Abstract
A total of 69 isolates of Enterococcus faecalis from broiler breeders demonstrating different lesion types and representing eight different flocks were characterized by multilocus sequence typing. Twenty isolates obtained from healthy birds representing two additional flocks were included for comparison. A total of 12 different sequence types (STs) was obtained. Correlation between ST and lesion type was not demonstrated. However, three STs (ST82, ST174, ST177) made up 81% of the isolates associated with lesions, indicating that these STs might be particularly associated with birds. In addition, ST174, the most frequently demonstrated ST, was only obtained from affected birds. Surprisingly, ST82, previously reported to be associated with amyloid arthropathy in layers worldwide, demonstrated a high degree of diversity as to lesion types, just as healthy carriers were demonstrated among broiler breeders. STs associated with healthy birds and lesions, respectively, did not demonstrate a phylogenetic relationship.
Introduction
Enterococci constitute a part of the normal microbiota of the gastrointestinal and urogenital tract of humans and animals (Facklam et al., Citation1999; Devriese et al., 2006). In chickens, Enterococcus faecalis and Enterococcus faecium have been found to constitute the dominan bacterial flora of the intestinal tract in 1-day-old chicks, while Enterococcus cecorum was found to be dominan in birds older than 12 weeks (Devriese et al., 2006). In addition, these organisms have the potential to cause clinical infections. In poultry, E. faecalis has been isolated from septicaemia (Chadfield et al., Citation2004), endocarditis (Jortner & Helmboldt, Citation1971), amyloid arthropathy (Landman et al., Citation1994), pulmonary hypertension syndrome (Tankson et al., Citation2001) and malabsorption and growth depression in chickens (Eyssen & Desomer, Citation1967).
E. faecalis also occurs as a potential pathogen in other animals, and is the most common Enterococcus sp. associated with infections in humans (Moellering et al., Citation1974; Caprioli et al., Citation1975; Garrison et al., Citation1982; Luginbuhl et al., Citation1987; Malani et al., Citation2002). In addition, it is an important cause of nosocomial infections (Emori & Gaynes, Citation1993). Owing to accumulation of antibiotic resistances, enterococcal infections represent a life-threatening challenge (Nallapareddy et al., Citation2002).
Very little is known about the pathogenesis and epidemiology of infections in animal species. E. faecalis affects avian species of all ages. However, the most serious infections have previously been associated with embryos and very young birds (Wages, Citation2003). Recent observations on the causes of mortality in broiler parent flocks have demonstrated an increased mortality due to E. faecalis (Chadfield et al., Citation2004; Bisgaard et al., Citation2010; Gregersen et al., Citation2010). Information on the epidemiology and lesion types associated with this organism in broiler parents, however, is almost non-existent.
Investigations on the epidemiology and population structure of E. faecalis of human origin have revealed a non-clonal population, although the use of eBURST outlined four major clonal complexes. Two of these complexes (CC2 and CC9) are particularly fit in the hospital environment (Ruiz-Garbajosa et al., Citation2006; Kawalec et al., Citation2007). In layer chickens, outbreaks of amyloid arthropathy (AA) were recently demonstrated to be associated with a particular multi locus sequence type (ST) of E. faecalis, ST82 (Petersen et al., Citation2009). Based upon pulsed field gel electrophoresis, a vancomycin-resistant clone of E. faecalis was demonstrated to persist in poultry in New Zealand, indicating that, once established, certain clones may be difficult to eradicate (Manson et al., Citation2004).
The aims of the present investigation therefore were to characterize isolates of E. faecalis obtained from different lesion types in broiler parents and cloacal swabs from healthy broiler breeders to investigate the diversity of E. faecalis in poultry, and to subsequently examine whether a correlation exists between exists the multilocus sequence typing (MLST)/phylogenetic lines outlined and lesion types. In addition, the antibiotic resistance profile was investigated for isolates of two closely related STs (ST16 and ST302) demonstrated among strains included in the study.
Materials and Methods
Flock data and isolates investigated
The occurrence and lesion type of E. faecalis infections in broiler parent flocks not affected by specific disease outbreaks and demonstrating “normal” production parameters, including mortality rate, were estimated based upon post-mortem results of dead birds representing normal mortality in flocks investigated during the past decade. A total of 69 isolates of E. faecalis, representing eight different flocks and birds demonstrating different lesion types, were subjected to further genetic characterization by MLST. Twenty isolates from 20 birds without lesions representing two additional broiler breeder flocks (10 birds per flock) were included for comparison.
Bacteriology
Organs demonstrating lesions typical of infection were decontaminated by searing before a sterile cotton swab was taken through the sterile surface and rotated within the lesion. Subsequently, the material on the swab was plated onto blood agar (Blood Agar Base, CM55; Oxoid, Basingstoke, UK) containing 5% sterile bovine blood. Isolates from plates demonstrating abundant growth of a pure culture were initially characterized and identified according to standard proceducers (Devriese et al., 2006). Isolates from affected organs were kept at –80°C in 15% glycerol until further characterization. Only a single isolate from affected birds was subsequently characterized. Cloacal swabs from healthy birds were plated on Slanetz agar (CM0377; Oxoid). Colonies with a red centre and a small greyish periphery, considered typical of E. faecalis, were subcultured, identified by standard procedures and kept at –80°C in 15% glycerol for further characterization. Only a single isolate from each bird was subsequently characterized.
Multilocus sequence typing
MLST was performed as previously described by Petersen et al. (Citation2009). The primers and polymerase chain reaction (PCR) conditions were as published at the multilocus sequence homepage for E. faecalis (http://efaecalis.mlst.net/), with the exception of primers published for the gyd gene. Use of published gyd primers did not generate a sequence from the PCR product. Instead, a new set of primers was designed (gydFnew, 5′-CAAACCATGAAACATTAACTGGA-3′ and gydRnew, 5′-AAGTTAGCGAAGTATTCTAAA GTACGA-3′) with an expected amplicon size of 597 bp, and PCR conditions remained unchanged. Amplification products were sequenced in both directions using the PCR primers at Macrogen Inc. (Seoul, Korea). Sequences obtained were assembled using CLC main workbench 5.2 software (CLC bio, Aarhus, Denmark) and compared with published alleles, and a ST was assigned to each strain (http://efaecalis.mlst.net/). A neighbour-joining tree was constructed based upon the concatenated sequences from the seven genes constituting each sequence type using MEGA 4.02 software (Tamura et al., Citation2007). To illustrate the allele-based evolutionary relatedness of E. faecalis, a population snapshot was made of all published STs using the eBURST programme available online (http://efaecalis.mlst.net/). Sequence and strain information have been deposited in the E. faecalis MLST database at the same website.
Antibiotic resistance
A high level of gentamicin resistance was recently demonstrated in ST16 from pigs and two Danish patients with infective endocarditis (Larsen et al., Citation2010). For the same reason, the antibiotic resistance for isolates of ST16 and the very closely related ST302 was determined. Vancomycin and high-level gentamicin resistance were carried out in a single concentration assay. The lack of bacterial growth in an overnight culture of brain heart infusion supplemented with 4 µg vancomycin/ml was evaluated as sensitive, while growth in an overnight culture of brain heart infusion containing 1024 µg gentamincin/ml was considered resistant. For the remaining antibiotics (amikacin, amoxicillin/clavulanic acid, ampicillin, cefazolin, cefoxitin, cefpodoxime, ceftiofur, cephalothin, chloramphenicol, clindamycin, enrofloxacin, erythromycin, gentamicin, imipenem, marbofloxacin, orbifloxacin, oxacillin + 2% NaCl, penicillin, rifampin, tetracycline, ticarcillin, ticarcillin + clavulanic acid, trimetroprim/suphamethoxazole), resistance was determined by the Sensititre® microbiology system (Trek Diagnostic Systems, West Sussex, UK) according to the manufacturer's instructions.
Results
Infections due to E. faecalis were demonstrated in 167 (5.4%) out of 3100 broiler parents received for routine post-mortem examination during the past decade. The occurrence of E. faecalis infections increased with the age of the birds, as did the occurrence of valvular endocarditis (). Pathological lesions included various combinations of septicaemia, amyloidosis, valvular endocarditis, salpingitis, peritonitis and arthritis. Lesion types observed are presented in . Unfortunately, data on post-mortem lesions registered did not allow investigation of the prevalence of the lesion types stated above, just as the possible seasonal and annual variations could not be calculated due to an uneven distribution of submissions.
Figure 1. Systemic infections and valvular endocarditis due to E. faecalis observed among 3100 broiler breeders subjected to post-mortem examination.
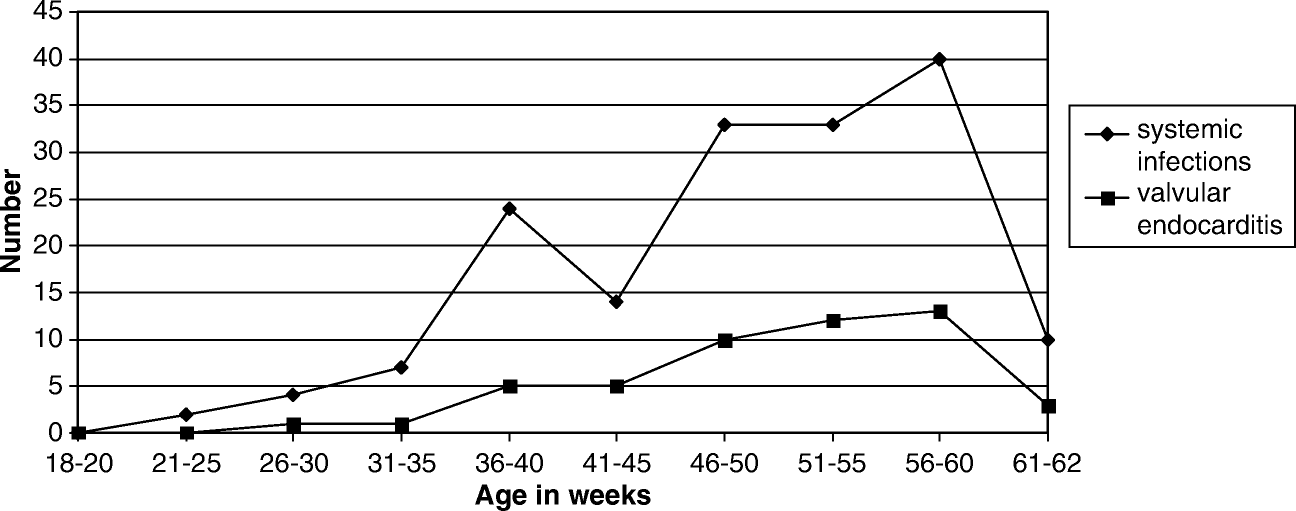
Table 1. STs of E. faecalis and lesions observed in broiler parents.
Eighty-nine isolates of E. faecalis (69 isolates from diseased birds and 20 isolates from healthy birds) were selected for MLST characterization. A total of 12 different STs of E. faecalis were demonstrated ( and ). Among these 12 STs, the allelic variation varied from four (gdh) to nine different allele types (xtp). Nine different sequences types (ST16, ST32, ST82, ST174, ST175, ST176, ST177, ST249 and ST255) () were demonstrated among the 69 strains of E. faecalis obtained from diseased birds, all of which have been reported previously in the E. faecalis MLST database (www.mlst.net). Isolates from healthy birds demonstrated seven different STs, four of which also were found among STs from diseased birds (ST16, ST32, ST82 and ST177) (). Two out of the seven STs obtained from healthy birds, ST302 and ST325, represent new STs. ST302 is closely related to ST16, and only differed with 1 bp in the Yilq gene (base pair 32 is cytosine in ST16 and thymine in ST302). ST325 is closely related to ST82, sharing all alleles except the xtp gene, in which allele 20 of the xtp gene in ST82 was replaced by allele type 2 in ST325.
Table 2. Distribution of STs of E. faecalis among flocks investigated.
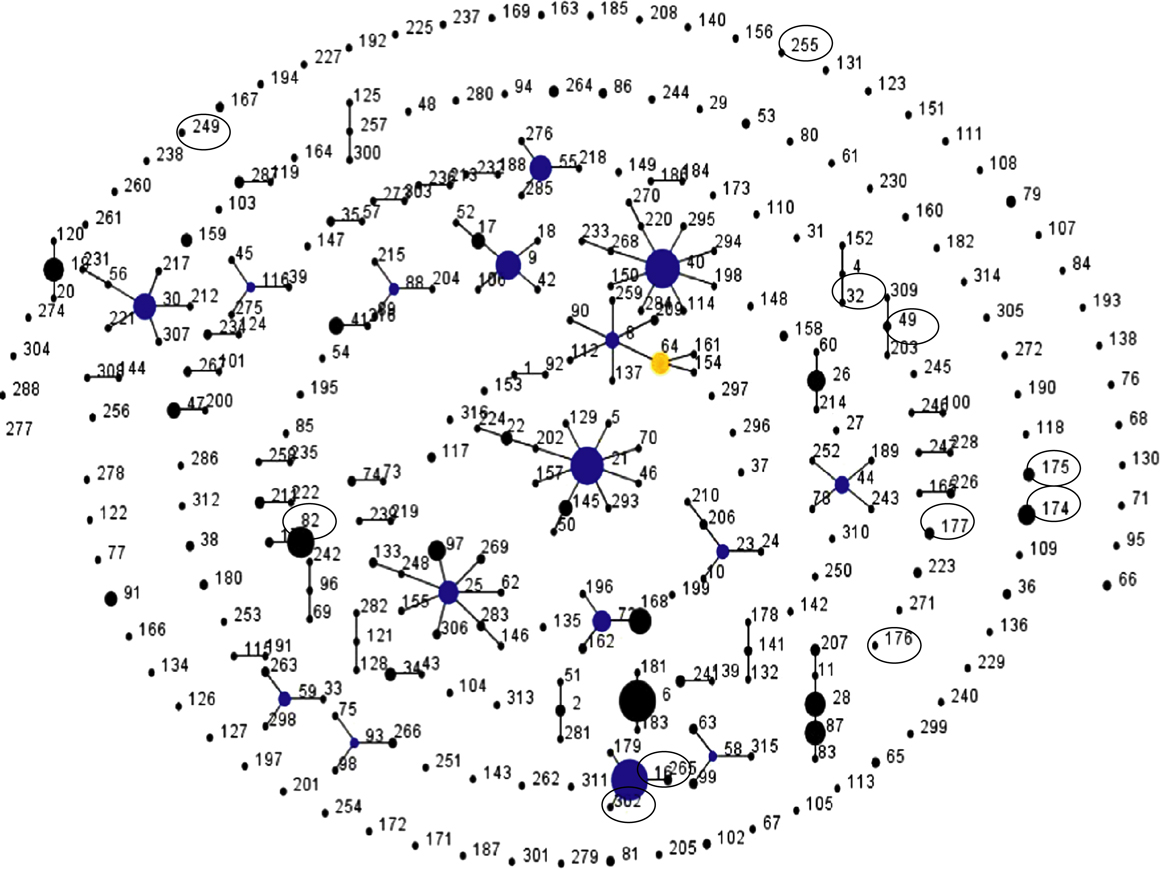
The analysis of the concatenated sequences of the sequence types revealed that, out of the 2631 bases analysed, differences were only demonstrated at a maximum of 25 positions (ST174 compared with ST249), whereas the phylogenetically related STs (ST302 and ST16) only differed in one position. The MLST types were clearly separated on a neighbour-joining tree (). eBURST analysis revealed that only ST16 and ST302 belonged to the same clonal complex, while the remaining 10 STs identified among the investigated isolates either belonged to other clonal complexes (e.g. ST82) or represented singletons (e.g. ST176) ().
Figure 2. Phylogenetic relationship between the concatenated sequences of the identified sequence types determined for the isolates of E. faecalis based on the neighbour-joining analysis. Sequence types without labels are found in both healthy and diseased birds.
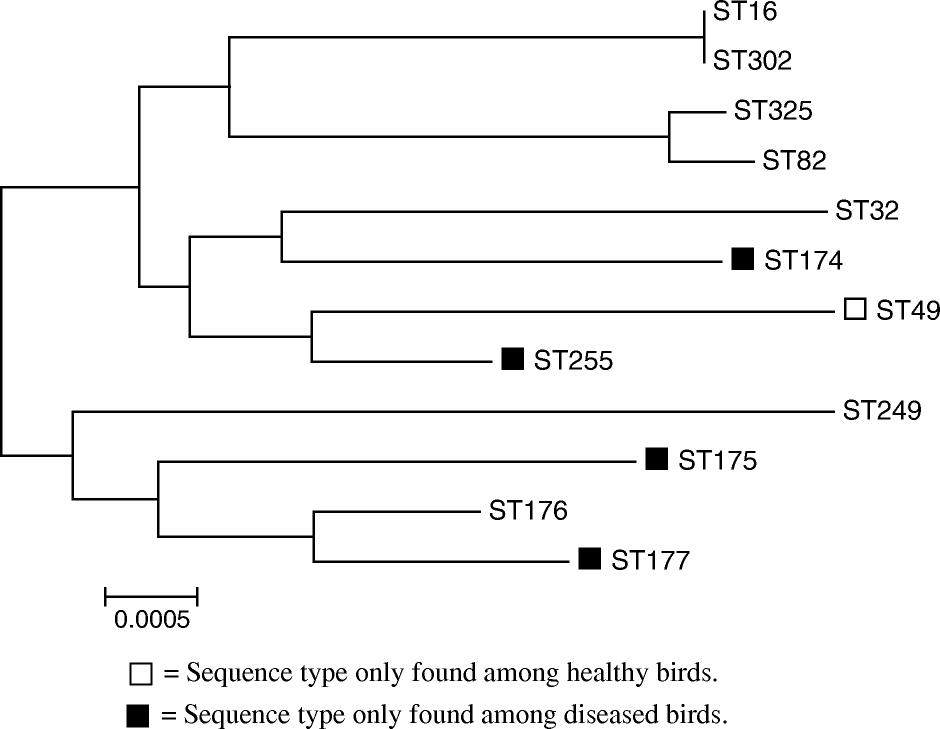
Figure 3. Population snapshot of all STs included in the MLST database for E. faecalis using the eBurst program. Each ST is represented as a node with a number (the ST number); the relative size of each node is indicative of its prevalence among the isolates in the MLST database. Black lines connect single locus variants (STs that differ in only one of the seven housekeeping genes). Clusters of linked STs correspond to clonal complexes. Primary founders (blue) are positioned centrally in the cluster, and subgroup founders are shown in yellow. STs encircled in figure are STs isolated from poultry identified in the present study. Among the STs identified in the present study, only ST16 and ST302 belong to the same clonal complex. (ST325 was not yet included in the E. faecalis eBurst MLST-based database at the time of submitting this paper.)
STs of E. faecalis isolated from different lesion types are outlined in . Overall, an increased number of lesion types were observed with an increased number of strains investigated. ST174 was associated with six different lesion types. In addition, this ST was demonstrated in eight out of eight flocks investigated. The second most frequently demonstrated ST (ST82) was associated with seven different lesion types in four out of eight flocks investigated, while the third most frequent ST (ST177) was associated with five different lesion types in six out of eight flocks. These three STs made up 81% of the isolates obtained from lesions and 15% of the isolates from healthy birds. With the exception of three STs (ST16, ST175 and ST255) totalling seven isolates (10%), all STs seem to have the capacity of inducing amyloidosis (). The prevalence of amyloidosis increased from 15% of the isolates associated with septicaemic lesions to 31% in case of endocarditis and septicaemia, and some 60% for salpingitis, peritonitis and septicaemia, arthritis, and arthritis accompanied by septicaemia ().
Table 3. Sequence types (ST) of E. faecalis and amyloidosis detected macroscopically.
Three isolates of ST16 and four isolates of ST302 were subjected to antibiotic resistance determination. All seven isolates were resistant to amikacin, cefoxitin, clindamycin, gentamicin and oxacillin + 2% NaCl. Six out of seven isolates were resistant to cephalothin. Vancomycin resistance was not observed. All seven isolates of ST16 and ST302 demonstrated high-level gentamicin resistance.
Discussion
The ability of E. faecalis to cause serious infections is connected to the inherent hardiness of the bacterium, which enables it to tolerate desiccation, persist in the environment and endure host defences (Kramer et al., Citation2006; Leblanc, Citation2006). In the present study, E. faecalis infections increased with the age of the broiler breeders and made up some 5% of the overall mortality of apparently healthy broiler breeder flocks. Litter quality is known to deteriorate with time, predisposing birds to foot pad lesions and subsequent bacteraemia, leading to endocarditis beyond a certain age (Haslam, Citation2001; Bilgili et al., Citation2009), as also demonstrated during the present investigation. Like in humans, the risk of bacterial endocarditis due to E. faecalis increases with age of poultry (Anderson et al., Citation2004).
Infections observed in the present study often seem to demonstrate a chronic pattern including infection of the reproductive tract confirming previous observations (Bisgaard & Dam, Citation1981). For the same reasons, vertical transmission may not be excluded, explaining the importance of this organism previously reported for young chickens (Wages, Citation2003).
Further investigations on the first week mortality in broilers are, however, needed to confirm the importance of vertical transmission. Lesions observed were diverse, and a correlation to the demonstrated STs was not observed. However, three STs (ST82, ST174 and ST177) made up 81% of the isolates from lesions investigated. Each of these STs was, however, associated with at least five different lesion types (). The lack of obvious correlation might be overlooked as a result of the limited number of isolates assigned to each ST. ST174, the most frequently demonstrated ST among diseased birds, was associated with septicaemia (with or without necroses and amyloidosis) in 15/24 (62.5%) of the birds included. Moreover, ST174 was only demonstrated among isolates from affected birds, indicating a high pathogenic potential of this ST. The lack of an apparent correlation between ST and lesion type was particularly surprising for ST82, previously demonstrated to be associated with amyloid arthropathy in layers worldwide (Petersen et al., Citation2009). Lesions associated with ST82 in this study were diverse and included various combinations of septicaemia, valvular endocarditis, amyloidoisis, salpingitis, peritonitis and arthritis. Overall, the present findings seem to indicate that most STs of E. faecalis have the potential to develop amyloidosis. However, the longer it takes to develop a lesion type, the greater the risk of developing amyloidosis.
MLST represents an outstanding tool for global and long-term epidemiological studies. However, considering the possible role of mobile elements in the evolution of virulence of E. faecalis (Mcbride et al., Citation2009), isolates of the same ST are likely to posses different virulence factors related to different pathological lesions in the host, explaining the lack of correlation between ST and lesion type. Isolates of E. faecalis from broiler parents without lesions also demonstrated heterogeneity, totalling seven STs. Out of these, ST16, ST32, ST82 and ST177 were also demonstrated among E. faecalis isolated from diseased birds in the present study. ST16 isolates have previously been reported to display major diversity as to the source of isolation and lesions (Ruiz-Garbajosa et al., Citation2006). Rapid emergence of vancomycin-resistant enterococci presents an increasingly significant threat to public health, since vancomycin-resistant enterococci frequently possess determinants conferring resistance to multiple classes of antimicrobial drugs (Moellering, Citation1992).
None of the present ST16 isolates were vancomycin resistant. All isolates were, however, resistant to gentamicin (minimum inhibitory concentration > 8 ). Recent studies of both porcine and human isolates of ST16 showed a minimum inhibitory concentration for gentamicin > 1024 µg gentamin/ml for these isolates, and it was suggested that pigs are the source of high-level gentamicin-resistant (HLGR) E. faecalis infections in humans (Larsen et al., Citation2010). Our findings of HLGR E. faecalis suggest that poultry also might be a source of HLGR E. faecalis infections in humans. Further investigations are needed, however, to establish whether the poultry isolates are related to those obtained from pigs and human. The analysis of the concatenated sequences of STs from both diseased and healthy birds (see ) underlines the genetic diversity of the isolates investigated, confirming previous observations by Bisgaard et al. (Citation2007). Moreover, a phylogenetic relationship was not observed among isolates obtained from healthy and diseased birds, respectively. However, demonstration of only 12 different STs among the 89 isolates characterized was surprising compared with another study in which 110 isolates of E. faecalis from different sources and geographic locations revealed the existence of no less than 55 different STs (Ruiz-Garbajosa et al., Citation2006). Only four (ST16, ST82, ST49 and ST249) out of 12 STs demonstrated in the present investigation have been reported on the MLST webpage from sources other than poultry. Although not absolute, our findings seem to indicate a certain degree of host specificity to poultry of selected phylogenetic lines, underlining that certain variants are better suited for a particular niche and proliferate and complete that niche, leading to well-defined genetic lineages of the species (Mcbride et al., Citation2009). A good example of an ST with a possible association with a specific niche is ST174, which was isolated from affected birds in eight out the eight flocks investigated, constituting 24/69 isolates from six different lesion types. In contrast, ST16 has been isolated from poultry, bulls, swine, and different lesion types in humans as well as from non-affected humans. Although the natural sources of infection in humans have remained speculative, food-producing animals (including poultry) are considered a possible source of infection (Templer et al., Citation2008). Further investigations are needed, however, to clarify the zoonotic potential of E. faecalis.
Acknowledgements
The present work was founded by the Chicken and Hen Infection Protection—CHIP project, Danish Agency for Science, Technology and Innovation (Journal number 07-024716). Katrine Madsen is thanked for skilful technical assistance.
References
- Anderson , D.J. , Sexton , D.J. , Murdoch , D.R. , Stout , J.E. , Cabell , C.H. , Reller , L.B. and Corey , G.R. 2004 . Risk factors for infective endocarditis in patients with enterococcal bacteremia: a case–control study . Infection , 32 : 72 – 77 .
- Bilgili , S.F. , Hess , J.B. , Blake , J.P. , Macklin , K.S. , Saenmahayak , B. and Sibley , J.L. 2009 . Influence of bedding material on footpad dermatitis in broiler chickens . Journal of Applied Poultry Research , 18 : 583 – 589 .
- Bisgaard , M. and Dam , A. 1981 . Salpingitis in poultry II. Prevalence, bacteriology, and possible pathogenesis in egg-laying chickens . Nordisk Veterinærmedicin , 33 : 81 – 89 .
- Bisgaard , M. , Bojesen , A.M. , Christensen , J.P. and Christensen , H. 2010 . Observations on the incidence and aetiology of valvular endocarditis in broiler breeders and detection of a newly reported taxon of Pasteurellaceae, Avibacterium endocarditis . Avian Pathology , 39 : 177 – 181 .
- Bisgaard M. Hedegaard L. Petersen A. Christensen H. Christensen J.P. Chadfield M. 2007 Genetic diversity and persistence of Enterococcus faecalis obtained from different pathological lesions in adult chickens as demonstrated by PFGE and MLST. Abstract and oral presentation In Proceedings of the XV Congress of the World Veterinary Poulty Association (10–15 September). Beijing, China
- Caprioli , T. , Zaccour , F. and Kasatiya , S.S. 1975 . Phage typing scheme for group D streptococci isolated from human urogenital tract . Journal of Clinical Microbiology , 2 : 311 – 317 .
- Chadfield , M.S. , Christensen , J.P. , Christensen , H. and Bisgaard , M. 2004 . Characterization of streptococci and enterococci associated with septicaemia in broiler parents with a high prevalence of endocarditis . Avian Pathology , 33 : 610 – 617 .
- Devriese L.A. Baele M. Butaye P. 2006 The genus Enterococcus In M. Dworkin S. Falkow E. Rosenberg K.-H. Schliefer E. Stackebrandt The Prokaryotes , 3rd edn 163 174 New York Springer
- Emori , T.G. and Gaynes , R.P. 1993 . An overview of nosocomial infections, including the role of the microbiology laboratory . Clinical Microbiology Reviews , 6 : 428 – 442 .
- Eyssen , H. and Desomer , P. 1967 . Effects of Streptococcus faecalis and a filterable agent on growth and nutrient absorption in gnotobiotic chicks . Poultry Science , 46 : 323 – 333 .
- Facklam , R.R. , Sahm , D.F and Teixeira , L.M. 1999 . “ Enterococcus ” . In Manual of Clinical Microbiology , 7th edn , Edited by: Murray , P.R. , Baron , E.J. , Pfaller , M.A. , Tenover , F.C. and Yolken , R.H. 297 – 205 . Washington , DC : ASM Press .
- Garrison R.N. Fry D.E. Berberich S. Polk H.C. Jr 1982 Enterococcal bacteremia: clinical implications and determinants of death Annals of Surgery 196 43 47
- Gregersen R.H. Christensen . H. Ewers C. Bisgaard M. 2010 Impact of Escherichia coli vaccine on parent stock mortality, first week mortality of broilers, and population diversity of E. coli in vaccinated flocks Avian Pathology in press.
- Haslam , S. 2001 . “ Legislation and poultry welfare in the UK ” . In Poultry Diseases , 5th edn , Edited by: Jordan , F. , Pattison , M. , Alexander , D. and Faragher , T. 287 – 295 . London : W.B. Saunders .
- Jortner , B.S. and Helmboldt , C.F. 1971 . Streptococcal bacterial endocarditis in chickens. Associated lesions of the central nervous system . Veterinary Pathology , 8 : 54 – 62 .
- Kawalec , M. , Pietras , Z. , Danilowicz , E. , Jakubczak , A. , Gniadkowski , M. , Hryniewicz , W. and Willems , R.J.L. 2007 . Clonal structure of Enterococcus faecalis isolated from Polish hospitals: characterization of epidemic clones . Journal of Clinical Microbiology , 45 : 147 – 153 .
- Kramer , A. , Schewebke , I. and Kampf , G. 2006 . How long do nosocomial pathogens persist on inanimate surfaces? A systematic review . BMC Infectios Diseases , 6 : 130
- Landman , W.J.M. , Gruys , E. and Dwars , R.M. 1994 . A syndrome associated with growth depression and amyloid arthropathy in layers: a preliminary report . Avian Pathology , 23 : 461 – 470 .
- Larsen J. Schønheyder H.C. Lester C.H. Olsen S.S. Porsbo L.J. Garcia-Migura L. et al. 2010 Porcine-origin gentamicin-resistant Enterococcus faecalis in humans, Denmark Emerging Infectious Diseases 16 682 684
- Leblanc , D.J. 2006 . “ Enterococcus ” . In The Prokaryotes , 3rd edn , Edited by: Dworkin , M. , Falkow , S. , Rosenberg , E. , Schliefer , K.-H. and Stackebrandt , E. 175 – 204 . New York : Springer .
- Luginbuhl , L.M. , Rotbart , H.A. , Facklam , R.R. , Roe , M.H. and Elliot , J.A. 1987 . Neonatal enterococcal sepsis: case–control study and description of an outbreak . Pediatric Infectious Disease Journal , 6 : 1022 – 1026 .
- Malani , P.N. , Kaufmann , C.A. and Zervos , M.J. 2002 . “ Enterococcal disease, epidemiology and treatment ” . In The Enterococci: Pathogenesis, Molecular Biology, and Antibiotic Resistance , Edited by: Gilmore , M.S. 385 – 408 . Washington , DC : ASM Press .
- Manson , J.M. , Keis , S. , Smith , J.M. and Cook , G.M. 2004 . Persistence of vancomycin-resistant enterococci in New Zealand broilers after discontinuation of avoparcinuse . Applied and Environmental Microbiology , 70 : 5764 – 5768 .
- Mcbride , S.M. , Coburn , P. , Baghdayan , A. , Willems , R. , Grande , M. , Shankar , N. and Gilmore , M. 2009 . Genetic variation and evolution of the pathogenicity island of Enterococcus faecalis . Journal of Bacteriology , 191 : 3392 – 3402 .
- Moellering R.C. Jr 1992 Emergence of Enterococcus as a significant pathogen Clinical Infecti Diseases 14 1173 1176
- Moellering R.C. Jr Watson B.K. Kunz L.J. 1974 Endocarditis due to group D streptococci: Comparison of disease caused by Streptococcus bovis with that produced by the enterococci The American Journal of Medicine 57 239 250
- Nallapareddy , S. , Duh , R.W. , Singh , K. and Murray , B. 2002 . Molecular typing of selected Enterococcus faecalis isolates: pilot study using multilocus sequence typing and pulsed-field gel electrophoresis . Journal of Clinical Microbiology , 40 : 868 – 876 .
- Petersen , A. , Christensen , H. , Philipp , H.-C. and Bisgaard , M. 2009 . Clonality of Enterococcus faecalis associated with amyloid arthropathy in chickens evaluated by multi locus sequence typing (MLST) . Veterinary Microbiology , 134 : 392 – 395 .
- Ruiz-Garbajosa , P. , Bonten , M.J. , Robinson , D.A. , Top , J. , Nallapareddy , S.R. Torres , C. 2006 . Multilocus sequence typing scheme for Enterococcus faecalis reveals hospital-adapted genetic complexes in a background of high rates of recombination . Journal of Clinical Microbiology , 44 : 2220 – 2228 .
- Tamura , K. , Dudley , J. , Nei , M. and Kumar , S. 2007 . MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0 . Molecular Biology and Evolution , 24 : 1596 – 1599 .
- Tankson , J.D. , Thaxton , J.P. and Vizzier-Thaxton , Y. 2001 . Pulmonary hypertension syndrome in broilers caused by Enterococcus faecalis . Infection and Immunity , 69 : 6318 – 6322 .
- Templer , S.P. , Rohner , P. and Baumgartner , A. 2008 . Relation of Enterococcus faecalis and Enterococcus faecium isolates from foods and clinical specimens . Journal of Food Protection , 71 : 2100 – 2104 .
- Wages , D.P. 2003 . “ Streptococcosis ” . In Diseases of Poultry , 11th edn , Edited by: Saif , Y.M , Barnes , H.J. , Glisson , J.R. , Fadly , A.M. , McDougald , L.R. and Swayne , D.E. 805 – 812 . Ames : Iowa State Press .