Abstract
The aim of the present study was the molecular identification of a common source of infection of Campylobacter coli in two grandparent breeder farms. Campylobacter jejuni and C. coli were isolated from well water and cloacal swabs from grandparent chickens. Colonies were genotyped using restriction fragment length polymorphism-flaA gene, pulsed field gel electrophoresis and multi-locus sequence typing. The same genotype of C. coli was found in both farms and in the well from which drinking water was supplied to the farms. The well water was epidemiologically linked as the source of C. coli infection. The molecular identification for epidemiological source-tracking of C. coli in breeder farms could aid in combating the colonization of this pathogen and therefore to reduce their incidence in human campylobacteriosis.
Introduction
Thermophilic members of Campylobacter are recognized as the main cause of bacterial acute gastroenteritis in man in most of the industrialized countries (Galanis, Citation2007). The most common species reported is Campylobacter jejuni (80 to 90% of cases) (Janssen et al., Citation2008) followed by Campylobacter coli (18.6%) (Gürtler et al., Citation2005). Chicken products remain the primary source of infection (Vellinga & Van Loock, Citation2002; Kapperud et al., Citation2003). The reduction and/or elimination of Campylobacter spp. from chicken products is a major strategy in the efforts to control this disease. Therefore, it is crucial to ascertain the sources of infection for chicken farms as well as finding control measures to prevent colonization. The tight biosecurity measures and the breeding conditions in grandparent breeder farms make them good scenarios in the search for possible sources of infection.
According to different studies, infection on chicken farms could enter the flock by vertical transmission from parent flocks (Cox et al., Citation2002), or by horizontal transmission via different sources (Pearson et al., Citation1993; van de Giessen et al., Citation1998; Petersen & Wedderkopp, Citation2001; Hiett et al., Citation2002; Hansson et al., Citation2007). Groundwater is frequently used as drinking water in poultry farms. Several studies have been conducted to prove whether contaminated groundwater was the source of Campylobacter spp. in poultry flocks, and most studies have shown evidence of the involvement of groundwater as a possible source of infection (Pearson et al., Citation1993; Stanley et al., Citation1998; Zimmer et al., Citation2003). Recently Ogden et al. (Citation2007), using multi-locus sequence typing (MLST) as a molecular marker, have found the same C. jejuni sequence types both in water tanks and broiler farms, showing the possibility of water as a source of infection in broilers.
The aim of the present study was to determine whether the drinking water was the source of C. coli infection in two grandparent heavy breeder flocks by demonstrating that the species and genotype of strains found in the drinking water were identical to those isolated from the chickens.
Materials and Methods
Rearing farms
The samples were taken in two rearing farms of grandparent heavy breeders located in a central region of Spain. The rearing farms are identified as R-1 and R-2, and the distance between the two is approximately 500 m. Farm R-1 is comprised of two sheds and Farm R-2 of three sheds. Around the rearing farms there are five production farms separated by a distance of about 3 km, without a common source of water, farm staff and equipment to the rearing farms. There are no other farms of other livestock in the region.
Both rearing and production farms have very strict biosecurity procedures. The vehicles entering the farm are thoroughly cleaned and disinfected. The farm staff follow tight biosecurity practices with showers and change of clothing, including boots, at the main entrance of the farm and another change of boots at each chicken house. Each farm has its own staff and equipment. Windows and ventilation system are covered by a mesh in order to avoid the entrance of insects and droppings of wild birds. The birds remain on the rearing farms from their first day of life until the age of 20 weeks, when they are moved to the production farms. The difference age between the flocks reared in the two rearing farms was 12 weeks. When the farm is empty, the litter is removed by lorry, the feed belts and bell drinkers are removed and thoroughly cleaned and disinfected.
The water used in both farms comes from a well with a depth of 15 m with the borehole covered with a metal lid. This well is located on Farm R-2, at a distance of about 20 m from the sheds. A pump at the top of the borehole fills a tank with a capacity of 17 m3. The pump starts to work when the water level drops and thus keeps the level constant. The well and tank are located inside a small building to prevent contamination of the water as a consequence of contact with wild birds or other animals. This tank is shared by both rearing farms. The drinking water is not used for human consumption.
The water is sanitized by an automatic chlorination system that works by pulse dosage. When the pump starts to work, the system pours the appropriate volume of a sodium hypochlorite solution into the water tank in order to achieve a level of free chlorine between 2 and 3 ppm at the end of the drinker lines. From the tank the water is distributed to both rearing farms through polyethylene pipes using a pressure pump that sends the water (pressure level, 4 to 5 bars) to a 500 l steel tank. When the water reaches the shed it is distributed through a polyvinyl chloride pipe at a minimum pressure of 5 bars. The quantity of water consumed in each shed is controlled by a flowmeter and there is one electrovalve that can be used to ration the water, depending on the needs of the poultry and seasonal variations. Inside the sheds there is one polyvinyl chloride pipe that branches into a number of pipes where the bell drinkers are located. There are four drinking lines inside each shed.
Water samples
One sample of 20 l water was taken aseptically from the pipe connected to the pump of the well that pours into the tank shared by the two rearing farms (R-1 and R-2). The sample was collected in two sterile 10 l opaque plastic containers and was transported at a temperature of 4°C to the laboratory and analysed within 6 h.
Analysis of water samples
The samples were analysed according to the Food and Drug Administration Analytical Manual (http://www.fda.gov/Food/ScienceResearch/LaboratoryMethods/BacteriologicalAnalyticalManualBAM/UCM072616). Briefly, the water was filtered using a peristaltic pump through positively charged 0.45 µm diameter Zetapor filters (Cuno, Meriden, CT, USA). The filters were cut up into small pieces and cultured in 100 ml Bolton broth (CM0983B; Oxoid, Madrid, Spain). The broth was incubated for 3 h at 30°C, then at 37°C for a further 2 h, and finally at 42°C for 48 h. All incubations were carried out under microaerobic conditions (Campygen 3.5 L; Oxoid).
After both 24 and 48 h of incubation, 100 µl Bolton broth was streaked onto one plate of Karmali agar (CM0935B; Oxoid) and another plate of Abeyta-Hunt-Bark agar (according to the Food and Drug Administration). The plates were incubated for 48-h periods at 42°C in a microaerobic environment. Subsequently, the suspected colonies were selected and streaked on blood agar plates (Mueller-Hinton agar supplemented with 5% sheep blood; Becton Dickinson GmBH, Heidelberg, Germany) for their identification using biochemical tests.
Cloacal swabs
Since 2001, samples have been taken for surveillance from 60 birds in each flock when the selection of males takes place (5 to 7 weeks of age) and when the birds are moved to the production farm (18 to 20 weeks of age). In the present study, a similar sampling procedure had been applied from Farm R-2 (6 weeks of age) and 1 week later from Farm R-1 (19 weeks of age). Related to the positive results obtained in the analyses from Farm R-1 and from the water samples collected 1 week later, another 60 cloacal swabs were taken from birds in Farm R-2 (9 weeks of age) 1 week after collecting the water samples. The swabs were placed in a Cary-Blair transport medium and sent to the laboratory. All samples were cultivated within 6 h. Each swab was streaked on Karmali agar and Abeyta-Hunt-Bark agar. The plates were incubated for 48 h at 42°C under microaerobic conditions. Subsequently, the suspect colonies were collected and streaked on blood agar plates for identification
Morphological, biochemical, and polymerase chain reaction identification
The suspect colonies, one selected from each cloacal swab, were identified as Campylobacter spp. when the organisms showed the typical curved or “seagull” morphology under microscopic observation, after staining for 3 min with 50% crystal violet in distilled water, and were positive in oxidase and catalase tests. Finally, the isolates were identified by means of the hippurate hydrolysis test. Colonies with a negative or doubtful result for the hippurate test were identified at the species level with a previously described multiplex polymerase chain reaction (PCR) (Persson & Olsen, Citation2005).
Genotyping
Molecular characterization of strains was carried out using a combination of: firstly, PCR amplification of the flaA gene, followed by restriction fragment length polymorphism (RFLP) of the PCR products obtained using DdeI (RFLP-flaA); secondly, DNA macrorestriction analysis by pulsed field gel electrophoresis (PFGE) using two different restriction enzymes (SmaI and KpnI); and thirdly, by MLST of seven housekeeping genes.
RFLP-flaA typing
The DNA template was obtained using a simple DNA extraction procedure (Yates et al., Citation2002). PCR amplification was performed with a Ready-to-Go system (GE Healthcare, Little Chalfont, UK). RFLP-flaA with the restriction enzyme DdeI (Roche, Madrid, Spain) was performed following the Campynet protocol (Harrington et al., Citation2003).
Pulsed field gel electrophoresis typing
Preparation of Campylobacter DNA was performed by the Pulsenet protocol (Ribot et al., Citation2001). PFGE by SmaI (Roche) was also performed by the Pulsenet protocol and by KpnI (Takara, Conda, Madrid, Spain) using the protocol of On et al. (Citation1998). PFGE profiles were assigned to pulsotypes on the basis of one or more band difference between strains.
Computer-assisted analysis of fingerprinting experiments
All of the fingerprinting experiments—RFLP-flaA, PFGE-SmaI and PFGE-KpnI—were analysed with a global comparison using an average estimation from experiments using the InfoQuest FP software (Bio-Rad, Madrid, Spain). The dendrogram was constructed using the unweighted pair group method of averages.
Multi-locus sequence typing amplification and sequencing
MLST was performed as previously described (Dingle et al., Citation2001). All allelic sequences were compared against the C. jejuni/C. coli MLST database (http://pubmlst.org/campylobacter/). Novel sequences were entered into the MLST database and assigned new allele numbers. Clonal complexes were defined as groups of two or more independent strains that shared identical alleles at five or more loci; each complex was named after the putative founder sequence type.
Results
The results reported here were obtained in 2004; all previous flocks since 2001 had been negative for campylobacter. A total of 91 isolates were obtained from the chicken cloacal swabs. The prevalence was about 90% in Farm R-1 (54 positive out of 60 samples) and 61.7% in Farm R-2 (37 out of 60 samples). The organisms all colonies showed the typical curved or seagull shape under microscopy, they were positive in oxidase and catalase tests, and were identified as C. jejuni (38 colonies) or C. coli (53 colonies). Our investigations also revealed that both species were found in all of the sheds at the two farms. One week after isolating Campylobacter spp. on Farm R-1 and 1 week before isolating Campylobacter spp. on Farm R-2, we isolated Campylobacter spp. from the well water. Twenty suspect colonies of Campylobacter spp. were chosen from the Karmali and Abeyta-Hunt-Bark agar plates, which had been cultured from Bolton broth inoculated with the 0.45 µm water filters. Thirteen and seven colonies were identified as C. jejuni and C. coli, respectively.
The number of C. jejuni and C. coli colonies, the genotypes found, and the number of strains belonging to each genotype are summarized in . In each farm, there were no differences among the distribution of the genotypes from the sheds. The relationship between different strains determined by PFGE using SmaI and KpnI and RFLP of the flaA gene is shown in . The C. coli genotype RFLP-flaA2-Sma2-Kpn2-ST827 was the only one found in both the well water and the two rearing farms. Two C. jejuni strains isolated from chickens on rearing Farm R-1 and 13 strains from Farm R-2 had the same genotype RFLP-flaA3-Sma3-Kpn3-ST46. However, strains of this genotype were not isolated from the drinking water.
Figure 1. Dendrogram showing the relatedness among the strains using all of the fingerprinting experiments with a global comparison. Dendrogram constructed with the unweighted pair group method of averages. CJ, C. jejuni; CC, C. coli; ST, sequence type using MLST; NS, number of strains isolated with this genotype; R-1 and R-2, farms; WW, well water.
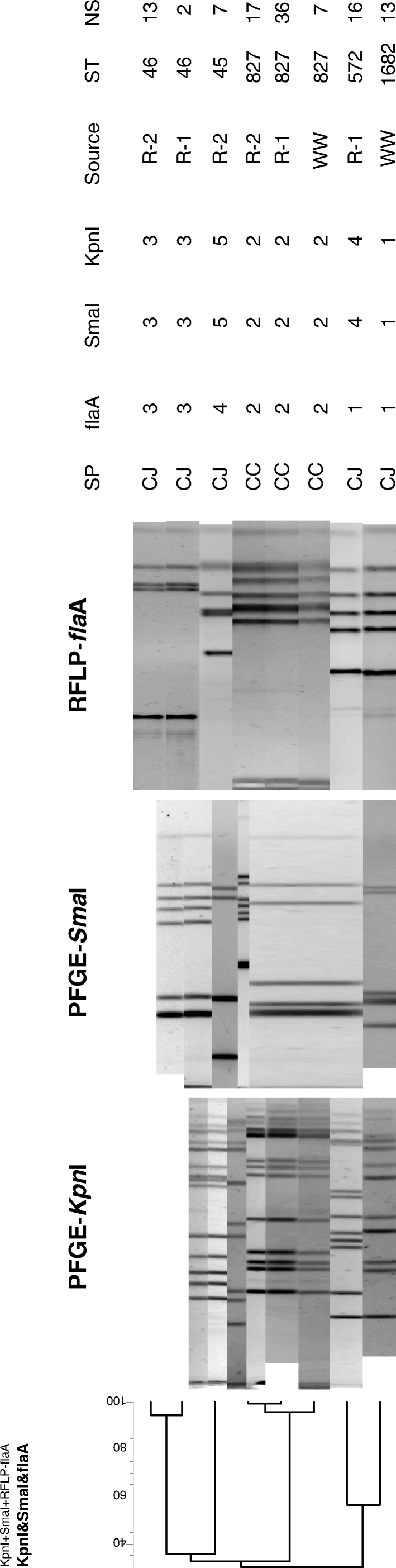
Table 1. Distribution of Campylobacter spp. genotypes isolates according to DdeI RFLP of the flaA gene, PFGE-SmaI, PFGE-KpnI and MLST alleles and corresponding sequence types.
Discussion
A C. coli clone was isolated from the water supply of two chicken rearing farms (Farms R-1 and R-2) during summer 2004. The same C. coli genotype had been isolated 1 week before from chicken cloacal swabs from Farm R-1. Farm R-2 was negative for Campylobacter spp. 1 week before the positive results in rearing Farm R-1, but 1 week after the water sampling the same C. coli genotype was isolated from chicken cloacal swabs from Farm R-2.
Chickens are not the main host for C. coli, which show a preference for swine or cattle (Botteldoorn et al., Citation2001; Oporto et al., Citation2007), although some studies have shown that C. coli is capable of colonizing chickens efficiently (Bull et al., Citation2006; Miller et al., Citation2006). This fact is proven in our study, in which C. coli appeared in a higher percentage of chickens than C. jejuni.
It would be difficult to identify the origin of the C. coli strain found in the drinking water. Firstly, the well and header tank are located inside a building and the borehole was fitted with a lid, which always remains closed. Therefore the contamination by Campylobacter from within the sheds or from non-water vectors would be difficult. Secondly, it is unlikely that contamination of the water came from the population of birds inside the poultry farm because the bacteria would have had to track back upstream against the water flow. Therefore, there is a strong possibility that the water was contaminated before entering the header tank.
The MLST data do not allow one to establish a host association between the C. coli strains isolated from water and birds. They belong to a clonal complex (ST-828 clonal complex) that includes strains isolated from many different sources, including cattle, chickens, pigs, and humans among others.
Most of the strains isolated from water belonged to the C. jejuni genotype fla1-Sma1-Kpn1-ST1682. The new ST1682 was detected for the first time in our study. This ST was not isolated from birds, and its ability to colonize birds has not yet been studied. C. jejuni strains with the fla3-Sma3-Kpn3-ST46 genotype were isolated from chickens from both rearing farms. The fact that they were isolated on both farms points to a common source. This genotype was not isolated from the water samples, either because it was not there, the concentration was too low for detection, or the strains were in a viable but non-culturable form.
It is very difficult to specify the possible origin of the remaining genotypes isolated from chickens in the two rearing farms (fla1-Sma4-Kpn4-ST572 and fla4-Sma5-Kpn5-ST45). The first genotype shares the same flaA type with one of the water genotypes, but the remaining markers (as seen in ) did not possess any similarity. However, it is likely that the water supply was also the source of these strains if we take into account the strict biosecurity measures on both farms.
With regard to the water sanitization measures, the water was treated with a sodium hypochlorite solution in order to achieve a level of free chlorine between 2 and 3 parts/106 at the end of the drinker lines. However, this treatment proved ineffective in preventing infection of the chickens or reducing the proportion of chickens colonized. These results are in accordance with previous studies about the influence of chlorination in delaying the onset or decreasing the prevalence of colonization in the treated flocks as compared with the control flocks (Stern et al., Citation2002). After the infection, the chlorination system was revised and the dosage was enhanced. The well was not closed because it is the only source of water for these farms. Since then, C. coli has not been isolated from the farms.
To our knowledge, this is the first time that the same C. coli strains have been isolated from groundwater and from chickens using the groundwater as drinking water. The strains isolated from birds and water belong to the same clone because the patterns obtained with four molecular methods are nearly the same, and therefore we have shown that drinking water is one of the sources of C. coli on chicken farms.
Acknowledgements
The present study was supported in part by grant P.I. 02/26 from the Instituto de Salud Carlos III (Spain). This publication used the Campylobacter Multi Locus Sequence Typing database developed by Keith Jolley and Man-Sue Chan from the University of Oxford. The authors gratefully thank Patricia Sánchez Barreno for her comments and suggestions, and Susana Pedraza Díaz for her critical revision of the manuscript.
Additional information
Notes on contributors
David Pérez-Boto
David Pérez-Boto and Francisco J. García-Peña contributed equally to this workReferences
- Botteldoorn , N. , Heyndrickx , M. , Rijpens , N. and Herman , L. 2001 . Prevalence of Salmonella, Campylobacter and VTEC on pig farms . Mededelingen (Rijksuniversiteit te Gent Fakulteit van de Landbouwkundige en Toegepaste Biologische Wetenschappen) , 66 : 373 – 380 .
- Bull , S.A. , Allen , V.M. , Domingue , G. , Jorgensen , F. , Frost , J.A. , Ure , R. and Humphrey , T.J. 2006 . Sources of Campylobacter spp. colonizing housed broiler flocks during rearing . Applied and Environmental Microbiology , 72 : 645 – 652 .
- Cox , N.A. , Stern , N.J. , Hiett , K.L. and Berrang , M.E. 2002 . Identification of a new source of Campylobacter contamination in poultry: transmission from breeder hens to broiler chickens . Avian Diseases , 46 : 535 – 541 .
- Dingle , K.E. , Colles , F.M. , Wareing , D.R. , Ure , R. , Fox , A.J. , Bolton , F.E. and Maiden , M.C. 2001 . Multilocus sequence typing system for Campylobacter jejuni . Journal of Clinical Microbiology , 39 : 14 – 23 .
- Galanis , E. 2007 . Campylobacter and bacterial gastroenteritis . Canadian Medical Association Journal , 177 : 570 – 571 .
- Gürtler , M. , Alter , T. , Kasimir , S. and Fehlhaber , K. 2005 . The importance of Campylobacter coli in human campylobacteriosis: prevalence and genetic characterization . Epidemiology and Infection , 133 : 1081 – 1087 .
- Hansson , I. , Vagsholm , I. , Svensson , L. and Olsson Engvall , E. 2007 . Correlations between Campylobacter spp. prevalence in the environment and broiler flocks . Journal of Applied Microbiology , 103 : 640 – 649 .
- Harrington , C.S. , Moran , L. , Ridley , A.M. , Newell , D.G. and Madden , R.H. 2003 . Inter-laboratory evaluation of three flagellin PCR/RFLP methods for typing Campylobacter jejuni and C. coli: the CAMPYNET experience . Journal of Applied Microbiology , 95 : 1321 – 1333 .
- Hiett , K.L. , Stern , N.J. , Fedorka-Cray , P. , Cox , N.A. , Musgrove , M.T. and Ladely , S. 2002 . Molecular subtype analyses of Campylobacter spp. from Arkansas and California poultry operations . Applied and Environmental Microbiology , 68 : 6220 – 6236 .
- Janssen , R. , Krogfelt , K.A. , Cawthraw , S.A. , van Pelt , W. , Wagenaar , J.A. and Owen , R.J. 2008 . Host–pathogen interactions in Campylobacter infections: the host perspective . Clinical Microbiology Reviews , 21 : 505 – 518 .
- Kapperud , G. , Espeland , G. , Wahl , E. , Walde , A. , Herikstad , H. , Gustavsen , S. and Digranes , A. 2003 . Factors associated with increased and decreased risk of Campylobacter infection: a prospective case-control study in Norway . American Journal of Epidemiology , 158 : 234 – 242 .
- Miller W.G. Englen M.D. Kathariou S. Wesley I.V. Wang G. Pittenger-Alley L.S Mandrell R.E. 2006 Identification of host-associated alleles by multilocus sequence typing of Campylobacter coli strains from food animals Microbiology 152 245 255
- Ogden , I.D. , MacRae , M. , Johnston , M. , Strachan , N.J. , Cody , A.J. , Dingle , K.E. and Newell , D.G. 2007 . Use of multilocus sequence typing to investigate the association between the presence of Campylobacter spp. in broiler drinking water and Campylobacter colonization in broilers . Applied and Environmental Microbiology , 73 : 5125 – 5129 .
- On , S.L. , Nielsen , E.M. , Engberg , J. and Madsen , M. 1998 . Validity of SmaI-defined genotypes of Campylobacter jejuni examined by SalI, KpnI, and BamHI polymorphisms: evidence of identical clones infecting humans, poultry, and cattle . Epidemiology and Infection , 120 : 231 – 237 .
- Oporto , B. , Esteban , J.I. , Aduriz , G. , Juste , R.A. and Hurtado , A. 2007 . Prevalence and strain diversity of thermophilic campylobacters in cattle, sheep and swine farms . Journal of Applied Microbiology , 103 : 977 – 984 .
- Pearson , A.D. , Greenwood , M. , Healing , T.D. , Rollins , D. , Shahamat , M. , Donaldson , J. and Colwell , R.R. 1993 . Colonization of broiler chickens by waterborne Campylobacter jejuni . Applied and Environmental Microbiology , 59 : 987 – 996 .
- Persson , S. and Olsen , K.E. 2005 . Multiplex PCR for identification of Campylobacter coli and Campylobacter jejuni from pure cultures and directly on stool samples . Journal of Medical Microbiology , 54 : 1043 – 1047 .
- Petersen , L. and Wedderkopp , A. 2001 . Evidence that certain clones of Campylobacter jejuni persist during successive broiler flock rotations . Applied and Environmental Microbiology , 67 : 2739 – 2745 .
- Ribot , E.M. , Fitzgerald , C. , Kubota , K. , Swaminathan , B. and Barrett , T.J. 2001 . Rapid pulsed-field gel electrophoresis protocol for subtyping of Campylobacter jejuni . Journal of Clinical Microbiology , 39 : 1889 – 1894 .
- Stanley , K. , Cunningham , R. and Jones , K. 1998 . Isolation of Campylobacter jejuni from groundwater . Journal of Applied Microbiology , 85 : 187 – 191 .
- Stern , N.J. , Robach , M.C. , Cox , N.A. and Musgrove , M.T. 2002 . Effect of drinking water chlorination on Campylobacter spp. colonization of broilers . Avian Diseases , 46 : 401 – 404 .
- Van de Giessen , A.W. , Tilburg , J.J. , Ritmeester , W.S. and Van der Plas , J. 1998 . Reduction of Campylobacter infections in broiler flocks by application of hygiene measures . Epidemiology and Infection , 121 : 57 – 66 .
- Vellinga , A. and Van Loock , F. 2002 . The dioxin crisis as experiment to determine poultry-related Campylobacter enteritis . Emerging Infectious Diseases , 8 : 19 – 22 .
- Yates , M.D. , Drobniewski , F.A. and Wilson , S.M. 2002 . Evaluation of a rapid PCR-based epidemiological typing method for routine studies of Mycobacterium tuberculosis . Journal of Clinical Microbiology , 40 : 712 – 714 .
- Zimmer , M. , Barnhart , H. , Idris , U. and Lee , M.D. 2003 . Detection of Campylobacter jejuni strains in the water lines of a commercial broiler house and their relationship to the strains that colonized the chickens . Avian Diseases , 47 : 101 – 107 .