Abstract
A study was conducted to determine the cytochrome (CYP) P450 enzymes responsible for the bioactivation of aflatoxin B1 (AFB1) into its epoxide form (AFBO) in duck liver microsomes. Six male and six female 6-week-old Pekin ducks were used. The biochemical toxicology strategies applied included the use of selective inhibitors, prototype substrate activity for specific human P450s, correlation between aflatoxin bioactivation and enzymatic activity of prototype substrates, and the expression of specific CYP450 enzymes using antibodies against human CYP450s. Enzymatic activity was detected for the duck orthologues CYP1A1/2, CYP2A6 and CYP3A4 but not for the CYP2D6 orthologue. Immunoreactive proteins for CYP1A1, CYP2A6 and CYP3A4 were also detected. Inhibition studies suggested that the duck turkey CYP2A6 orthologue and, to a lesser extent, the CYP1A1 orthologue are involved in the bioactivation of AFB1. Correlation studies, however, suggest that CYP3A4, CYP2A6 and CYP1A1/2 are all involved in AFBO formation. The finding that four CYP enzymes may be involved in AFB1 bioactivation in ducks could explain the high sensitivity of this species to AFB1. Further studies are needed to fully elucidate the phase I hepatic metabolism of AFB1 in ducks, the only poultry species that develops hepatic cancer from AFB1 exposure.
Introduction
Mycotoxins are secondary metabolites produced by toxigenic strains of different species of fungi and are of toxicological concern because they can affect human health and livestock and poultry productivity. Hundreds of potentially toxic secondary metabolites can be produced by filamentous fungi. The toxigenic genera Aspergillus, Penicillium and Fusarium have been shown to produce more than 300 mycotoxins (Bünger et al., Citation2004).
Aflatoxin B1 (AFB1) is one of the most important mycotoxins due to its hepatotoxic and carcinogenic effects in animals and humans (McLean & Dutton, Citation1995; Wild & Turner, Citation2002). Aspergillus flavus and Aspergillus parasiticus are the most common fungi responsible for its production (Leeson et al., Citation1995). AFB1 is bioactivated by hepatic cytochrome P450 enzymes (CYP450) to a highly reactive electrophilic metabolite known as aflatoxin-8,9-exo-epoxide (AFBO). This unstable metabolite reacts with cellular macromolecules such as proteins causing cytotoxicity and DNA causing genotoxicity (Doi et al., Citation2002). AFBO is known to bind to guanine residues in nucleic acids leading to DNA mutations and liver cancer in humans, primates and ducks (Eaton & Gallagher, Citation1994; Verma, Citation2004; Do & Choi, Citation2007).
The presence of specific CYP450 enzymes capable of biotransforming AFB1 to AFBO associated with poor conjugation with glutathione is considered to be an important factor in the sensitivity of a species to AFB1. In humans, CYP1A2, CYP2A6 and CYP3A4 have been identified as the enzymes responsible for the bioactivation of AFB1 into the AFBO (Guengerich et al., Citation1996, Citation1998; Gallagher et al. Citation1996). In poultry, adverse health effects due to aflatoxins have been reported in turkeys (Diaz et al., Citation2009), quails (Oliveira et al., Citation2002), chickens (Kadian et al., Citation1988) and ducks (Barraud et al., Citation1999). Among domestic fowl, ducks are the most sensitive species to the acute effects of aflatoxins with a median lethal dose for ducklings of 0.34 to 0.56 mg/kg body weight compared with 6.5 to 16.5 for chicks (Leeson et al., Citation1995). Ducks are also the only poultry species that develop hepatic tumours from chronic or sub-chronic aflatoxin exposure, an effect that is also seen in rats, primates and humans (Leeson et al., Citation1995). Although the AFB1 bioactivation to AFBO is relatively well understood in mammals, studies in poultry are still needed. We previously reported differences in the rate of biotransformation of AFB1 into the epoxide form in commercial poultry species including ducks, chickens, turkeys and quails (Lozano & Diaz, Citation2006). The highly sensitive duck produced twice as much AFBO compared with the less sensitive chicken. However, no studies were conducted to determine the specific CYP enzymes responsible for this bioactivation reaction. Furthermore, no information is available regarding which CYPs are responsible for the metabolism of AFB1 in ducks.
Chemical inhibitors have been used to define catalytic specificity of CYP450 enzymes. Most of the early generation inhibitors (e.g. SKF 525A, metyrapone) are not highly selective, but more recently developed inhibitors have shown considerable enzyme selectivity (Halpert et al., Citation1994). A major advantage of using selective P450 inhibitors is that the fractional inhibition of a reaction in microsomes or another crude preparation indicates the extent to which a specific P450 is responsible for the metabolic reaction being studied (Halpert et al., Citation1994). It is important to note, however, that human P450 inhibitors do not necessarily exhibit the same selectivity when used with microsomes obtained from another species (Eagling et al., Citation1998). Thus caution needs to be taken when interpreting results obtained in animal studies.
The aim of the present study was to further investigate the phase I metabolism of AFB1 in the most sensitive poultry species, the duck. The main objective was to identify the specific hepatic CYP450s responsible for the bioactivation of AFB1 into its epoxide form. The selective inhibitors of CYP1A1/2, CYP1A2, CYP2A6, CYP2D6 and CYP3A4 were used as probes and AFBO production was correlated with prototype substrate activities. In addition, the expression of specific CYP450 enzymes in duck liver microsomes was investigated using an immunoblotting technique.
Materials and Methods
Reagents
AFB1, AFB2α, TRIS, Tween 20, glucose 6-phosphate sodium salt, glucose 6-phosphate dehydrogenase, ethylenediaminetetraacetic acid (EDTA), bicinchoninic acid, copper sulphate, sucrose, glycine, NADP sodium salt hydrate, glycerol, bovine serum albumin, methoxyresorufin, α-naphthoflavone, 8-methoxypsoralen, oleandomycin triacetate (troleandomycin) and 7-hydroxycoumarin were purchased from Sigma Chemical Co (St Louis, Missouri, USA). Sodium chloride and magnesium chloride hexahydrate were from Mallinckrodt Baker (Phillipsburg, New Jersey, USA). Sodium dihydrogen phosphate monohydrate and di-sodium hydrogen phosphate anhydrous were from Merck (Darmstadt, Germany). Furafylline, nifedipine, oxidized nifedipine, and purified human CYP1A1, CYP1A2, CYP2A6 and CYP3A4 enzymes were purchased from BD-Biosciences (San Jose, California, USA). Debrisoquine sulphate, 4-hydroxydebrisoquine sulphate, coumarin, ethoxyresorufin and resorufin sodium salt were purchased from MP Biomedicals (Solon, Ohio, USA). Primary antibodies (rabbit anti-human CYP450) against CYP1A1, CYP1A2, CYP2A6 and CYP3A4 were purchased from AbCam (Cambridge, Massachusetts, USA). Low molecular range protein standards, immunoblot polyvinylidene fluoride (PVDF) membranes (7×8.4 cm), filter paper (7.5×10 cm), 30% acrylamide/bisacrylamide solution (29:1), ammonium persulphate, TEMED, bromophenol blue, Opti-4CN goat anti-rabbit detection kit, and sodium dodecyl sulphate (SDS) were purchased from BioRad (Hercules, California, USA). Methanol, acetonitrile, water and other solvents used in preparing mobile phases were all high-performance liquid chromatography (HPLC) grade.
Liver samples
All experiments were carried out at 4°C. Twelve healthy 6-week old Pekin ducks (six males and six females) were humanely sacrificed and their livers extracted immediately. The livers were immediately washed with cold phosphate-buffered saline (PBS) buffer (20 mM phosphates, pH 7.4, 100 mM NaCl) and stored at −70°C until processing.
Microsome extraction
Frozen liver was thawed and approximately 2.5 g was carefully minced and homogenized with 10 ml cold PBS buffer (20 mM phosphate buffer, pH 7.4, with 1 mM EDTA and 250 mM sucrose) for 30 sec using a tissue homogenizer (IKA Ultra-Turrax, Staufen, Germany). The homogenate was then centrifuged at 9000×g for 30 min at 4°C. The supernatant (approximately 12 ml) was collected and transferred to ultracentrifuge tubes kept at 4°C and centrifuged for 90 min at 100,000×g. Supernatants (cytosolic fraction) were collected for future studies and the resulting pellet was resuspended in 3 ml storage buffer (20 mM phosphate buffer, pH 7.4, 1 mM EDTA, 250 mM sucrose and 20% glycerol) and aliquoted in microcentrifuge tubes. Aliquots were stored at −70°C until use. An aliquot was used to determine the protein content by the bicinchoninic acid protein quantification method according to Redinbaugh & Turley (Citation1986).
Microsomal incubations
In vitro microsomal incubations were carried out in 1.5 ml microcentrifuge tubes at 39°C. Incubations contained 5 mM glucose 6-phosphate, 0.5 mM NADP+, 0.5 IU glucose 6-phosphate dehydrogenase, 2 µl (128 µM) AFB1 or of the different substrates or inhibitors (all dissolved in dimethylsulphoxide (DMSO)), 50 µg microsomal protein and incubation buffer (50 mM phosphate buffer, pH 7.4, 5 mM MgCl and 0.5 mM EDTA) to a final volume of 250 µl. The final DMSO concentration in the incubations never exceeded 1% except for troleandomycin and furafylline inhibitors where the DMSO concentration was 2% due to their low solubility in the aqueous incubation buffer. Reactions were stopped after 10 min of incubation with 250 µl ice cold acetonitrile (Blanchard, Citation1981) and centrifuged at 12,000×g for 10 min. Depending on the specific detector response of the substrate and/or product from the incubation, dilutions of the supernatant were made before injection into the HPLC as described below.
Enzymatic activity of selected CYP450 prototype substrates
The following prototype enzymatic activities were determined using HPLC: 7-ethoxyresorufin-O-deethylase (EROD) for CYP1A1/2, 7-methoxyresorufin-O-deethylase (MROD) for CYP1A2, coumarin 7-hydroxylase for CYP2A6, debrisoquinone 4-hydroxylase for CYP2D6, and nifedipine oxidase for CYP3A4. The amount of product formed by each enzymatic activity was quantified by HPLC using a Shimadzu Prominence System (Shimadzu Scientific Instruments, Columbia, Maryland, USA) equipped with a DGU-20A3 Degasser, an LC-20AB pump, a SIL-20A HT autosampler, a CTO-20A column oven, an SPD-20AV UV–vis detector, an RF-10AXL fluorescence detector, and a CBM-20A bus module, all controlled by “LC Solutions” software. All separations were carried out with an Alltech Alltima HP C18, 5 µm column, 150 mm×3.0 mm (Alltech Associates Inc., Deerfield, Illinois, USA).
Chromatographic conditions
Determination of 7-ethoxyresorufin and its product resorufin (EROD activity) was carried out at room temperature, at a flow rate of 0.3 ml/min, using an isocratic mobile phase consisting of 30% phosphate buffer (20 mM phosphate, pH 7.4) and 70% methanol. The analytes were monitored using fluorescence detection at excitation and emission wavelengths of 530 nm and 580 nm, respectively (Leclercq et al., Citation1996). The incubation supernatant was diluted 1:10 with water, and 5 µl dilute sample was injected into the chromatograph. The same conditions were used for the determination of 7-methoxyresorufin and its product resorufin (MROD activity), except that only 2 µl dilute sample was injected into the chromatograph. Determination of coumarin and its hydroxylated metabolite 7-hydroxycoumarin (CYP2A6 activity) was carried out at room temperature, at a flow rate of 0.4 ml/min, using an isocratic mobile phase consisting of 30% acetonitrile and 70% water. 7-Hydroxycoumarin was monitored using fluorescence detection at excitation and emission wavelengths of 325 nm and 452 nm, respectively, while coumarin was monitored using UV detection at 325 nm (Fink & Koehler, Citation1970). Supernatants were diluted 1:100 with water and 5 µl dilute sample were injected into the chromatograph. Debrisoquine and 4-hydroxydebrisoquine were determined at room temperature, at a flow rate of 0.3 ml/min, using an isocratic mobile phase consisting of 30% methanol, 70% water and 0.1% formic acid. Analytes were monitored by fluorescence detection at excitation and emission wavelengths of 210 nm and 290 nm, respectively (Pereira et al., Citation2000; Granvil et al., Citation2002), and 5 µl supernantants were analysed by HPLC without further dilution. Determination of nifedipine and its metabolite oxidized nifedipine (CYP3A4 activity) was carried out at room temperature, at a flow rate of 0.5 ml/min using an isocratic mobile phase consisting of 32% acetonitrile and 68% water. The analytes were monitored by UV detection at 270 nm (Shim et al., Citation1988) and 10 µl supernatant was injected directly into the chromatograph without further dilution. Each enzymatic activity was correlated with the production of AFBO by each individual sample. AFB1 and AFBO determination was carried out at 40°C, at a flow rate of 0.35 ml/min. The AFBO production was monitored as the AFB1–dhd adduct, which was quantitated using AFB2a as standard (given the similar spectral properties of AFB1-dhd and AFB2α), as described by Lozano & Diaz (Citation2006). The compounds were separated using a linear gradient of A (water–0.1% formic acid) and B (methanol–0.1% formic acid) as follows: 0 min, 30% B; 2 min, 30% B; 7 min, 55% B; and 7.01 min, 30% B. The two analytes were monitored by fluorescence detection at excitation and emission wavelengths of 365 nm and 425 nm, respectively (Gallagher et al., Citation1996; Lozano & Diaz, Citation2006). A 1:100 dilution was made, from which 2 µl was injected into the chromatograph.
Determination of enzymatic kinetic parameters
In order to estimate the apparent K M and V max of the selected CYP450 orthologues, four of the five prototype substrates specific for human CYP orthologues were selected. Debrisoquine 4-hydroxylase activity was excluded from these experiments because no activity was detected in the samples analysed. Each substrate was tested in three randomly selected microsome samples from both male and female ducks in duplicate. For EROD activity (CYP 1A1/2), concentrations ranging from 4.8 to 0.3 µM 7-ethoxyresorufin were used; for MROD activity (CYP1A2), concentrations from 5.88 to 0.36 µM 7-metoxyresorufin were used; for 7-coumarin hydroxylase activity (CYP 2A6), concentrations from 80 to 5 µM coumarin were used; and for nifedipine oxidation (CYP3A4), concentrations from 71.0 to 4.4 µM nifedipine were used. To investigate AFB1 epoxidation activity, concentrations from 256 to 16 µM AFB1 were used.
Inhibition studies
Four different inhibitors were tested using the same incubation conditions described above at a constant concentration of 128 µM AFB1 and variable concentrations of the inhibitor. α-Naphthoflavone and 8-methoxypsoralen were dissolved in 1 µl DMSO while furafylline and troleandomycin were dissolved in 4 µl DMSO and added at the following concentration ranges: α-naphthoflavone (for CYP1A1/2) from 22.95 to 1.43 µM, furafylline (for CYPIA2) from 384.2 to 24 µM, 8-methoxypsoralen (for CYP2A6) from 92.5 to 2.8 µM, and troleandomycin (for CYP3A4) from 122.8 to 7.6 µM. Each inhibitor was tested in duplicate in three male and three female microsome samples.
Immunoblot (western blot)
The immunoblot determination of the P450 orthologues was conducted in microsomes kept in storage buffer from all birds used in the experiment (n=12) using 100 µg microsomal protein for CYP1A1, CYP1A2 and CYP2A6, and 30 µg for CYP3A4. The samples were preheated at 92°C for 5 min with 5 µl sample buffer (Tris–HCl 7 mM, pH 6.8, containing 1.5% SDS, 20% glycerol, 5% β-mercaptoethanol and 0.02% bromophenol blue) and run in a SDS-PAGE gel (T = 10%, C = 3.3%) (Laemmli, Citation1970) at 150 V constant voltage in a Tris–glycine running buffer (Tris 25 mM, glycine 192 mM, pH 8.8) for 80 min. Proteins were then transferred to a PVDF membrane at a constant voltage of 100 V for 2.5 h in Dunn–Carbonate buffer (NaHCO3 10 mM, Na2CO3 3 mM, pH 9.9). After transfer, the membrane was washed three times with PBS–Tween 0.1% (5 min each wash) and blocked with PBS–Tween–5% powdered milk for 1 h at room temperature and constant shaking. After washing, the membrane was incubated with the primary antibody (rabbit anti-CYP450) prepared at a dilution of 1:1000 in PBS–Tween–5% powdered milk overnight at 4°C with constant shaking. Finally, the PVDF membrane was washed three times with PBS–Tween 0.1% (5 min each wash) and incubated in secondary antibody (goat anti-rabbit horseradish peroxidase) prepared in a 1:2000 dilution in PBS–Tween–5% powdered milk for 1 h at room temperature and washed three times with PBS–Tween 0.1% (5 min each wash). Detection was carried out with the Opti-4CN goat anti-rabbit detection kit until band colours were visible and the reaction was stopped by washing the membrane repeatedly with MilliQ water. Quantification of the bands was done using a BioRad Gel Doc XR system supplied with the BioRad Quantity One 1-D analysis software version 4.6.3.
Statistical analysis
The enzymatic parameters K M and V max were determined by non-linear regression using the Marquardt method adjusting the data to Michaelis–Menten enzyme kinetics using the equation: v=V max[S]/K M+[S], where v is the enzyme reaction velocity, [S] represents substrate concentration, V max represents maximal velocity and K M represents the Michaelis–Menten constant (Marangoni, Citation2003; Bisswanger, Citation2008). Non-linear regression fitting was accomplished with the use of the following weighting function: ω = 1/y i α, where y i is the velocity for each substrate concentration and α the magnitude of the relationship between residuals and variance (Kakkar et al., Citation1999; Marangoni, Citation2003). The P450-mediated production of AFBO in the presence of different concentrations of each inhibitor was expressed as mean percentage of inhibition of the corresponding control±standard deviation, for each inhibitor concentration. Pearson correlation coefficients were calculated to determine the correlation between the activity of the P450 orthologues and the production of AFBO. In all cases, normality, homoscedasticity and independence of residuals were verified. Calculations were performed using the Statistical Analysis System software (SAS Institute, Inc., Citation2008).
Results
Kinetic parameters using model substrates
No debrisoquinone 4-hydroxylase activity (CYP2D6) was detected in any of the liver microsome samples analysed. However, enzymatic activity for the other four model substrates evaluated was detected in all samples. summarizes the results of the enzymatic kinetic parameters for 7-ethoxyresorufin O-deethylation, methoxyresorufin O-deethylation, coumarin 7-hydroxylation, and nifedipine oxidation. No significant (P<0.05) differences in the enzymatic activities were observed between sexes and the data from both male and female ducks were pooled. In the case of AFB1 epoxidation, the apparent V max values were not different between sexes (2.75 vs. 2.89 nmol/mg/min, respectively) but there was a significant difference (P<0.05) in the apparent K M values, which corresponded to 44.4 µM and 25.0 µM for males and females, respectively.
Table 1. Enzymatic parameters for selected P450 enzymatic activities in duck liver microsomesa.
Inhibition studies
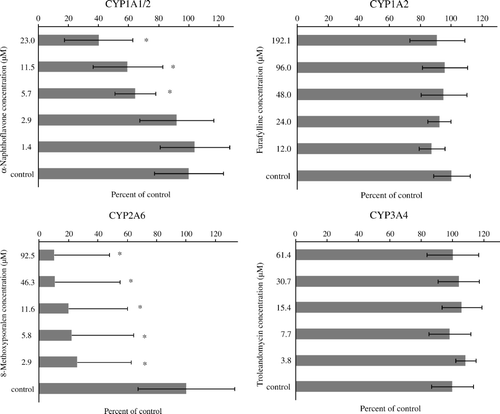
and show the effect of the four chemical inhibitors tested on P450-mediated AFBO production. 8-MOP (CYP2A6 inhibitor) and, to a lesser extent, α-naphthoflavone (CYP1A1/2 inhibitor) affected AFBO production, but no inhibition was seen for the CYP1A2 or CYP3A4 inhibitors (). The percentage inhibition caused by 8-MOP was significant (P <0.05) at all concentrations of inhibitor tested, whereas only the highest concentrations of α-naphthoflavone tested (≥5.7 µM) caused a significant inhibition on AFBO production (). α-Naphthoflavone inhibits both CYP1A1 and CYP1A2 activities (Halpert et al., Citation1994; Klaassen, Citation2008), whereas furafylline is a strong mechanism-based inhibitor of CYP1A2 but not of CYP1A1 in humans and rats (Sesardic et al., Citation1990; Kunze & Trager, Citation1993). AFBO formation was significantly decreased by α-naphthoflavone but not by furafylline, suggesting that the inhibition observed with α-naphthoflavone was due to the effect of the inhibitor on CYP1A1, the enzyme not sensitive to furafylline.
Figure 1. Effect of selected chemical inhibitors on cytochrome P450-mediated AFB1 epoxide production in duck liver microsomes. Each graph corresponds to six individual birds per experiment (three males and three females). Each graph header indicates the enzymatic activity affected by the inhibitor.
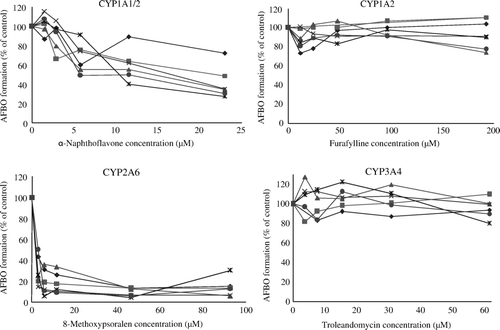
Figure 2. Effect of selected chemical inhibitors on cytochrome P450-mediated AFB1 epoxide production in duck liver microsomes. Each bar corresponds to the mean±standard deviation of six observations per experiment (three males and three females). Each graph header indicates the enzymatic activity affected by the inhibitor. *Significantly different (P < 0.05) from control.
Correlation of model substrate activity and aflatoxin B1 epoxidation
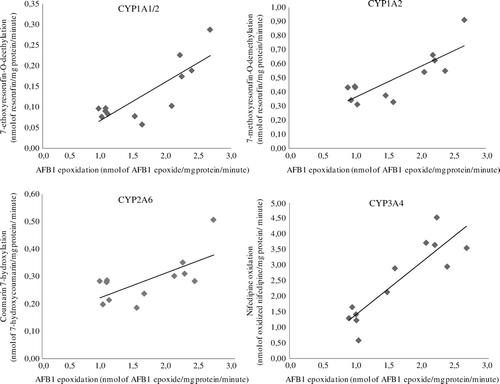
shows the relationship between AFBO formation and model substrate biotransformation. A significant positive linear relationship was observed for all activities tested but the correlation was highest for nifedipine oxidation, corresponding to the putative activity of the CYP3A4 orthologue. The calculated Pearson correlation coefficients for AFB1 epoxidation and EROD, MROD, coumarin 7-hydroxylation and nifedipine oxidation activities were 0.82, 0.82, 0.67 and 0.88, respectively. A very high correlation was observed between EROD and MROD activities (0.95).
Figure 3. AFB1 epoxidation versus model substrate enzymatic activity in duck liver microsomes (n = 12). The enzyme associated with the model enzymatic activity indicated in the y-axis is shown on top of each graph. A positive significant relationship (P < 0.05) was observed for all enzymatic activities tested.
Immunoblot
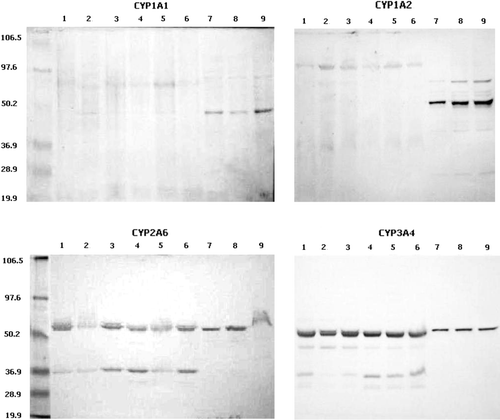
shows the protein blots for the duck CYP1A1, CYP1A2, CYP2A6 and CYP3A4 orthologues. Microsomal proteins reacting with polyclonal antibodies against human CYP2A6 and CYP3A4 were detected in all samples tested. CYP1A1 immunoreactive proteins were detected only in a few samples and no CYP1A2 was detected in the samples. It is important to note, however, that the absence of immunoreactive bands could be related to the low sensitivity of the detection technique (colorimetric) and the usually low expression of these proteins in all animal species (Klaassen, Citation2008) rather than to the actual absence of these proteins.
Figure 4. Protein blot of selected duck CYP450 orthologues. Lanes 1 to 6, bands obtained with six different samples of duck microsomes; lanes 7 to 9, microsomes containing cDNA-expressed human CYP450 enzymes as indicated in each graph. Molecular weight standards are shown with their corresponding molecular weight value.
Discussion
Demonstration or confirmation of a given P450 enzymatic activity is the first step to investigate the role of an enzyme in a specific reaction. The investigation on the apparent K M and V max parameters showed the presence of enzymatic activity towards four of the five model substrates tested and suggested the existence of duck P450 orthologues of the mammalian CYP1A1/2, CYP2A6 and CYP3A4 enzymes (). The high correlation coefficients (>92%) shown in , which were accompanied by low values for standard error (<20%, data not shown), indicate the presence of enzymes that have a strong adjustment to Michaelis–Menten enzyme kinetics. The evidence of specific enzymatic activity was supported by the finding of immunoreactive proteins for CYP1A1, CYP2A6 and CYP3A4. Our findings support previous studies where CYP1A1/2 and CYP3A4 activities were reported in ducks. Dealkylation of substituted alkylresorufins (CYP1A1/2 activity) has been reported in Mallard ducks by Riviere (Citation1992), whereas Kennedy et al. (Citation1996) reported basal EROD (CYP1A1/2) activity in Campbell and Pekin ducks. Raynal et al. (Citation2001) reported EROD, MROD (CYP1A2), and ethylmorphine and erythromycin N-demethylase enzymatic activities (CYP3A4) in Mallard ducks. However, no CYP2A6 activity has been reported previously in ducks.
With regard to AFB1 biotransformation, the present study suggests that CYP2A6 and CYP3A4 and, to a lesser extent, the CYP1A1/2 orthologues play a role in the bioactivation of AFB1 into its epoxide form. Evidence is provided both through the use of specific inhibitors and by correlation studies between model enzymatic activities and AFB1 activation. The strong inhibition of AFBO formation caused by 8-MOP and the high correlation between nifedipine and AFB1 oxidation (r=0.88) supports the notion that CYP2A6 and CYP3A4 are the most important hepatic enzymes responsible for the bioactivation of AFB1 in the duck. The positive, highly significant correlation between AFBO formation and EROD and MROD activities (0.82 in both cases) also suggests a role for CYP1A activity in AFB1 activation. Our results are consistent with the role of the human orthologues CYP2A6, CYP3A4 and CYP1A2 as activators of AFB1 (Omiecinski et al., Citation1999), but there are no previous reports on AFB1 activation by CYP1A1. CYP1A1, however, has been reported to be an activator of other carcinogens such as benzo[a]pyrene. One of the reactions catalysed by CYP1A1 is the formation of an epoxide group similar to the one formed in the AFB1 metabolite, AFBO. Substrates reported to be preferentially epoxidated by CYP1A1 include polycyclic aromatic hydrocarbons, co-planar polyhalogenated biphenols, dibenzofurans and dioxins (Klaassen, Citation2008). In relation to the role of CYP3A4 in AFBO formation, it is important to note that even though a strong positive relationship was observed between AFB1 oxidation and nifedipine oxidation, AFBO formation was not inhibited by troleandomycin, a known inhibitor of the human CYP3A4 (Omiecinski et al., Citation1999). A possible explanation for this apparent inconsistency may be that the duck CYP3A4 orthologue does not exhibit the same sensitivity to specific inhibitors as the human orthologue. Klein et al. (Citation2000) showed inhibition of AFBO formation in turkey liver microsomes using 17α-ethynylestradiol. It is possible that the CYP3A4 duck orthologue might be sensitive to 17α-ethynylestradiol, as is the turkey orthologue, but more studies are needed to verify this hypothesis. Unfortunately we were unable to obtain 17α-ethynylestradiol due to commercial restrictions.
Raynal et al. (Citation2001) reported that fumonisin B1 exposure in ducks significantly increases the hepatic activities of the CYP1A1/2 and CYP3A4 orthologues, without affecting growth. This finding might be very significant in the light of our present findings because the induction of these enzymatic activities by fumonisin B1 may result in more bioactivation of AFB1 and a greater toxicological response in ducks fed diets containing both mycotoxins. Both fumonisin B1 and AFB1 are common co-contaminants of maize in many parts of the world (Leeson et al., Citation1995).
Finally, the finding that female ducks have a significantly lower K M for AFB1 oxidation compared with males suggest that females could be more sensitive to AFB1 than males. Lower K M values indicate a higher affinity for the substrate and potentially more AFB1 bioactivation. However, more studies are needed to determine whether female ducks exhibit greater sensitivity than males in vivo.
In summary, the results of the present study suggest that the duck CYP2A6 and 3A4 orthologues, and to a lesser extent the CYP1A1/2 orthologues, are involved in the bioactivation of AFB1 into AFBO. The finding that four different CYPs can bioactivate AFB1 in a single species could potentially explain the high sensitivity of ducks to this mycotoxin. In other species, CYP1A2 produces aflatoxin M1 and CYP3A4 aflatoxin Q1 (Leeson et al., Citation1995), metabolites much less toxic than the epoxide. More studies are needed to further clarify the role of each of these enzymes in the biotransformation of AFB1 in ducks, a species of particular interest due to its high sensitivity to both acute and chronic AFB1 toxicity.
Acknowledgements
Funding was provided by the International Foundation for Science, Stockholm, Sweden (Grant No. B-3094).
References
- Barraud , L. , Guerret , S. , Chevallier , M. , Borel , C. , Jamard , C. , Trepo , C. , et al. 1999 . Enhanced duck hepatitis B virus gene expression following aflatoxin B1 exposure . Hepatology , 29 , 1317 1323 .
- Bisswanger , H. 2008 . Enzyme Kinetics. Principles and Methods , 2nd edn , Weinheim : Wiley-VCH Verlag GmbH & Co .
- Blanchard , J. 1981 . Evaluation of the relative efficacy of various techniques for deproteinizing plasma samples prior to high-performance liquid chromatography analysis . Journal of Chromatography , 226 : 455 – 460 .
- Bünger , J. , Westphal , G. , Mönnich , A. , Hinnendahl , B. , Hallier , E. and Müller , M. 2004 . Cytotoxicity of occupationally and environmentally relevant mycotoxins . Toxicology , 202 : 199 – 211 .
- Diaz , G.J. , Cortés , A. and Botero , L. 2009 . Evaluation of the ability of a feed additive to ameliorate the adverse effects of aflatoxins in turkey poults . British Poultry Science , 50 : 240 – 250 .
- Do , J.H. and Choi , D. 2007 . Aflatoxins: detection, toxicity, and biosynthesis . Biotechnology and Bioprocess Engineering , 12 : 585 – 593 .
- Doi , A.M. , Patterson , P.E. and Gallagher , E.P. 2002 . Variability in aflatoxin B1–macromolecular binding and relationship to biotransformation enzyme expression in human prenatal and adult liver . Toxicology and Applied Pharmacology , 181 : 48 – 59 .
- Eagling , V.A. , Tjia , J.F. and Back , D.J. 1998 . Differential selectivity of cytochrome P450 inhibitors against probe substrates in human and rat liver microsomes . British Journal of Clinical Pharmacology , 45 : 107 – 114 .
- Eaton , D.L. and Gallagher , E.P. 1994 . Mechanisms of aflatoxin carcinogenesis . Annual Review of Pharmacology and Toxicology , 34 : 135 – 172 .
- Fink , D.W. and Koehler , W.R. 1970 . pH effects on fluorescence of umbelliferone . Analytical Chemistry , 42 : 990 – 993 .
- Gallagher , E. , Kunze , K.L. , Stapleton , P.L. and Eaton , D.L. 1996 . The kinetics of aflatoxin B1 oxidation by human cDNA-expressed and human liver microsomal cytochromes P450 1A2 and 3A4 . Toxicology and Applied Pharmacology , 141 : 595 – 606 .
- Granvil , C.P. , Krausz , K.W. , Gelboin , H.V. , Idle , J.R. and Gonzales , F.J. 2002 . 4-Hydroxylation of debrisoquine by human CYP1A1 and its inhibition by quinidine and quinine . Journal of Pharmacology and Experimental Therapeutics , 301 : 1025 – 1032 .
- Guengerich , F.P. , Johnson , W.W. , Shimada , T. , Ueng , Y. , Yamazaki , H. and Langouët , S. 1998 . Activation and detoxication of aflatoxin B1 . Mutation Research , 402 : 121 – 128 .
- Guengerich , F.P. , Johnson , W.W. , Ueng , Y. , Yamazaki , H. and Shimada , T. 1996 . Involvement of cytochrome P450, glutathione S-transferase and epoxide hydrolase in the metabolism of aflatoxin B1 and relevance to risk of human liver cancer . Enviromental Health Perspectives , 104 : 557 – 562 .
- Halpert , J.R. , Guengerich , F.P. , Bend , J.R. and Correia , M.A. 1994 . Selective inhibitors of cytochromes P450 . Toxicology and Applied Pharmacology , 125 : 163 – 175 .
- Kadian , S.K. , Monga , D.P. and Goel , M.C. 1988 . Effect of aflatoxin B1 on the delayed hypersensitivity and phagocytic activity of reticuloendotelial system in chickens . Mycopathologia , 104 : 33 – 36 .
- Kakkar , T. , Boxenbaum , H. and Mayersohn , M. 1999 . Estimation of Ki in a competitive enzyme-inhibition model: comparisons among three methods of data analysis . Drug Metabolism and Disposition , 27 : 756 – 762 .
- Kennedy , S.W. , Lorenzen , A. , Jones , S.P. , Hahn , M.E. and Stegeman , J.J. 1996 . Cytochrome P4501A induction in avian hepatocyte cultures: a promising approach for predicting the sensitivity of avian species to toxic effects of halogenated aromatic hydrocarbons . Toxicology and Applied Pharmacology , 141 : 214 – 230 .
- Klaassen , C.D. . 2008 . Casarett and Doull′s Toxicology: The Basic Science of Poisons , 7th edn New York McGraw Hill Medical.
- Klein , P. , Buckner , R. , Kelly , J. and Coulombe , R. 2000 . Biochemical basis for the extreme sensitivity of turkeys to aflatoxin B1 . Toxicology and Applied Pharmacology , 165 : 45 – 52 .
- Kunze , K.L. and Trager , W.F. 1993 . Isoform-selective mechanism-based inhibition of human cytochrome P450 1A2 by furafylline . Chemical Research in Toxicology , 6 : 649 – 656 .
- Laemmli , U. 1970 . Cleavage or structural proteins during the assembly of the head of bacteriophage T4 . Nature , 227 : 680 – 685 .
- Leclercq , I. , Desager , J.P. , Vandenplas , C. and Horsmans , Y. 1996 . Fast determination of low level cytochrome P-450 1A1 activity by high-performance liquid chromatography with fluorescence or visible absorbance detection . Journal of Chromatography B , 681 : 227 – 232 .
- Leeson , S. , Diaz , G.J. and Summers , J.D. 1995 . Poultry Metabolic Disorders and Mycotoxins , 249 – 280 . Guelph , ON : University Books .
- Lozano , M.C. and Diaz , G.J. 2006 . Microsomal and cytosolic biotransformation of aflatoxin B1 in four poultry species . British Poultry Science , 47 : 734 – 741 .
- Marangoni , A.G. 2003 . Enzyme Kinetics:A Modern Approach , Hoboken : John Wiley & Sons .
- McLean , M. and Dutton , M.F. 1995 . Cellular interactions and metabolism of aflatoxin: an update . Pharmacology and Therapeutics , 65 : 163 – 192 .
- Oliveira , C.A.F. , Rosmaninho , J.F. , Butkeraitis , P. , Corrêa , B. , Reis , T.A. , Guerra , J.L. , et al. 2002 Effect of low levels of dietary aflatoxin B1 on laying japanese quail . Poultry Science , 81 , 976 980 .
- Omiecinski , C.J. , Remmel , R.P. and Hosagrahara , V.P. 1999 . Concise review of the cytochrome P450s and their role in toxicology . Toxicological Sciences , 48 : 151 – 156 .
- Pereira , V.A. , Auler , J.O. Jr , Carmona , M.J. , Mateus , F.H. , Lanchote , V.L. , Breimer , D.D. and Santos , S.R.C.J. 2000 . A micromethod for quantitation of debrisoquine and 4-hydroxydebrisoquine in urine by liquid chromatography . Brazilian Journal of Medical and Biological Research , 33 : 509 – 514 .
- Raynal , M. , Bailly , J.D. , Bernard , G. and Guerre , P. 2001 . Effects of fumonisin B1 present in Fusarium moniliforme culture material on drug metabolizing enzyme activities in ducks . Toxicology Letters , 121 : 179 – 190 .
- Redinbaugh , M.G. and Turley , R.B. 1986 . Adaptation of the bicinchoninic acid protein assay for the use with microtiter plates and sucrose gradient fractions . Analytical Biochemistry , 153 : 267 – 271 .
- Riviere , J.L. 1992 . Hepatic microsomal monooxygenase activities in natural populations of the Mallard Duck Anas platyrhynchos, the Tufted Duck Aythya fuligula and the Great Crested Grebe Podiceps cristatus . Ecotoxicology , 1 : 117 – 135 .
- SAS Institute Inc . 2008 . SAS/STAT® 9.2 User's Guide Cary , NN SAS Institute, Inc .
- Sesardic , D. , Boobis , A.R. , Murray , B.P. , Murray , S. , Segura , J. , De La Torre , R. and Davies , D.S. 1990 . Furafylline is a potent and selective inhibitor of cytochrome P450IA2 in man . British Journal of Clinical Pharmacology , 29 : 651 – 663 .
- Shim , S.C. , Pae , A.N. and Lee , Y.J. 1988 . Mechanistic studies on the photochemical degradation of nifedipine . Bulletin of the Korean Chemical Society , 9 : 271 – 332 .
- Verma , R.J. 2004 . Aflatoxin causes DNA damage . International Journal of Human Genetics , 4 : 231 – 236 .
- Wild , C.P. and Turner , P.C. 2002 . The toxicology of aflatoxins as a basis for public health decisions . Mutagenesis , 17 : 471 – 481 .