Amino acid residues of HN protein of different NDV strains are shown in a single-letter code. Residues that are identical to the majority are indicated by a dot (.). The HRA and HRB (amino acid residues 74 to 110) and C-terminal extension are indicated above the amino acid sequences. Variation at amino acid residue 81 in HRA is indicated above amino acid sequences. A total of 13 cysteine residues in the HN glycoprotein of NDV strains, at residues 123, 172, 186, 196, 238, 247, 251, 344, 455, 461, 465, 531, and 542, are marked by ▪ above the amino acid sequences. Three domains related to epitopes on HN protein of NDV, including site 23, sites 1 and 14, a C-terminal domain composed of residues 494, 513 to 521, and sites 12 and 2, are indicated by boxes. Three key amino acid residues at positions 401 (E), 416 (R), and 526 (Y) of HN glycoprotein required for receptor binding are indicated by * above the amino acid sequences. Phylogenetic lineages created on the basis the F gene of NDV are indicated on the left-hand side. The majority indicates the common HN sequences of NDV.
Examination of the amino acid sequences of the HN glycoprotein of NDV isolates revealed that there was a total of 13 cysteine residues conserved in HN glycoprotein of recent Taiwanese NDV strains, at amino acid residues 123, 172, 186, 196, 238, 247, 251, 344, 455, 461, 465, 531, and 542 (). Three amino acid residues at positions 401 (E), 416 (R), and 526 (Y) of the HN glycoprotein that were reported to be key residues for receptor binding (Connaris et al., Citation2002) were also conserved in all recent Taiwanese and previously published NDV isolates (). Examination of the antigenic sites on the HN glycoprotein of NDV isolates revealed that there was a total of six amino acid residue substitutions at positions 197 (R to K), 200 (S to L), 2003 (H to Y), 347 (E to G), 352 (R to T), and 521 (S to N) (). These mutations may affect the antigenicity of this protein. It is interesting to note that there was only one mutation at HRA of HN. Most of the recent Taiwanese isolates possessed amino acid residue 81M in the HRA of the stalk of HN ().
By comparing the amino acid residues of the C-terminal HN extension of NDV, they were classified into at least four groups, including amino acid residues of 571, 577, 580 and 616 ( and ). This grouping seemed not to be related to genotype typing based on the nucleotide sequences of the F or HN gene. Inter-relationships, especially between Taiwanese NDV isolates, appear to be associated with lineages having the same C-terminal HN extensions (571 amino acid residues) ( and ). Comparison of sequences at the cleavage site of the F protein and the C-terminal extension of the HN protein indicated that HN gene relationships revealed by phylogenetic analyses were also maintained in comparisons between the F gene cleavage sites ().
Table 2. HA titre and amino acid sequences at residue 81 in HRA of NDV strains.
Real-time RT-PCR
To determine the copy number of different NDV strains used in the HA test, a real-time PCR was established. In the real-time PCR assay, NDV genomic RNA diluted serially 101 to 108 copies/µl was detected. PCR products (246 bp) were separated on a 1.5% agarose gel stained with ethidium bromide (a). A standard curve (b) was created with 10-fold dilutions of NDV genomic DNA (101 to 108 genome copies) to quantify NDV. Linear regression of the Ct values and the quantity of RNA revealed a good negative linearity (r=–1, error=0.127, slope=–3.520, and intercept=37.73). The standard curve showed good correlation between copy number and Ct values. The melting points of PCR products from different NDV isolates within run tests are shown in .
Figure 2. 2a: Real-time RT-PCR fluorescence curve derived from serially diluted NDV genomic RNA. Following amplification, the real-time RT-PCR products were analyzed by agarose gel electrophoresis. 2b: Standard curve of real-time PCR. Serially diluted NDV genomic RNA was amplified and analyzed in real time. The threshold cycle (Ct) values were plotted against the copy number to construct the standard curve, r=–1.0. The NDV RNA copy number was determined spectrophotometrically.
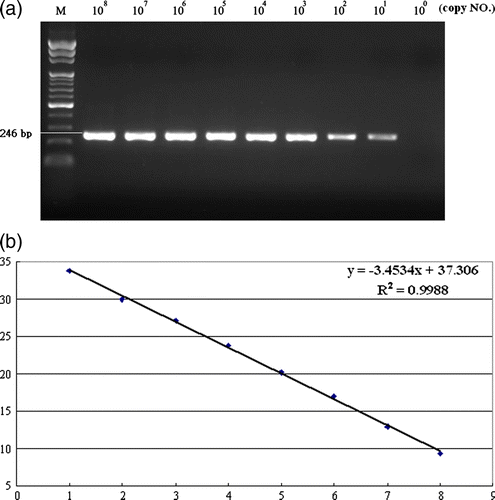
In this report, we found that the HN of recent NDV isolates carrying a substitution at amino acid residue 81M had lower HA titres than that of NDV HN containing 81V or 81I (). To ensure the presence of 81M-HN mutated NDV used in HA test, real-time RT-PCR was established and carried out to determine the copy number of each virus. According to the results of melting curve analysis, NDV strains were in the range of 84.4 to 85.6 (). The specificity of the real-time LC RT-PCR was 100% since negative control or some avian viruses showed no detectable fluorescent signals. The PCR products amplified from all tested NDV isolates were 246 bp in length as expected (data not shown). However, the PCR products were sequenced to confirm the specificity of the assay. No cross-reactions were found with non-NDV isolates. The assays were extremely reproducible with coefficient of variation ranging between 1.4% and 0.6%. In this study, the copy number of NDV isolates used in HA tests was obtained using real-time PCR ().
81M-mutated NDV HN isolates exhibited diminished haemadsorption
Since recent Taiwanese NDV isolates exhibited low levels of HA (), it was of interest to determine whether a substitution at amino acid residue 81 (I to M) in the heptad repeat regions A of the stalk would affect the level of HA. HA is caused by the adsorption of the virus to specific receptors on red blood cells to form a lattice network between the cells (Kimball, Citation1990). Two mutants, TW/02-302 HNM81I and TW/84-1211 HNI81M, were therefore generated to elucidate the mechanism by which the heptad repeat domain of the HN protein may contribute to HA. The HN and mutated HN genes were transfected into Cos-7 cells. The expression of HN and mutant HN proteins in cells was detected by western blot assay (data not shown). HA activities of 81I-mutated and 81M-mutated HN proteins were determined by a haemadsorption assay. Chicken red blood cells were bound to the surface of Cos-7 cells expressing either M81I-mutated or I81M-mutated HN proteins. In cells expressing I81M-mutated HN, there was a decrease in the level of red blood cell binding, while M81I-mutated HN protein bound at greater levels than the I81M HN mutant (). In cells expressing vector alone (pcDNA3.1), virtually no red blood cell binding was observed. Indeed, I81M-mutated HN exhibited a significant reduction in haemadsorption activity. M81I-mutated HN had greater HA activity than that of I81M-mutated HN protein (). In addition, these mutations had little or no effect on the NA activities of the M81I-mutated and I81M-mutated HN glycoprotein.
Figure 3. Haemadsorption activity of HN mutants was determined at 4°C by the ability of the expressed protein to adsorb chicken red blood cells. Two mutants, including TW/02-302 HN M81I and TW/84-1211 HN I81M created from NDV TW/02-302 HN M81 and TW/84-1211 HN I81 strains, are shown. The HN mutants (TW/02-302 HN M81I and TW/84-1211 HN I81M) showed significantly increased or reduced haemadsorption activity (P<0.05) when compared with TW/02-302 HN M81 and TW/84-1211 HN I81 strains. Haemadsorption activity was quantitated by measurement of the absorbance at 540 nm minus the background obtained with cells expressing the vector alone. The results shown represent the means of three independent experiments. Error bars represent the mean±standard deviation.
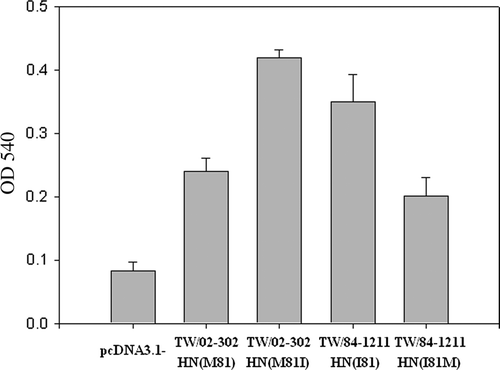
HA activities of the mutant proteins varied greatly, with I81M having only 50% of wild-type HA activity (). These data further elucidated that recent Taiwanese NDV isolates carrying the 81M-HN protein have lower HA titres than NDV strains with 81V-HN or 81I-HN proteins.
Discussion
Substitution of residue M81 in the heptad repeat region of the stalk domain of the HN glycoprotein resulted in a marked reduction in haemagglutinin activity. A heptad repeat is a structural motif in which a hydrophobic amino acid is repeated every seven (heptad) residues; such motifs are designated by the letters ‘a’ to ‘g’ (Lupas, Citation1996). Heptad repeats, which contain hydrophobic or neutral residues in the ‘a’ and ‘d’ positions of the repeat, can form alpha helices and are able to interact with other heptad repeats by forming coiled coils (Chambers et al., Citation1990), which are involved in protein–protein interactions . The hydrophobic ‘a’ position amino acids were the first residues chosen for mutagenesis because the ‘a’ positions of heptad repeats region in the stalk domain of the HN glycoprotein are often important for mediating protein–protein interactions. Thus, mutations in the heptad repeats region in the stalk domain of the HN glycoprotein may cause a more deleterious effect on HA than mutations in other positions of the helices. Indeed, we found that altering the protein in the ‘a’ position (81M) negatively affected its HA ability. Recent Taiwanese isolates with an amino acid change (81M) at the heptad repeat region in the stalk domain of the HN glycoprotein had lower HA than those without the change.
In the present study, mutagenesis analysis of the HN protein demonstrated that mutation of residue 81M within the heptad repeat region abolished the HA activity and further explained why recent Taiwanese isolates showed only low levels of HA activity. This study provides first evidence that a specific amino acid residue change within the heptad repeated region in the stalk domain affects the HA of the HN glycoprotein. This suggests that quantification of NDV based on HA tests may not be accurate, and that real-time PCR may be a better approach to determine the copy number of NDV and to demonstrate the presence of NDV .
The genes encoding the HN proteins of some NDVs, especially avirulent or low-virulence isolates, have extended reading frames in comparison with the corresponding genes of reference virulent isolates (Millar et al., Citation1986; McGinnes et al., Citation1987; Sato et al., Citation1987; Gotoh et al., Citation1988). This feature has been used to group isolates of NDV on the basis of the length of this extension. In the present report, we show that the recent virulent Taiwanese isolates have HN proteins consisting of only 571 amino acid residues, while the avirulent strain V4 has an HN protein of 616 amino acid residues (Simmons, Citation1967).
Previous studies suggested that monoclonal antibodies to three overlapping antigenic sites (12, 2, and 23) on HN glycoprotein suppressed the NA activity of the virus (Iorio et al., Citation1986, Citation1989), and monoclonal antibodies to two sites (1 and 14) inhibited HA and caused virus neutralization predominantly by preventing viral attachment to chicken cells (Iorio & Bratt, Citation1984; Iorio et al., Citation1986). The region of amino acid residues 341 to 355 in antigenic sites (1 and 14) of the HN glycoprotein was also defined as a linear epitope, and residues 352 to 355 are required for antibody recognition (Iorio et al., Citation1991). In the present study, six amino acid residue substitutions at positions 97, 200, 2003, 347, 352, and 521 in the HN glycoprotein of recent Taiwanese NDV isolates occurred in the antigenic sites and may lead to antigenic change and alter the characteristic of the NDV HN glycoprotein.
It was also found that all of the HN glycoproteins of recent Taiwanese NDV strains contain 13 cysteine residues, which are well conserved among the strains; except for cysteine 123, which is replaced by tryptophan in some strains. Previous reports indicated that six intra-molecular disulphide bonds and one inter-molecular disulphide bond at cysteine 123 form a covalent homodimer. Although this cysteine residue is not conserved in all NDV strains, the protein still forms a dimer and tetramer, suggesting that the inter-molecular disulphide bond only stabilizes the oligomeric structures (McGinnes & Morrison, Citation1994) and may not be involved in the function of HN.
In the present study, we provided evidence that a point mutation at M81 in the heptad repeat region in the stalk domain of the HN glycoprotein of recent Taiwanese strains can drastically diminish the HA activity of the virus. These data further elucidate why recent Taiwanese isolates exhibited low HA activity as compared with other NDV reference strains that carry residue I81or V81 in the heptad repeat region in the stalk domain of the HN glycoprotein.
Acknowledgements
This research was supported by a research grant from the Council of Agriculture (97AS-14.2.1-BQ-B1), Taiwan.
References
- Alexander , D.J. 1989 . “ Newcastle disease ” . In A Laboratory Manual for the Isolation and Identification of Avian Pathogens , 3rd edn , Edited by: Purchase , H.G. , Arp , L.H. , Domermuth , C.H. and Pearson , J.E. 114 – 120 . Kennett Square , PA : American Association of Avian Pathologists .
- Chambers , P. , Pringle , C.R. and Easton , A.J. 1990 . Heptad repeat sequences are located adjacent to hydrophobic regions in several types of virus fusion glycoproteins . Journal of General Virology , 71 : 3075 – 3080 .
- Collins , P.L. and Mottet , G. 1991 . Homooligomerization of the hemagglutinin-neuraminidase glycoprotein of human parainfluenza virus type 3 occurs before the acquisition of correct intramolecular disulfide bonds and mature immunoreactivity . Journal of Virology , 65 : 2362 – 2371 .
- Connaris , H. , Takimoto , T. , Russell , R. , Crennell , S. , Moustafa , I. , Portner , A. and Taylor , G. 2002 . Probing the sialic acid binding site of the hemagglutinin-neuraminidase of Newcastle disease virus: identification of key amino acids involved in cell binding, catalysis, and fusion . Journal of Virology , 76 : 1816 – 1824 .
- Deng , R. , Wang , Z. , Glickman , R.L. and Iorio , R.M. 1994 . Glycosylation within an antigenic site on the HN glycoprotein of Newcastle disease virus interferes with its role in the promotion of membrane fusion . Virology , 204 : 17 – 26 .
- Gotoh , B. , Sakaguchi , T. , Nishikawa , K. , Inocencio , N.M. , Hamaguchi , M. , Togoda , T. and Nagi , Y. 1988 . Structural features unique to each of the three antigenic sites on hemagglutinin-neuraminidase protein of Newcastle disease virus . Virology , 163 : 174 – 182 .
- Hodder , A. , Selleck , P. , White , J. and Gorman , J. 1993 . Analysis of pathotype-specific structural features and cleavage activation of Newcastle disease virus membrane glycoproteins using antipeptide antibodies . Journal of General Virology , 74 : 1081 – 1091 .
- Iorio , R.M. and Bratt , M.A. 1984 . Monoclonal antibodies as functional probes of the HN glycoprotein of Newcastle disease virus. Antigenic separation of the hemagglutinating and neuraminidase sites . Journal of Immunology , 133 : 2215 – 2219 .
- Iorio , R.M. , Borgman , R.L. , Glickman , R.L. , Riel , A.M. and Bratt , M.A. 1986 . Genetic variation within a neutralizing domain on the haemagglutinin-neuraminidase glycoprotein of Newcastle disease virus . Journal of General Virology , 67 : 1393 – 1403 .
- Iorio , R.M. , Glickman , R.L. , Riel , A.M. , Sheehau , J.P. and Bratt , M.A. 1989 . Functional and neutralization profile of seven overlaping antigenic sites on the the HN glycoprotein of Newcastle disease virus: monoclonal antibodies to some sites prevent viral attachment . Virus Research , 13 : 245 – 262 .
- Iorio , R.M. , Syddall , R.J. , Sheehan , J.P. , Bratt , M.A. , Glickman , R.L. and Riel , A.M. 1991 . Neutralization map of the hemagglutinin-neuraminidase glycoprotein of Newcastle disease virus: domains recognized by monoclonal antibodies that prevent receptor recognition . Journal of Virology , 65 : 4999 – 5006 .
- Ke , G.M. , Chang , H.L. , Ke , L.Y. , Ji , W.T. , Chulu , J.L.C. , Chang , T.J. and Liu , H.J. 2006 . Development of a quantitative light cycler real-time RT-PCR for detection of avian reovirus . Journal of Virological Methods , 133 ( 1 ) : 6 – 13 .
- Ke , G.M. , Liu , H.J. , Lin , M.Y. , Chen , J.H. , Tsai , S.S. and Chang , P.C. 2001 . Molecular characterization of Newcastle disease viruses isolated from recent outbreaks in Taiwan . Journal of Virological Methods , 79 : 1 – 11 .
- Ke , G.M. , Yu , S.W. , Ho , C.H. , Chu , P.Y. , Ke , L.Y. , Lin , K.H. , Lin , M.Y. Liu , H.J. 2010 . Characterization of newly emerging Newcastle disease viruses isolated during 2002–2008 in Taiwan . Virus Research , 147 : 247 – 257 .
- Kimball , J.W. 1990 . Introduction to Immunology , 3rd edn , 42 – 46 . New York : Macmillan Publishing Company .
- Lin , M.Y. , Liu , H.J. and Ke , G.M. 2003 . Genetic and antigenic analysis of Newcastle disease viruses from recent outbreak in Taiwan . Avian Pathology , 32 : 345 – 350 .
- Lupas , A. 1996 . Coiled coils: new structures and new functions . Trends in Biochemical Science , 21 : 375 – 382 .
- McGinnes , L.W. and Morrison , T.G. 1994 . The role of the individual cysteine residues in the formation of the mature, antigenic HN protein of Newcastle disease virus . Virology , 200 : 470 – 483 .
- McGinnes , L.W. , Wilde , A. and Morrison , T.G. 1987 . Nucleotide sequence of the gene encoding the Newcastle disease virus hemagglutinin-neuraminidase protein and comparison of other paramyxovirus hemagglutinin-neuraminidase sequences . Virus Research , 7 : 187 – 202 .
- Millar , N.S. , Chambers , P. and Emmerson , P.T. 1986 . Nucleotide sequence analysis of the hemagglutinin-neuraminidase gene of Newcastle disease virus . Journal of General Virology , 67 : 1917 – 1927 .
- Mirza , A.M. , Sheehan , J.P. , Hardy , L.W. , Glickman , R.L. and Iorio , R.M. 1993 . Structure and function of a membrane anchor-less form of the hemagglutinin-neuraminidase glycoprotein of Newcastle disease virus . Journal of Biological Chemistry , 258 : 21425 – 21431 .
- Morrison , T.G. and McGinnes , L.W. 1989 . Avian cells expressing the Newcastle disease virus HN protein are resistant to NDV infection . Virology , 171 : 10 – 17 .
- Ng , D.T.W. , Randall , R.E. and Lamb , R.A. 1989 . Intracellular maturation and transport of the SV5 type II glycoprotein hemagglutinin-neuraminidase: specific and transient association with GRP78-Bip in the endoplasmic reticulum and extensive internalization from the cell surface . Journal of Cell Biology , 109 : 3273 – 3289 .
- Sato , H. , Hattori , S. , Ishida , N. , Imamara , Y. and Kawakita , M. 1987 . Nucleotide sequence of the hemagglutinin-neuraminidase strain D26: evidence for a longer coding region with a carboxyl terminal extension as compared to virulent strains . Virus Research , 8 : 217 – 232 .
- Scheid , A. and Choppin , P.W. 1973 . Isolation and purification of the envelope proteins of Newcastle disease virus . Journal of Virology , 11 : 263 – 271 .
- Seal , B.S. , King , D.J. and Bennett , J.D. 1995 . Characterization of Newcastle disease virus isolated by reverse transcription PCR coupled to direct nucleotide sequencing and development of sequence database for pathotype prediction and molecular analysis . Journal of Clinical Microbiology , 33 : 2624 – 2630 .
- Simmons , G.C. 1967 . The isolation of Newcastle disease in Queensland . Australian Veterinary Journal , 43 : 29 – 30 .