Abstract
Necrotic enteritis is a potentially fatal multifactorial disease of chickens, which under commercial conditions is often associated with increased levels of mortality and reduced bird performance. The safety and efficacy of a Clostridium perfringens type A alpha-toxoid (Netvax™) formulated as an oil emulsion was investigated, following maternal immunization of broiler breeder hens, housed under commercial conditions, by the intramuscular route. A total of 11,234 hens were vaccinated across two integrated poultry sites. The vaccine was safe with no systemic reactions or adverse effects on bird performance detected. Vaccination resulted in a significant increase in anti-alpha toxin antibody in the hen that was maintained throughout the study, and subsequently transferred to their progeny throughout the laying period via egg yolk. Chicks hatched from eggs produced from vaccinated hens were shown to have reduced mortality specifically related to progeny flocks where gross gut lesions associated with necrotic enteritis were observed in control chicks. Further, whilst C. perfringens was isolated from control chicks with necrotic enteritis lesions, no such isolations were made at these time points from chicks from vaccinated hens. These results indicate that, under commercial conditions, maternal vaccination with Netvax™ can help to control losses related to necrotic enteritis.
Introduction
Necrotic enteritis (NE) is a complex enteric disease of poultry associated with Clostridium perfringens type A. Global costs to the industry have been estimated at up to $2 billion per annum (Kaldhusal & Lovland, Citation2000; McDevitt et al., Citation2006). Control of the disease has been achieved through the routine prophylactic administration of antimicrobial drugs either in-feed or in-water (Ficken & Wages, Citation1997); however, as a result of an increasing concern about the contribution of such practices to antimicrobial resistance problems, there is growing pressure to ban this practice. In consequence, interest in alternative approaches including vaccination has been increasing.
C. perfringens is a commensal in the gut of vertebrates including poultry (Songer, Citation1996; Porter, Citation1998); however, data suggest that only specific strains produce NE (reviewed by Cooper & Songer, Citation2009). Even when such strains are present, development of clinical NE appears to be at least partially dependent on the presence of pre-disposing factors such as prior damage to the mucosal surface of the intestine or a diet containing fishmeal or high levels of indigestible polysaccharides, as found in rye, wheat and barley (reviewed by Williams, Citation2005).
Intestinal necrosis has been induced through the inoculation of young birds with crude broth cultures of C. perfringens containing alpha-toxin (Al-Sheikhly & Truscott, Citation1977; Truscott & Al-Sheikhly, Citation1977), and in germ-free chickens the effect of a similar challenge was neutralized by prior treatment with anti-alpha-toxin antibodies (Fukata et al., Citation1988). In contrast, Keyburn et al. (Citation2006) found that lesions typical of NE were still observed in conventional birds following challenge with mutants constructed with deletions in the alpha-toxin gene, raising questions around the role of alpha-toxin in the disease process. Nevertheless, progeny chicks from hens vaccinated with cell-free supernatant toxoids derived from C. perfringens type A or type C were shown to be protected against sub-clinical NE (Løvland et al., 2004); however, such preparations would contain antigens other than alpha-toxin. Partial protection of chicks against NE, however, has been demonstrated following vaccination with either recombinant alpha-toxin (Kulkarni et al., Citation2007; Cooper et al., Citation2009) or Salmonella vectored alpha-toxin (Kulkarni et al., Citation2008; Zekarias et al., Citation2008). On the contrary, Thompson et al. (Citation2006) showed that oral immunization with live alpha-toxin-deficient mutants of C. perfringens can still protect against experimentally induced NE. Antigens other than alpha-toxin may therefore also be able to play a role as key immunogens in a vaccine (Kulkarni et al., Citation2008; Jiang et al., Citation2009).
The objective of the current study was to extend previous observations on the performance of cell-free supernatant toxoids under experimental conditions and to investigate the safety and efficacy of an experimental vaccine against NE for the passive protection of broiler chicks housed under commercial conditions.
Materials and Methods
The study comprised the immunization of broiler breeder hens at each of two commercial sites and the follow-up of progeny birds from three hatches at Site 1 and two hatches at Site 2. Both the hatcheries and the rearing farms were blinded to the immunization status of the flocks from which the chicks originated, as were the laboratories at which samples were analysed. Follow-up of progeny birds from additional hatches was restricted by the availability of suitable commercial broiler farms able to both meet the standards of good clinical practice and provide the support required within the study protocol. A summary of the study design is presented in . A total of 11,234 pullets received the test vaccine, with 9304 birds in the control group.
Table 1. Summary of study design.
Vaccine
The vaccine comprised an experimental batch of Netvax™ containing C. perfringens type A toxoid at a concentration of 3 total combining power units (with reference to the Fifth International Standard for Gas-Gangrene Antitoxin (C. perfringens alpha-antitoxin), Equine code PE) per dose emulsified in a light mineral oil. The vaccine (0.5 ml) was administered by intramuscular injection into the thickest part of the breast muscle, with the first dose administered into the left breast and the second into the right breast. Control birds did not receive any vaccine.
Husbandry
Two integrated poultry companies were used in the study, the first (Site 1) located in Germany and the second (Site 2) located in Italy.
At Site 1, 1-day-old ISA 957 broiler breeder chicks were supplied by the hatchery to the rearing farm. The entire flock was managed following the standard guidelines provided by the hatchery, including a routine vaccination programme. The pullets were initially housed at a density of 16 birds/m2 in a building divided into two pens. Before vaccination at 11 weeks they were transferred to the laying house, and vaccinates and controls were kept in separate pens at a stocking density of 8 birds/m2. Eggs from each treatment group were collected over 5-day to 7-day periods from hens at 30, 35 and 45 weeks of age and were transferred to the hatchery where they were set in separate trays and hatched under the same conditions. One-day-old broiler chickens from week 30, week 35 and week 45 hatches were placed at one of two different commercial farms. At each farm, treatment groups were located in the same house but were separated by nets. Birds were sent for slaughter at 56 to 64 days of age
At Site 2, 1-day-old Ross 508 broiler breeder chicks were supplied by the hatchery to the rearing farm. The entire flock was managed following the standard guidelines provided by the hatchery, including a routine vaccination programme. The pullets were initially housed at a density of 14 to 17 birds/m2 in a three-floor building, with four pens per floor. At 21 weeks of age the birds were moved to the broiler breeder farm, where they were placed in two separated one-floor houses on the same site at a stocking density of 9 to 10 birds/m2. Eggs from each treatment group were collected over a 7-day period from hens at 27 and 32 weeks of age and were transferred to the hatchery where they were set in separate trays and hatched under the same conditions. One-day-old broiler chickens from week 27 and 32 hatches were placed at one of two different commercial farms. At each farm, the two treatment groups were housed in separated houses in a multi-floor building. Females were sent for slaughter at 37 to 38 days of age, and males were sent for slaughter at 57 days of age.
At each site, standard coccidiostat-free diets (prestarter, Site 1 only; starter, grower and finisher, both sites) were fed to the progeny chicks from the two treatment groups.
Pre-vaccination examinations
Broiler breeder pullets were observed for general physical condition the day preceding each vaccination. Blood samples were collected from 25 pullets from each group before each vaccination.
Clinical observations
Clinical observations were conducted just before each vaccination, twice during the first 24 h following each vaccination and on a daily basis for 14 days after each vaccination. Following this initial 14-day daily observation period, the health status of the birds was recorded on a weekly basis until week 45 at Site 1 and week 32 at Site 2. Mortality was recorded on a daily basis during the whole study period starting from the day of first vaccination.
Injection site reaction
The presence of injection site reactions was monitored individually in 50 randomly selected pullets from each treatment group, twice weekly for 3 weeks after each vaccination, by inspection of the body surface around the injection site. These observations were extended until the injection site reaction disappeared if the local signs persisted beyond the initial 3-week observation period. At the conclusion of the injection site observation period (week 30), the injection sites were examined in 50 randomly selected birds from each treatment group for signs of tissue fibrosis by incision of the breast musculature, following euthanasia. In addition, parent hens that died during the production period were examined for visible macroscopic reactions and tissue fibrosis at the site of injection.
The occurrence of systemic reactions or other adverse events were monitored during the entire study period.
Egg production and viability
Data were collected throughout the study period by the hatchery as per usual management practices.
Samples for evaluation of specific antibody titres
On weeks 30, 35 and 42 at Site 1 and on weeks 27 and 32 at Site 2, the following samples were collected: blood samples were collected from 25 randomly selected pullets/hens per treatment group; and 60 randomly selected eggs laid on a single day in that week were collected for the determination of IgY titres.
Broiler chickens
The general health of the broiler chickens was monitored from placement until slaughter. Weight was measured on a weekly basis, by weighing 100 chicks in groups of 10, from each treatment group using calibrated scales. Mortality was recorded on a daily basis except for chicken broilers from the week 30 hatch at Site 1, where dead birds were kept by the farmer and counted by the investigator during routine visits. Feed consumption was determined based on each site's routine procedures. Concomitant veterinary treatments administered to the chickens outside of routine farming practices for each site were recorded.
Clinical observations for signs of NE (depression, ruffled feathers, inappetance, closed eyes, immobility and dark-coloured diarrhoea) were performed on a daily basis for the first 4 weeks after hatch and thereafter on a weekly basis. In the event of any clinical signs being observed, five sick birds were necropsied and any lesions were examined and scored. A number of birds from the alternate flock (vaccinate or control) were also examined at this time. In addition, all birds that died during the study (either natural or euthanized) were necropsied and lesions scored, except where the cause of death was well established and not related to NE. Gut lesions were scored according to the following: 0 = no C. perfringens-associated gross gut lesions; 1 = less than 10 C. perfringens-associated gross gut lesions; 2 = at least 10 C. perfringens-associated gross gut lesions; 3 = presence of at least one C. perfringens-associated gut lesion with a maximum extension larger than the circumference of the gut mucosa.
On days where lesions associated with NE were observed in any bird, a bacteriological analysis of the gut contents was conducted in all birds examined on that day as follows. Two grams of caecal contents were weighed and diluted to 10 − 2, 10 − 4 and 10 − 6. Then 100 µl aliquots of these dilutions were plated on blood agar plates. The plates were incubated anaerobically at 37°C for 18 to 24 h, and then colonies typical of C. perfringens were counted.
Blood samples were collected from 25 randomly selected chicks from each treatment group at 7 days of age.
Blood samples for serology
A minimum of 2 ml blood was collected in 4 ml serum separator tube vacutainers from the brachial vein. Blood samples were allowed to clot at ambient temperature for a minimum of 2 h prior to separation of the serum following centrifugation. Serum was stored frozen until testing for the presence of specific antibody by enzyme-linked immunosorbent assay (ELISA).
Egg collection
Eggs were collected and stored at 2 to 8°C before processing. For each treatment, eggs were pooled into groups of 10 and the IgY extracted from the egg yolk using a chicken IgY purification kit (Pierce Eggcellent Chicken IgY purification Kit). Purified IgY was stored below –15°C until testing for the presence of specific antibody by ELISA and haemolysis inhibition assay (HIA).
Enzyme-linked immunosorbent assay
A modification of the procedure described by Heier et al. (Citation2001) was followed. Briefly, ELISA plates were coated overnight at 2 to 8°C with 1 µg/ml phospholipase C type XIV (Sigma). Plates were blocked by incubation with phosphate-buffered saline containing 3% bovine serum albumin and 1% Tween 20 for 30 min at 37°C. Serial two-fold dilutions of test samples were added to the plate from an initial 1-in-10 dilution and incubated at 37°C for 1 h. At the end of the incubation, plates were washed and an optimal dilution of an anti-chicken IgG peroxidase conjugate (Sigma) was added to each well. Plates were incubated at 37°C for 30 min and bound conjugate was detected using TMB (SureBlue Reserve®TMB); optical densities were determined at 450 nm (OD450 nm). The cut-off value for the assay was determined as the mean OD450 nm+12 standard deviations for a panel of 50 sera from specific pathogen free birds reared under barrier containment, and a cut-off reference control developed to give an equivalent OD450 nm value. Appropriate positive, negative and cut-off reference controls were included on each plate. End points for control and test samples were calculated relative to the mean OD450 nm for the cut-off reference control.
Haemolysis inhibition assay
Test samples were heat treated at 56°C for 30 min. Serial two-fold dilutions of test samples in phosphate-buffered saline (pH 7.3) containing 1% bovine serum albumin were performed in V-bottomed 96-well plates, to give a final volume of 50 µl/well. An equal volume of C. perfringens alpha-toxin (selected as the highest dilution of toxin giving complete haemolysis of sheep red blood cells) was then added to each well and the plates incubated with shaking for 1 h at 37°C. Then 25 µl of 0.5% suspension of washed sheep red blood cells were added to each well and, following incubation at 37°C for 3 h (no shaking), the end point for each sample was calculated as the reciprocal of the highest dilution scored positive for the inhibition of haemolysis.
Data analysis
ELISA data were transformed by log2 (titre/5) prior to analysis. The value 5 was used for titres reported as <10. Separate analyses were performed for each time point. For HIA results, data were transformed by log2.
Statistical analysis was performed using SAS version 8.2 (SAS Institute, Cary, North Carolina, USA). Wilcoxon exact rank-sum tests were performed to compare differences in titres for chicks in control and vaccinated groups. Differences in mortality percentages of chicks in vaccinated and control groups were analysed by site and hatch using StatXact 6. P values were determined using the GLIMMIX SAS macro with binomial error and the logit link using SAS version 8.2. Weights for chicks were analysed using SAS PROC MIXED. Separate analyses were performed for site, hatch, and day. Statistical significance was declared for P<0.05.
Results
Safety
Local injection site reactions comprising generalized swelling, induration and or discolouration of the muscle tissue were observed in up to 12% of the birds at Site 1 (maximum size 60x20 mm2) and in up to 2% of the birds at Site 2 (maximum size 3x12 mm2) examined at any time point in the first 3 weeks following the initial vaccination. Following the second vaccination, up to 4% of the birds examined at Site 1 at any time point in the first 3 weeks also showed similar reactions (maximum size 25x20 mm2), but no reactions were observed over this time period at Site 2. At both sites tissue fibrosis was observed in a small number of birds 32 to 35 weeks after initial vaccination, but at Site 1 where the hens were kept to 42 weeks after initial vaccination no reactions were observed in the 50 birds examined at this time point. Further, no macroscopic lesions were observed following incision of the injection site in the breast musculature in birds that died or were euthanized during the laying period. No systemic reactions were observed in any of the hens at either site.
Egg production and hatchability throughout the laying cycle were comparable between vaccinated and control hens on each site, and in general were within the norms expected for each hatchery ().
Table 2. Egg production and hatchability.
Antibody responses
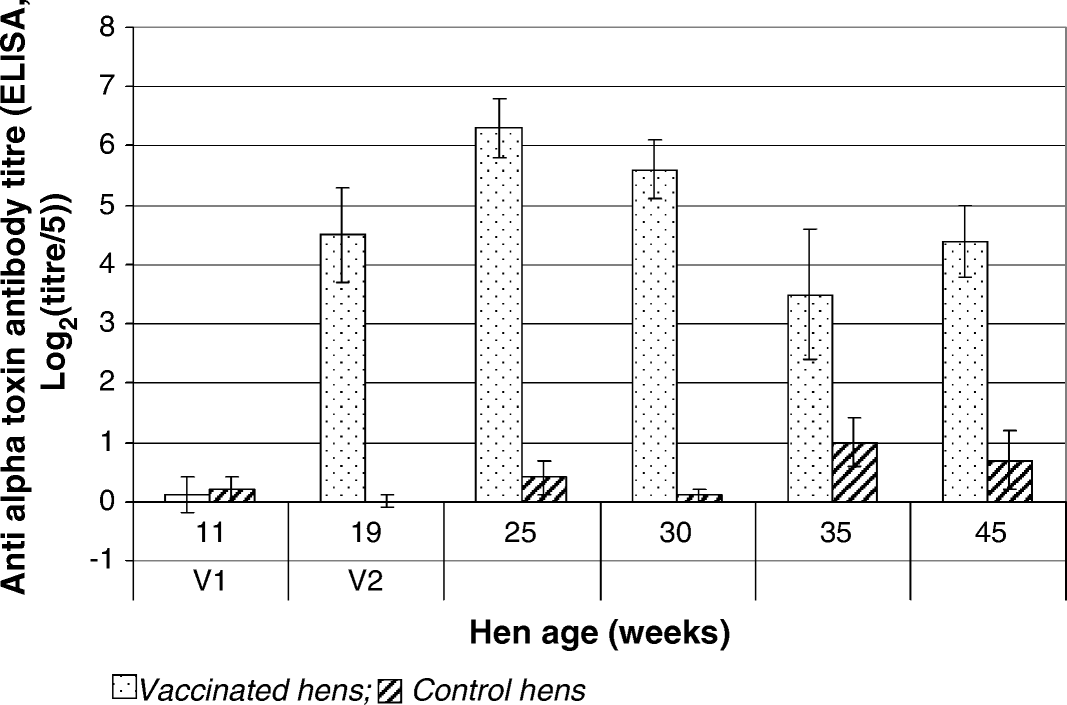
In the broiler breeder hens at Site 1, at the time of initial vaccination (11 weeks of age) mean serum antibody titres (ELISA) against C. perfringens alpha-toxin were at equivalent low levels in both vaccinated and control birds (). Following vaccination, mean specific antibody titres increased, reaching a peak (6.3 log2 (titre/5) 6 weeks after second vaccination (25 weeks of age)), after which the titres slowly fell throughout the rest of the laying cycle to approximately 4.0 log2 (titre/5) at 45 weeks of age. Mean specific antibody titres in control birds remained low (<2.0 log2 (titre/5)) throughout the study, with the highest levels (approximately 1.0 log2 (titre/5)) being recorded in the late phase of the study at 35 and 45 weeks of age.
Figure 1. Serum antibody titres against C. perfringens type A alpha-toxin determined by ELISA following vaccination of broiler breeder hens at Site 1.
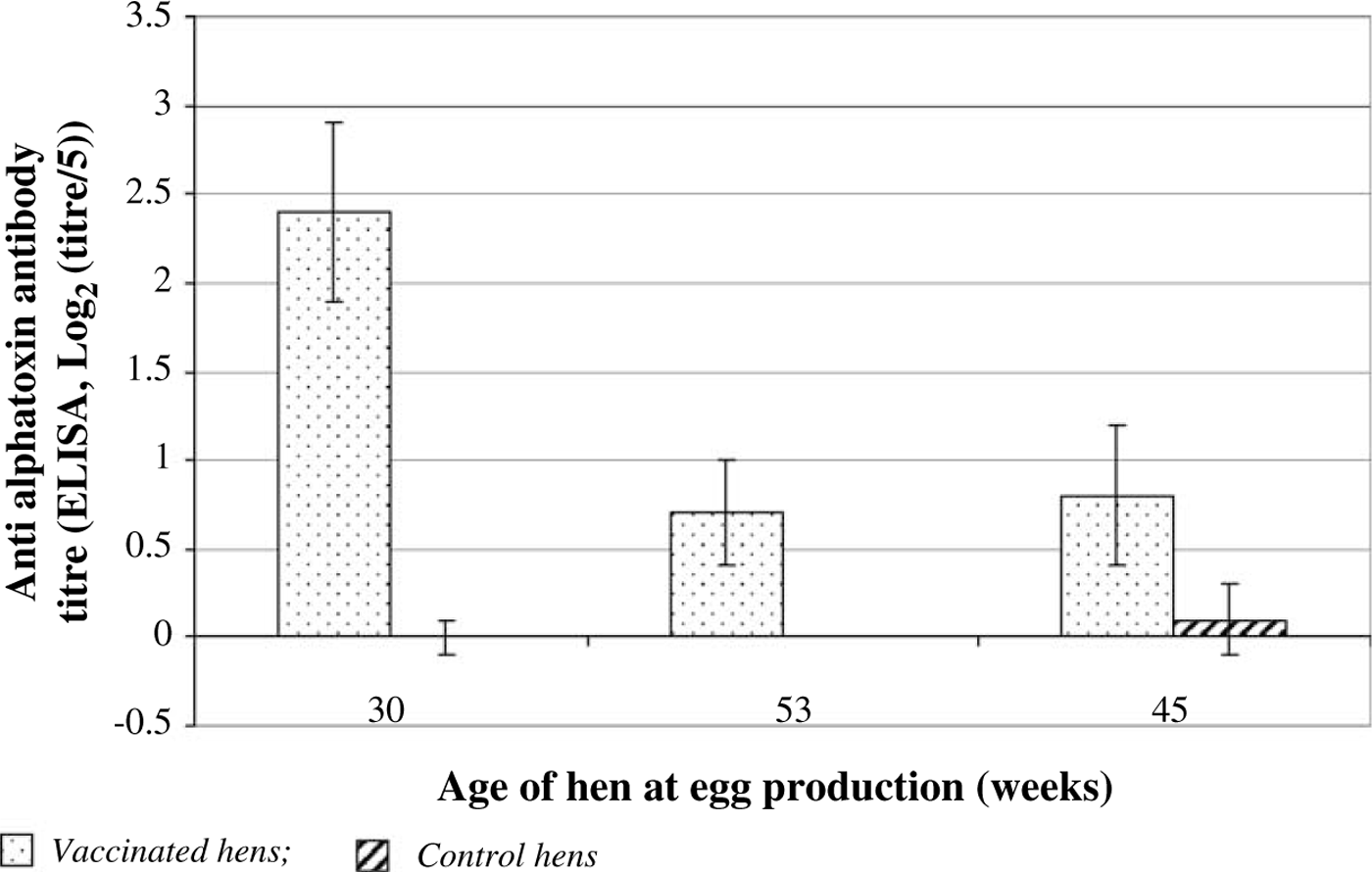
Mean specific antibody titres (ELISA) in pools of IgY from eggs collected from hens at 25, 30, 35 or 45 weeks of age at Site 1 showed an eight-fold decrease in antibody levels over time (a). Measure of antibody functionality by HIA, however, showed an approximate two-fold reduction in specific titre between eggs collected from hens at 25 and 30 weeks of age, titres remaining stationary at approximately 8.0 log2 in eggs collected at the remaining time points (b). As indicated by non-overlapping 95% confidence intervals, specific antibody levels in eggs from vaccinated birds were consistently significantly higher than those from control birds.
Figure 2. IgY titres against C. perfringens type A alpha-toxin determined by ELISA and haemolysis inhibition from eggs collected from vaccinated or control hens at 25, 30, 35 and 45 weeks of age at Site 1.
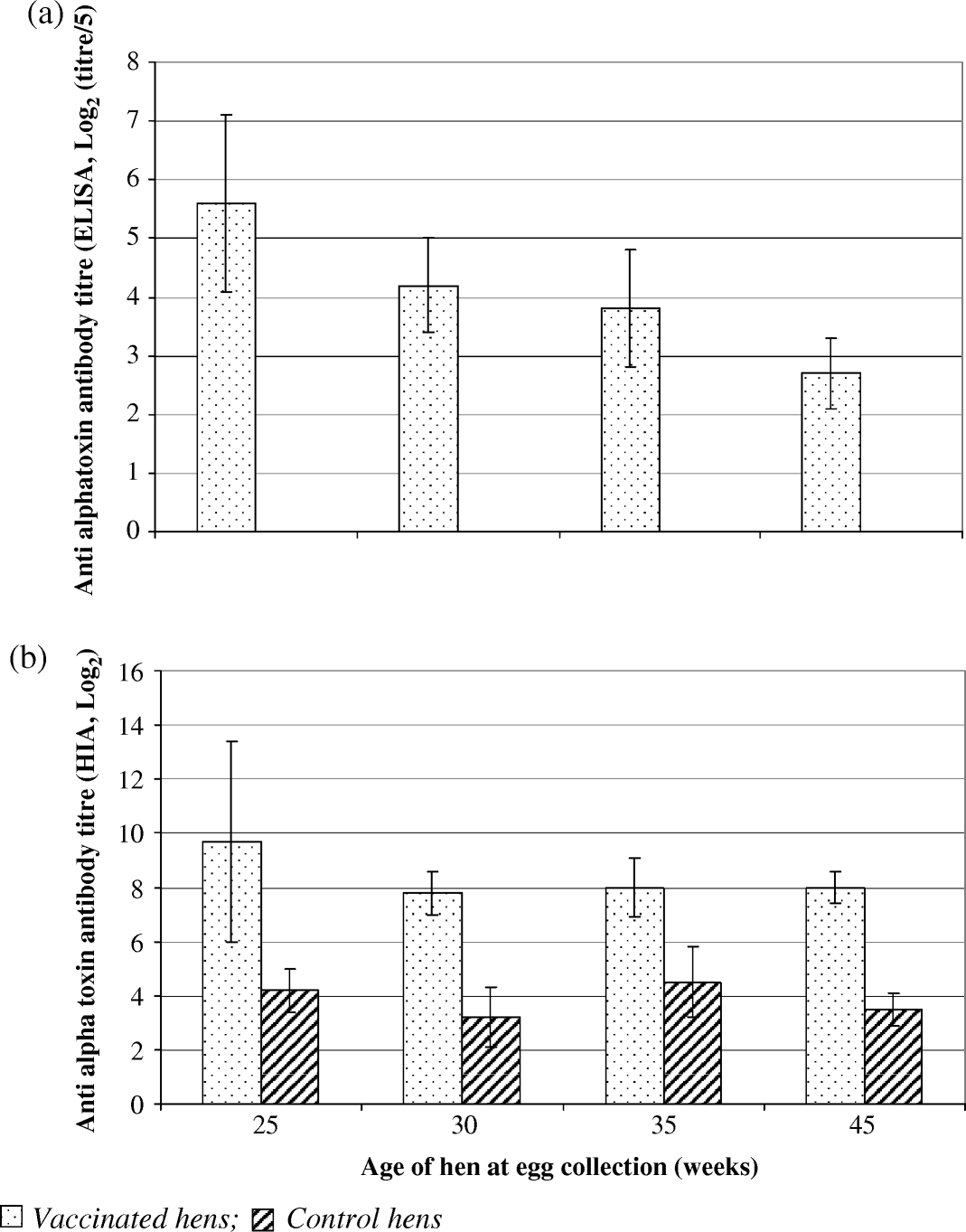
Mean antibody levels in chicks, 7 days after hatching from eggs laid by vaccinated hens, showed a two-fold reduction in titre between eggs laid by 30-week-old hens and those laid by 35-week-old hens, although no further decline was seen up to 45 weeks of age (). Mean antibody levels remained significantly higher than that detected in chicks hatched from eggs laid by control hens at each of the time points investigated. Similar results for hen serum, egg IgY and chick serum were also seen on Site 2 (data not shown).
Concomitant treatments
At Site 1, Baytril (10 mg/kg) was orally administered in drinking water to the chicks for the week 35 hatch from vaccinated hens at 6, 7 and 8 days old only, in order to treat a yolk sac infection. At Site 2, amoxicillin (30 g/100 l) was orally administered in drinking water to the chicks from control hens as therapy for the onset of NE, for the week 27 hatch at 24, 25 and 26 days of age and for the week 32 hatch at 25, 26, 27 and 28 days of age
Clinical efficacy
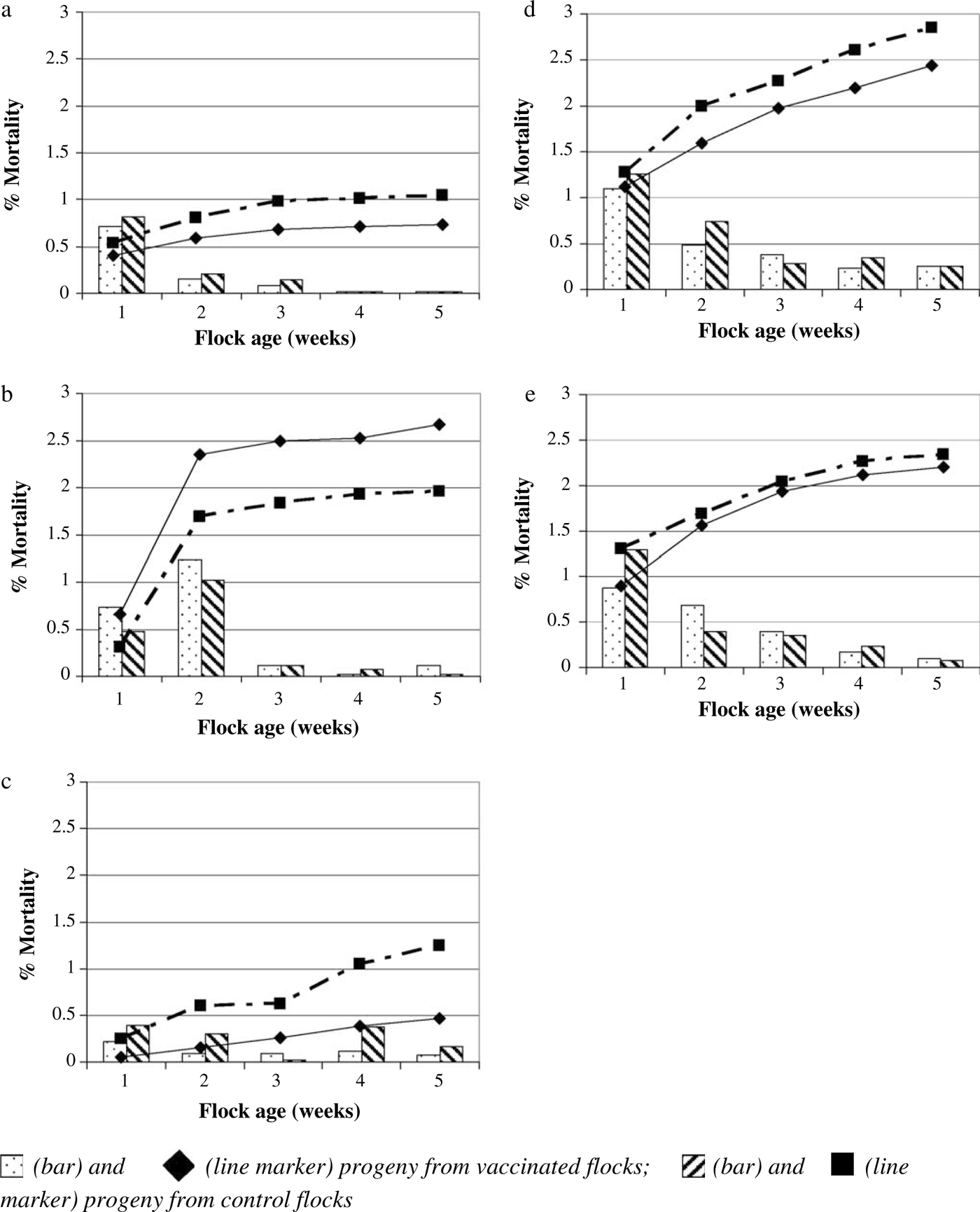
With the exception of the 35-week hatch at Site 1, where an increase in mortality associated with a yolk sac infection was reported, chick mortality by week was observed to decrease over time to a low plateau (). Progeny from the control broiler breeder flocks at the week 45 hatch at Site 1 and the week 27 hatch at Site 2, however, showed a small increase in this trend during week 4 (c, d). The pattern of accumulated mortality remained similar between progeny from vaccinated and control broiler breeder flocks, although, apart from the 35-week hatch at Site 1, overall mortality was consistently lower in the broilers from the vaccinated hens than in the control hens for all hatches.
Figure 4. Percentage mortality by week (bars) or accumulated throughout the study (lines) in progeny from vaccinated and control broiler breeder flocks at Site 1 (4a: 30-week hatch, 4b: 35-week hatch, 4c: 45-week hatch) and at Site 2 (4d: 27-week hatch, 4e: 32-week hatch).
Differences in percentage chick mortality from day of birth until sent for slaughter () were statistically significant at Site 1 for the 45-week hatch (P = 0.0037) and at Site 2 for the 27-week hatch (P = 0.0082). The percentage chick mortality from day of birth until day 21 () was also statistically significant at Site 1 for the 45-week hatch (P = 0.0456). This significant reduction in mortalities at Site 1 was also accompanied by a significantly higher mean weight at the end of the study in chicks from vaccinated hens compared with those from control hens (P = 0.0006).
Table 3. Comparison of mortality, gut lesions and bird performance in progeny from vaccinated and non-vaccinated broiler breeders.
The numbers of birds examined for the presence of gut lesions associated with NE, for all sites throughout the study, are presented in . No gross gut lesions were observed in any of the birds from vaccinated hens at either Site 1 or Site 2 ( and 4). Similarly, no C. perfringens-associated gross gut lesions were observed in birds hatched to control hens in weeks 30 or 35 at Site 1. In birds hatched to control hens at week 45, however, clinical signs of disease (ruffled feathers) were observed in a proportion of birds at 25 to 26 days of age and 66.6% (4/6) of birds examined post-mortem on day 26 had positive gut scores of either 2 (three birds) or 3 (one bird), giving a mean lesion score of 1.5 for all six birds. At Site 2, clinical signs of disease (depression, ruffled feathers and inappetance) were again observed in birds hatched to control hens, at around 24 to 26 days of age in flocks from both hatches. For the 27-week hatch, 92.3% (12/13) of birds examined on day 25 were found to have C. perfringens-associated gross gut lesions (mean lesion score 1.15) and 91.7% (11/12) on day 26 (mean lesion score 0.92), giving an overall mean lesion score of 1.1 (20 x score of 1, three x score of 2). For the 32-week hatch, 100% (12/12) of birds examined on day 25 were found to have C. perfringens-associated gross gut lesions (mean lesion score 1.58) and 60.0% (6/10) on day 26 (mean lesion score 0.7), giving an overall mean lesion score of 1.1 (11 x score of 1, seven x score of 2).
Table 4. Numbers of birds examined for the presence of NE gut lesions per week.
Confirmation of the diagnosis of NE was obtained by culturing gut or hepatic tissues from the birds in both vaccinate and control groups necropsied on the days when specific gut lesions where observed (i.e. Site 1, week 45 hatch, day 26; Site 2, week 27 and week 32 hatches, days 25 and 26) in order to identify the presence of C. perfringens or other pathogens. At both Sites 1 and 2 no C. perfringens was isolated from any of the birds hatched from vaccinated hens. In contrast, C. perfringens was isolated from gut samples of 1/4 birds from the week 45 hatch at Site 1, hatched to control hens at 26 days of age when lesions of NE were observed, and in 100% (25/25 Site 2, week 27 hatch and 10/10 Site 2, week 32 hatch) hatched to control hens at 26 days of age when lesions of NE were observed ().
Bird performance
Feed conversion ratios () were comparable between progeny from either vaccinated or control hens, and for each site were within the norms accepted by that producer.
For Site 1 the birds were slaughtered between 56 and 64 days of age. There were no significant differences in terms of rejects for broilers hatched from either vaccinated or control hens. Rejects before slaughter due to either low bodyweight (<800 g) or death during transport were as follows: week 30 hatch, controls 0.16%, vaccinates 0.15%; week 35 hatch, controls 0.16%, vaccinates 0.07%; and week 45 hatch, controls 0.29%, vaccinates 0.09%. Rejects after slaughter because of a poor-quality carcass were as follows: week 30 hatch, controls 0.81%, vaccinates 1.14%; week 35 hatch, controls 0.39%, vaccinates 0.34%; and week 45 hatch, controls 0.38%, vaccinates 0.41%. These figures were comparable with the values previously established for birds from this producer. At Site 2, males and females were slaughtered separately (females at 37 to 38 days of age, and males at 57 days of age). Yields (as defined by carcass weight divided by live body weight) were as follows: week 27 hatch, male controls 63.1%, male vaccinates 63.2%; female controls 64.0%, female vaccinates 64.0%; and week 32 hatch, male controls 63.3%, male vaccinates 63.5%; female controls 64.2%, female vaccinates 64.3%—these were comparable with the established norms for this producer.
Discussion
The present study investigated the safety and efficacy of a novel vaccine based on C. perfringens type A alpha-toxoid for the passive protection of broiler chickens against NE. The study was conducted at two commercial sites and involved a total of 11,234 vaccinated and 9304 control broiler breeders and the clinical observation of five batches of the broiler progeny.
Overall the vaccine appeared to be safe, with no systemic reactions or adverse effects on bird performance or reproduction observed. Differences in the incidence and size of local injection site reactions were noted between the two sites, and this may reflect differences in the breed of the birds. In general, the reactions were regarded as mild and appeared to resolve over time. Tissue fibrosis was observed in a small number of birds 30 to 35 weeks after initial vaccination, but it is impossible to correlate this with the use of Netvax™, since the birds received other injectable vaccines as part of the standard procedures at both locations.
As reported by other workers in laboratory studies (Løvland et al., 2004), we have confirmed that, under field conditions, vaccination of broiler breeder hens with a conventional C. perfringens alpha-toxoid vaccine induces the production of specific anti-alpha-toxin antibodies in the circulation of the hen, which remain at significant levels throughout the laying cycle. We have further demonstrated that specific antibodies are transferred from the circulation of the hen to egg yolk, resulting in elevated levels of such antibodies in the circulation of 7-day-old chicks hatched from eggs laid by broiler hens up to at least 45 weeks of age.
Passively acquired antibody is critical for the protection of newly hatched chicks, since during the first few weeks of life their immune system is not fully developed. Thus, although the first surface IgY-positive lymphocytes can be detected around the time of hatch (Kincade & Cooper, Citation1971; Lawton et al., Citation1975), Lawrence et al. (Citation1981) did not detect IgY secreting B cells in chick plasma until 6 days after hatching, and maturation of T and B lymphocytes in the gut-associated lymphoid tissue is not complete until about 2 weeks of age (Bar-Shira et al., Citation2003). It has been recognized that the principal mechanism for the transfer of IgY (IgG) from the hen to the circulation of the chick is via egg yolk and that the total levels observed in egg yolk are directly proportional to those seen in the hens’ serum (Loeken & Roth, Citation1983; Al-Natour et al., Citation2004). Alpha-toxin-specific IgY in egg yolk as measured by ELISA was found to demonstrate a declining trend over time, which was not observed when measured by HIA. This may reflect a difference in the relative sensitivities of the two test procedures. Alternatively, since the relationship between total IgY in hen serum and egg IgY appears to extend to the relative levels of antibodies against specific antigens (Hamal et al., Citation2006), this could reflect a maturation in the hens’ antibody response favouring functional (HIA) antibody over total anti-alpha-toxin antibody.
In poultry, C. perfringens infections may cause either a clinical or a sub-clinical form of disease. The clinical form of the disease results in increased mortality associated with gross lesions occurring predominantly in the jejunum (Wages & Opengart, Citation2003). It has been proposed that clinical disease results from the adhesion of C. perfringens to damaged mucosa facilitating bacterial proliferation and toxin production (Kageyama et al., Citation1987; Baba et al., Citation1992), and Si et al. (2007) demonstrated a positive correlation between the incidence of NE and both the level of alpha-toxin expression and increased C. perfringens proliferation. An average C. perfringens count of 5 log10 colony-forming units (CFU)/g appeared to be associated with a threshold for the development of NE with a lesion score of 2. In this study, in the control birds for both hatches at Site 2, clinical signs of NE were consistently observed during week 4 with median counts of approximately 3 log10 CFU/g associated with mean NE lesion scores of approximately 1.5. In both instances, however, treatment with amoxicillin was initiated immediately on the appearance of clinical signs and this would have controlled the proliferation of the organism and consequently minimized the development of intestinal lesions. No gross lesions associated with NE or clinical signs, however, were observed in the progeny of vaccinated hens. In addition, these chicks were also found to have an overall lower rate of mortality, with a statistically significant reduction associated with those time points at which gross gut lesions were also observed, thus providing strong evidence of efficacy against clinical disease under commercial conditions. Further evidence of clinical efficacy is provided by the failure to isolate detectable numbers of C. perfringens at any time from chicks from vaccinated hens, although this is in contrast to the findings of Løvland et al. (2004), where C. perfringens was isolated from the progeny of alpha-toxoid vaccinated hens with a median count of at least 4 log10 CFU/g from 21 days of age. The antigen content of this vaccine was, however, only 0.25 total combining power units/dose and the adjuvant was aluminium hydroxide gel rather than an oil emulsion, possibly resulting in the lower level of efficacy observed against clinical disease. In contrast, the sub-clinical form of the disease results in a reduced ability to digest and absorb food leading to lower body weight gain and increased feed conversion ratio (Elwinger et al., Citation1992; Løvland & Kaldhusdal, 2001). Whilst overall, final body weights were favourable for those birds hatched from eggs laid by vaccinated hens, there was no trend to improved feed conversion ratio (FCR). It is therefore likely that larger-scale trials may be required to confirm any effect on subclinical disease.
The results of this study support other evidence suggesting that alpha-toxin can act as a key protective immunogen for the prevention of NE in chickens (Kulkarni et al., Citation2007; Zekerias et al., 2008; Cooper et al., Citation2009), although it should be recognized that alternative immunogens have also been demonstrated to have a potential role in protection (Kulkarni et al., Citation2008; Jiang et al., Citation2009; Kulkarni et al., Citation2010) and that these may also be present in a cell-free supernatant toxoid.
Vaccination of broiler breeder hens with Netvax™ and the subsequent transfer of maternal immunity has thus been shown, under commercial conditions, to provide a safe and efficacious mechanism to reduce both the mortality and the severity of gut lesions associated with NE in their progeny.
Acknowledgements
The authors thank Beth Cook and David Lane for their technical assistance in the preparation and testing of serum and egg IgY samples, and Dr Dianne Sweeney for performing the statistical analysis.
References
- Al-Natour , M.Q. , Ward , L.A. , Saif , Y.M. , Stewart , B. and Keck , L.D. 2004 . Effect of different levels of maternally derived antibodies on protection against infectious bursal disease virus . Avian Diseases , 48 : 177 – 182 .
- Al-Sheikhly , F. and Truscott , R.B. 1977 . The interaction of Clostridium perfringens and its toxins in the production of necrotic enteritis of chickens . Avian Diseases , 21 : 256 – 263 .
- Baba , E. , Wakeshima , H. , Fukui , K. , Fukata , T. and Arakawa , A. 1992 . Adhesion of bacteria to the cecal mucosal surface of conventional and germ-free chickens infected with Eimeria tenella . American Journal of Veterinary Research , 53 : 194 – 197 .
- Bar-Shira , E. , Sklan , D. and Friedman , A. 2003 . Establishment of immune competence in the avian GALT during the immediate post hatch period . Developmental and Comparative Immunology , 27 : 147 – 157 .
- Cooper , K.K. and Songer , J.G. 2009 . Necrotic enteritis in chickens: a paradigm of enteric infection by Clostridium perfringens type A . Anaerobe , 15 : 55 – 60 .
- Cooper , K.K. , Trinh , J. and Songer , J.G. 2009 . Immunization with recombinant alpha toxin partially protects broiler chicks against experimental challenge with Clostridium perfringens . Veterinary Microbiology , 133 : 92 – 97 .
- Elwinger , K. , Schneitz , C. , Berndtson , E. , Fossum , O. , Teglof , B. and Engstom , B. 1992 . Factors affecting the incidence of necrotic enteritis, caecal carriage of Clostridium perfringens and bird performance in broiler chicks . Acta Veterinaria Scandinavica , 33 : 369 – 378 .
- Ficken , M.D. and Wages , D.P. 1997 . “ Necrotic enteritis ” . In Diseases of Poultry , 10th edn , Edited by: Calnek , B.W. , Barnes , H.J. , Beard , C.W. , McDougald , L.R. and Saif , Y.M. 261 – 264 . Ames , IA : Mosby-Wolfe .
- Fukata , T. , Hadate , Y. , Baba , E. , Uemura , T. and Arakawa , A. 1988 . Influence of Clostridium perfringens and its toxin in germ-free chickens . Research in Veterinary Science , 44 : 68 – 70 .
- Hamal , K.R. , Burgess , S.C. , Pevzner , I.Y. and Erf , G.F. 2006 . Maternal antibody transfer from dams to their egg yolks, egg whites and chicks in meat lines of chickens . Poultry Science , 85 : 1364 – 1372 .
- Heier , B.T. , Løvland , A. , Soleim , K.B. , Kaldhusdal , M. and Jarp , J. 2001 . A field study of naturally occurring specific antibodies against Clostridium perfringens alpha toxin in Norwegian broiler flocks . Avian Diseases , 45 : 724 – 732 .
- Jiang , Y. , Kulkarni , R.R. , Parreira , V.R. and Prescott , J.F. 2009 . Immunization of broiler chickens against Clostridium perfringens-induced necrotic enteritis using purified recombinant immunogenic proteins . Avian Diseases , 53 : 409 – 415 .
- Kageyama , A. , Fukata , T. , Baba , E. and Arakawa , A. 1987 . The influence of various bacteria on the cecal mucosa of monoflora chickens infected with Eimeria tenella. A scanning electron microscopic study . Zentralblatt für Bakteriologie, Mikrobiologie und Hygiene , A265 : 353 – 359 .
- Kaldhusdal , M. and Løvland , A. 2000 . Necrotic enteritis (4): the economical impact of Clostridium perfringens is greater than anticipated . World Poultry , 16 : 50 – 51 .
- Keyburn , A.L. , Sheedy , S.A. , Ford , M.E. , Williamson , M.M. , Awad , M.M. , Rood , J.I. and Moore , R.J. 2006 . Alpha-toxin of Clostridium perfringens is not an essential virulence factor in necrotic enteritis in chickens . Infection and Immunity , 74 : 6496 – 6500 .
- Kincade , P.W. and Cooper , M.D. 1971 . Development and distribution of immunoglobulin-containing cells in the chicken. an immunofluorescent analysis using purified antibodies to µ, γ and light chains . The Journal of Immunology , 106 : 371 – 382 .
- Kulkarni , R.R. , Parreira , V.R. , Sharif , S. and Prescott , J.F. 2007 . Immunization of broiler chickens against Clostridium perfringens-induced necrotic enteritis . Clinical and Vaccine Immunology , 14 : 1070 – 1077 .
- Kulkarni , R.R. , Parreira , V.R. , Sharif , S. and Prescott , J.F. 2008 . Oral immunisation of broiler chickens against necrotic enteritis with an attenuated Salmonella vaccine vector expressing Clostridium perfringens antigens . Vaccine , 26 : 4194 – 4203 .
- Kulkarni , R.R. , Parreira , V.R. , Jiang , Y.-F. and Prescott , J.F. 2010 . A live oral recombinant Salmonella enterica serovar Typhimurium vaccine expressing Clostridium perfringens antigens confers protection against necrotic enteritis in broiler chickens . Clinical and Vaccine Immunology , 17 : 205 – 214 .
- Lawrence E.C. Arnaud-Battandier F. Grayson J. Koski I.R. Dooley N.J. Muchmore A.V. Blaese R.M. 1981 Ontogeny of humoral immune function in normal chickens: a comparison of immunoglobulin-secreting cells in bone marrow, spleen, lungs and intestine Clinical and Experimental Immunology 43 50 457
- Lawton , A.R. , Kincade , P.W. and Cooper , M.D. 1975 . Sequential expression of germ lines in development of immunoglobulin class diversity . Federaltion Proceedings , 34 : 33 – 39 .
- Loeken , M.R. and Roth , T.F. 1983 . Analysis of maternal IgG subpopulations which are transported into the chicken oocyte . Immunology , 49 : 21 – 28 .
- Løvland , A. and Kaldhudsal , M. 2001 . Severely impaired production performance in broiler flocks with high incidence of Clostridium perfringens-associated hepatitis . Avian Pathology , 30 : 73 – 81 .
- Løvland , A. , Kaldhusdal , M. , Redhead , K. , Skjerve , E. and Lillehaug , A. 2004 . Maternal vaccination against subclinical necrotic enteritis in broilers . Avian Pathology , 33 : 83 – 92 .
- McDevitt , R.M. , Brooker , J.D. , Acamovic , T. and Sparks , N.H.C. 2006 . Necrotic enteritis: a continuing challenge for the poultry industry . World's Poultry Science Journal , 62 : 221 – 247 .
- Porter , R.E. Jr . 1998 . Bacterial enteritides of poultry . Poultry Science , 77 : 1159 – 1165 .
- Si , W. , Gong , J. , Han , Y. , Yu , H. , Brennan , J. , Zhou , H. and Chen , S. 2007 . Quantification of cell proliferation and alpha-toxin gene expression of Clostridium perfringens in the development of necrotic enteritis in broiler chickens . Applied and Environmental Microbiology , 73 : 7110 – 7113 .
- Songer , J.G. 1996 . Clostridial enteric diseases of domestic animals . Clinical Microbiological Reviews , 9 : 216 – 234 .
- Thompson , D.R. , Parreira , V.R. , Kulkarni , R.R. and Prescott , J.F. 2006 . Live attenuated vaccine-based control of necrotic enteritis of broiler chickens . Veterinary Microbiology , 113 : 25 – 34 .
- Truscott , R.B. and Al-Sheikhly , F. 1977 . Reproduction and treatment of necrotic enteritis in broilers . American Journal of Veterinary Research , 38 : 857 – 861 .
- Wages , D.P. and Opengart , K. 2003 . “ Necrotic enteritis ” . In Diseases of Poultry , 11th edn , Edited by: Saif , Y.M. , Barnes , H.J. , Glisson , J.R. , Fadly , A.M. , McDougald , L.R. and Swayne , D.E. 781 – 785 . Ames : Iowa State Press .
- Williams , R.B. 2005 . Intercurrent coccidiosis and necrotic enteritis of chickens: rational, integrated disease management by maintenance of gut integrity . Avian Pathology , 34 : 159 – 180 .
- Zekarias , B. , Mo , H. and Curtiss III , R. 2008 . Recombinant attenuated Salmonella enterica serovar Typhimurium expressing the carboxy-terminal domain of alpha toxin from Clostridium perfringens induces protective responses against necrotic enteritis in chickens . Clinical and Vaccine Immunology , 15 : 805 – 816 .