Abstract
Infection with the zoonotic bacterium Erysipelothrix rhusiopathiae causes severe disease outbreaks (erysipelas) in poultry flocks. As this bacterium has been isolated from the poultry red mite (Dermanyssus gallinae), this parasite has been suggested as a possible means of transmission of E. rhusiopathiae on and between poultry farms. To further elucidate the capacity of the mite as a reservoir, we analysed and compared 56 bacterial isolates from laying hens and nine isolates from mites by pulsed-field gel electrophoresis (PFGE), using the restriction enzyme SmaI. The isolates originated from one outbreak in a laying hen flock housed in an indoor litter-based aviary system. Except for two isolates, a homogeneous banding pattern was obtained from all isolates analysed, suggesting that a single strain was the cause of the outbreak. Another finding was that isolates from individual hens could exhibit slightly different PFGE patterns. Mites collected from the same house at the end of the production period of the following flock were negative for the presence of E. rhusiopathiae. An increasing number of erysipelas outbreaks as well as escalating problems with D. gallinae are expected in other European countries related to the forthcoming changes in housing systems for laying hens. Consequently, further studies are needed to investigate the importance of erysipelas in poultry and the importance of D. gallinae in the transmission of E. rhusiopathiae.
Introduction
Infections with the Gram-positive bacterium Erysipelothrix rhusiopathiae occur worldwide in many species, including humans (Wang et al., Citation2010). In poultry, disease outbreaks (erysipelas) of economic significance are infrequently reported in species other than turkeys (Bricker & Saif, Citation2008). Recently, outbreaks in chickens have also been reported from a few European countries (Eriksson et al., Citation2003; Købke et al., Citation2005; Mazaheri et al., Citation2005). Pulsed-field gel electrophoresis (PFGE) is a method used to study the genetic and epidemiologic relatedness of bacterial isolates recovered from such outbreaks (Tenover et al., Citation1995). This method has previously been successfully used for studies of E. rhusiopathiae (Opriessnig et al., Citation2004; Købke et al., Citation2005; Eriksson et al., Citation2009).
The poultry red mite (Dermanyssus gallinae, De Geer), a blood-feeding ectoparasite, has been suggested to be a vector of a variety of pathogens in chickens including E. rhusiopathiae (Chirico et al., Citation2003; Valiente Moro et al., Citation2005). The mite is distributed globally on poultry farms, where it may cause animal welfare problems as well as economic losses (Sparagano et al., Citation2009). The parasites hide in cracks and crevices in the poultry house, escaping both cleaning and eradication attempts (Nordenfors & Höglund, Citation2000).
In this paper we describe an outbreak of erysipelas in a D. gallinae-infected flock of laying hens housed in an indoor litter-based aviary system. The main objective of the study was to determine whether there was a clonal relationship between isolates of E. rhusiopathiae from laying hens and mites, using PFGE analysis. The second aim was to investigate whether D. gallinae could act as a reservoir of the bacterium on poultry farms over time.
Materials and methods
Flock data
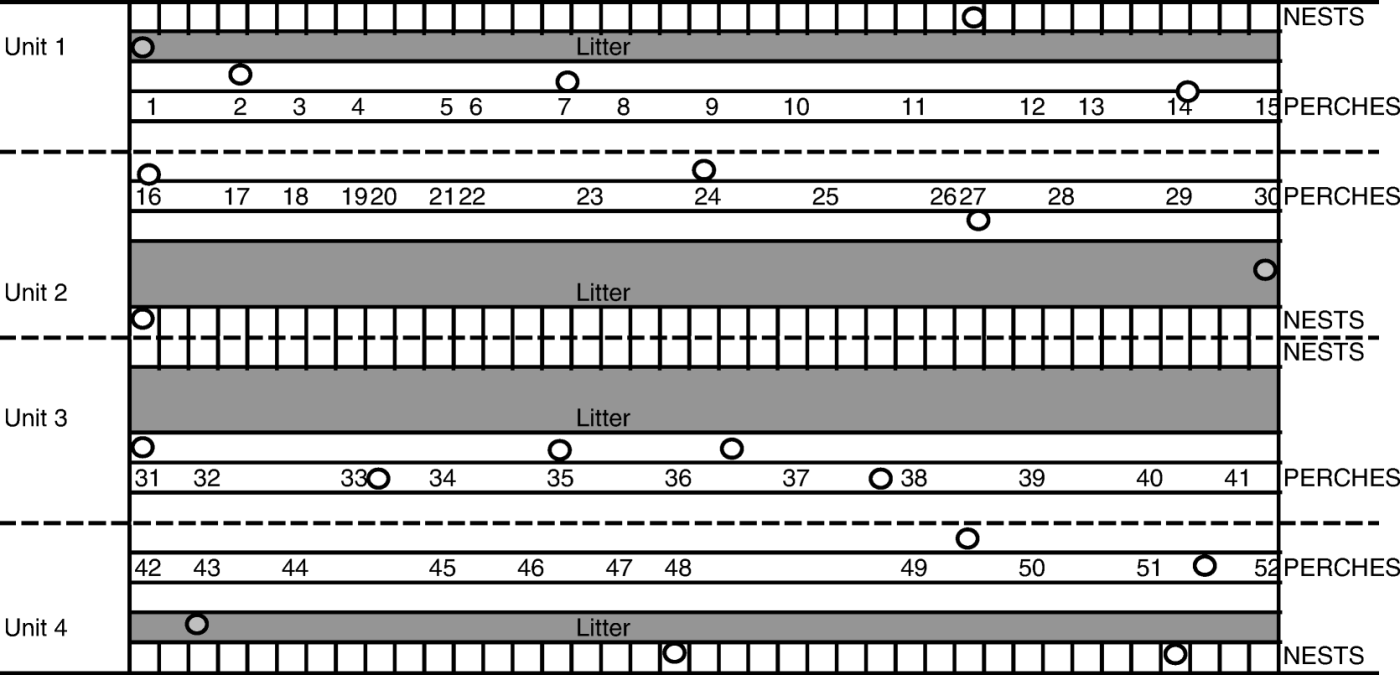
In April 2008 a sudden increase in mortality and a decrease in egg production were observed in a 62-week-old Swedish laying hen flock, originally consisting of 6030 birds. Necropsies were performed at a regional laboratory, where E. rhusiopathiae was isolated. The flock was kept in a house previously used for pig production. In 1994 the house was reconstructed into an indoor litter-based system, housing laying hens for 10 years. After that, the facility was empty for 2 years until the flock affected by the erysipelas outbreak was introduced in May 2007. The house consisted of four units, which on some levels were separated only by wire netting (). Hens of the hybrid Lohmann LSL were housed in Units 1 and 2, and Super Nick hens in Units 3 and 4. According to the farmer, infection with E. rhusiopathiae had not previously been diagnosed in flocks in this house.
Figure 1. Schematic illustration of sampling locations within the poultry house. Circles, dead hens for necropsy and isolation of E. rhusiopathiae were collected; numbers, location of mite traps. Traps were pooled as follows; Pool 1 (Traps 1 to 5), Pool 2 (Traps 6 to 10), Pool 3 (Traps 11 to 15), Pool 4 (Traps 16 to 21), Pool 5 (Traps 22 to 25), Pool 6 (Traps 26 to 30), Pool 7 (Traps 31 to 35), Pool 8 (Traps 36 to 41), Pool 9 (Traps 42 to 46), and Pool 10 (Traps 47 to 52).
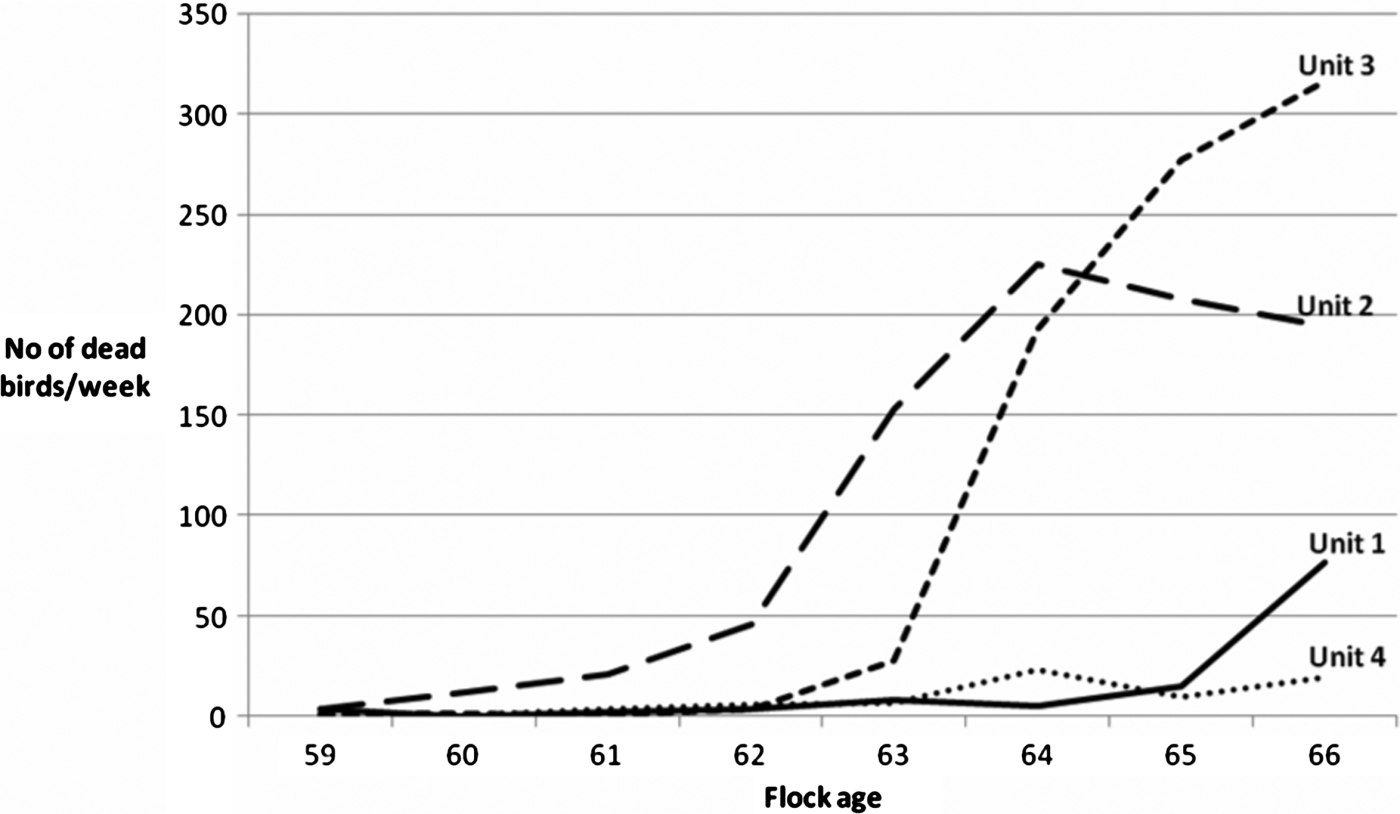
The outbreak was first noted in Unit 2 and subsequently spread to the other units as well. Mortality data, reflecting the progression of the outbreak, are shown in . Altogether 1861 hens died between 60 and 66 weeks of age. The flock was euthanized at 69 weeks of age, 3 months before schedule. After that, the house was cleaned and washed thoroughly followed by disinfection. No acaricides were applied after the outbreak. The next flock of laying hens in the house was vaccinated against erysipelas on their arrival at the farm in mid-July 2008, with an inactivated vaccine (Nobilis® Erysipelas vet; Intervet/Schering-Plough Animal Health, Boxmeer, The Netherlands). No signs of disease were noted in this flock.
Isolation of E. rhusiopathiae
Laying hens. In May 2008, during the outbreak, five dead laying hens were collected from each of the four units of the house. The distribution of the collected laying hens within the house is shown in . Hens were sent for examination at the National Veterinary Institute (SVA, Uppsala, Sweden). The liver and spleen from each bird were subjected to bacteriological analysis as previously described (Eriksson et al., Citation2009). E. rhusiopathiae colonies were identified by morphology, Gram staining and biochemical tests (Quinn et al., Citation1994). Three bacterial isolates from each positive organ were stored in serum broth composed of horse serum (SVA, Bro, Sweden), cattle broth (SVA, Uppsala, Sweden) and 15% glycerol (Merck, Darmstadt, Germany) at –70°C, until further investigation.
Mites
During the outbreak (May 2008) and at the end of the production period of the following flock (September/October 2009), mites were collected with 52 corrugated cardboard traps and plastic traps (Nordenfors & Chirico, Citation2001) placed on the perches at various locations in the house (). The traps were sent to SVA, where they were pooled into 10 pools as described in and stored at 4°C. Bacteriological examinations of the mites collected during the outbreak were performed on arrival and after 4 months of storage. Mites collected from the following flock in the house were examined bacteriologically monthly four times, 1 to 4 months after arrival at the laboratory. No surface disinfection of the mites was performed before the analysis, since the objective was to observe whether D. gallinae could act as reservoirs, and hence both the external and internal carriage of bacteria was of interest.
About 50 engorged mites were collected from each pool, crushed in a mortar and put in test tubes containing 5 ml crystal-violet sodium azide (2%) broth at 37°C for 48 h as previously described by Brännström et al. (Citation2010). The broth (100 µl) was then spread on blood agar plates made of Blood Agar Base no. 2 (Oxoid, Basingstoke, UK) with 5% horse blood. After a second incubation, E. rhusiopathiae was identified and stored as described above for the isolates from the layers.
DNA preparation and pulsed-field gel electrophoresis
Sixty-five E. rhusiopathiae isolates were analysed by PFGE. Nine isolates originated from mites, and the rest from the liver and spleen from the 20 laying hens. To further investigate the bacterial population within the same individual, 16 hens were represented by two isolates each and four hens contributed four isolates each. Three blood agar plates with overnight cultures of bacteria were harvested for each isolate and suspended in 330 µl lysis buffer (10 mM Tris–HCl, pH 8.0, 1 M NaCl, 200 mM ethylenediamine tetraacetic acid, 0.5% sodium lauroyl sarcosyl and 0.2% sodium deoxycholate). Suspensions were immediately mixed with an equal volume of 2% low melting point agarose (Agarose prep; GE Healthcare, Amersham, UK) and left to solidify in agarose plug moulds for 10 min. DNA preparation was further performed according to the previously described method (Eriksson et al., Citation2009). Genomic DNA was digested with 40 units of restriction enzyme SmaI (New England BioLabs, Ipswich, Massachusetts, USA) according to the instructions of the manufacturer. DNA fragments were separated in 1% agarose gel (Agarose NA; GE Healthcare) in 0.5x TBE buffer (45 mM Tris-borate, 1 mM ethylenediamine tetraacetic acid) for 24 h at pulse switch time ramped from 0.5 to 40 sec in a CHEF DRII apparatus (BioRad, Hercules, California, USA). PFGE patterns were analysed with the BioNumerics version 5.5 software (Applied Maths, Sint-Martens-Latem, Belgium). Cluster analysis was performed with the unweighted pair group method with arithmetic mean, dice coefficient and 0.5% optimization with 2% band position tolerance.
Results
Isolation of E. rhusiopathiae
Laying hens. At necropsy, all 20 hens displayed lesions consistent with septicaemia caused by E. rhusiopathiae infection. E. rhusiopathiae was isolated from both liver and spleen from all 20 birds.
Mites
From the mites collected in May 2008, E. rhusiopathiae could only be isolated from Pool 5, when analysed on arrival at the laboratory. However, E. rhusiopathiae was isolated from Pools 1, 2, 3, 5, 6, 7, 8 and 10 when examined in October 2008, after 4 months of storage at 4°C. Nine isolates, one from Pool 5 isolated on arrival and one from each positive pool isolated in October, were stored for further analyses. From mites collected in September and October 2009, no E. rhusiopathiae could be isolated at any of the four examination occasions, 1 to 4 months after collection.
Pulsed-field gel electrophoresis
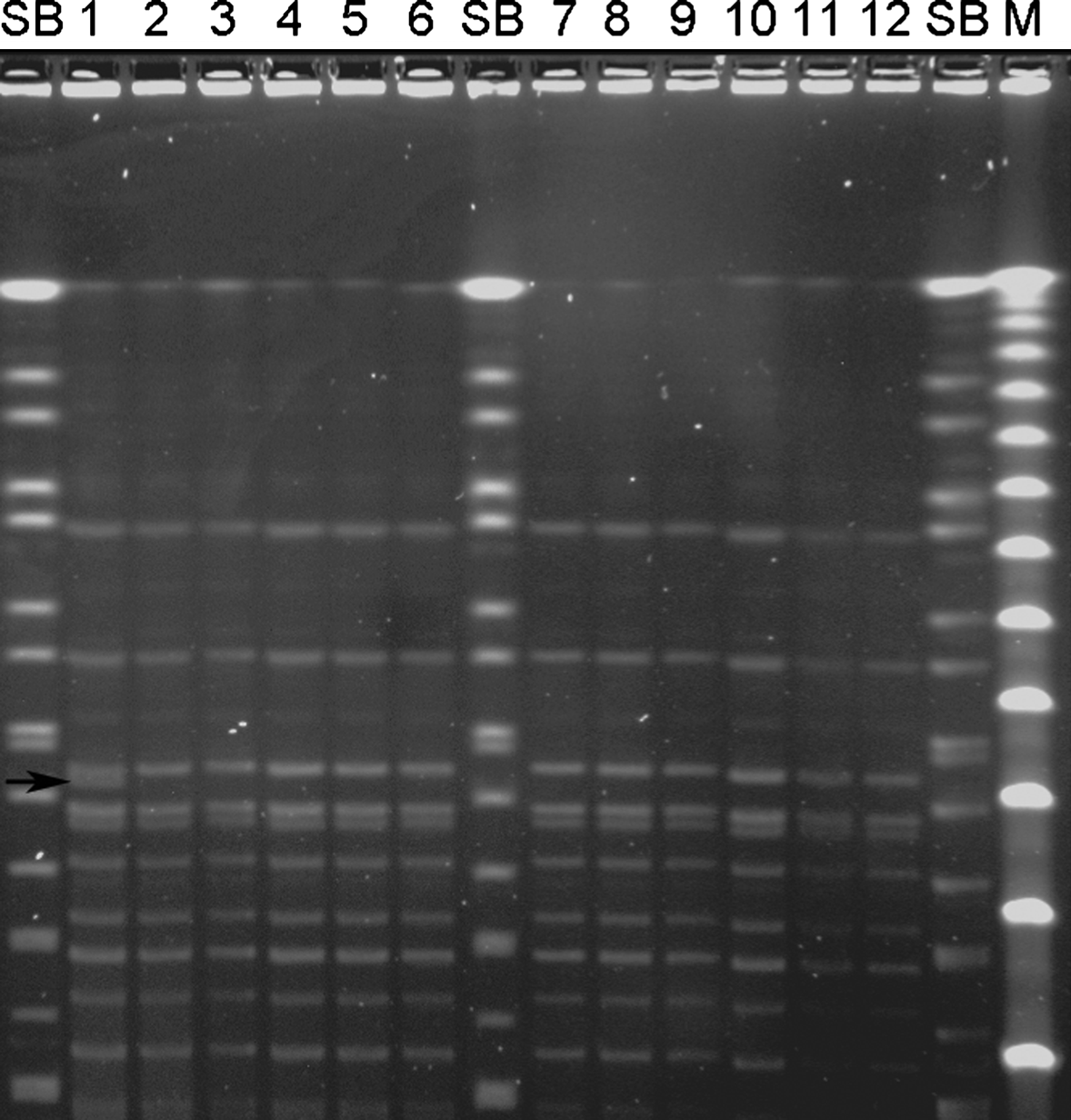
All isolates were visually identical except for two isolates, from the spleens of two different hens, which had an extra band of 145 and 150 kbp, respectively. However, all isolates shared a similarity greater than 93% upon cluster analyses. A picture of a PFGE gel with 10 of the isolates from hens and two isolates from mites is presented in .
Figure 3. PFGE patterns of SmaI digests of a subset of E. rhusiopathiae strains from a laying hen flock affected by erysipelas. SB, Salmonella Braenderup (H9812) size standard restricted with XbaI; M, Lambda PFG Marker (New England BioLabs, Ipswich, Massachusetts, USA). The pulse time was ramped from 0.5 to 40 sec for 24 h at 6 V/cm and 14°C. Lanes 1 to 10, strains isolated from the spleen or liver from two different hens in Unit 2 (lanes 1 to 4), two different hens in Unit 3 (lanes 5 to 8) and one hen in Unit 4 (lanes 9 and 10). Lanes 11 and 12, strains isolated from mites from Unit 1. Note the identical patterns except for lane 1, which is one of the two isolates with an extra band found in the present study. The arrow indicates the extra band of 150 kb.
Discussion
In the present study, we investigated whether there was a clonal relationship between isolates of E. rhusiopathiae collected from laying hens and D. gallinae during an outbreak of erysipelas. We also tried to assess whether the mite could act as a reservoir of the bacterium by analysing mites collected 1 year after the outbreak when the subsequent flock was kept in the house.
Previous studies of E. rhusiopathiae strains have shown that the species is genetically diverse (Opriessnig et al., Citation2004; Eriksson et al., Citation2009), and a genetic instability of E. rhusiopathiae over time has been proposed (Cross & Claxton, Citation1979; Eriksson et al., Citation2009). The isolates typed by PFGE in this study shared, with two notable exceptions, a homogeneous banding pattern (), and thus we can conclude that hens and mites were exposed to the same strain of the bacterium. This also suggests that a single strain was introduced and then spread between units. The two isolates with slightly different PFGE banding patterns might reflect mutational changes occurring in a rapidly growing bacterial population. It is also possible that different strains were introduced into the flock, but that one strain was better adapted for growth in this environment or was introduced at an earlier time point. It is noteworthy that isolates from one individual hen could have slightly different PFGE banding patterns.
Concluding that the same strain was infecting both mites and hens, there is the question as to whether the mite could act as a reservoir of the bacterium. According to Brännström et al. (Citation2010), D. gallinae is not an efficient biological vector of E. rhusiopathiae under experimental conditions. Nevertheless, the mite cannot be excluded as a potential vector of E. rhusiopathiae, since Valiente Moro et al. (Citation2007) have shown experimentally that ingestion of Salmonella-contaminated D. gallinae may lead to infection in chickens.
In the present study, parasites from Pool 5 collected during the outbreak were culture-positive for E. rhusiopathiae soon after arrival at the laboratory, while the other positive pools were confirmed after storage for 4 months at 4°C. This could be explained either by replication of E. rhusiopathiae within the parasites or by reduced numbers of viable competing bacteria after cold storage. The possible survival and multiplication of E. rhusiopathiae in mites requires further study. We confirmed that D. gallinae mites were present in the facility 1 year after the outbreak; however, they were no longer carriers of E. rhusiopathiae. In Sweden, mites can be spread between farms with live chickens and with egg trays, since egg producers commonly share the same egg-packing facility (Øines & Brännström, Citation2010). If this parasite is a reservoir of bacteria, then there is a potential risk of also spreading E. rhusiopathiae between farms.
Other possible reservoirs of E. rhusiopathiae that should be considered are other arthropods, such as flies, which previously have been suggested to be able to act as mechanical vectors of E. rhusiopathiae (Wellmann, Citation1955), and darkling beetles (Alphitobius diaperinus), which have been shown to transmit other bacterial species between successive flocks (Hazeleger et al., Citation2008). Rodents and other animals on the farm may also be important vectors and reservoirs that require further study.
The high mortality and decreased egg production observed during the weeks between the start of this outbreak and the euthanasia of the remaining laying hens correspond with what has been previously described (Eriksson et al., Citation2003; Mazaheri et al., Citation2005; Bricker & Saif, Citation2008). No detailed investigations into the source of this outbreak were performed. E. rhusiopathiae is ubiquitous in nature (Wang et al., Citation2010) and is said to survive in soil for a long time (Brooke & Riley, Citation1999). Furthermore, it has been estimated that 30 to 50% of healthy pigs carry E. rhusiopathiae in lymphoid tissues (Wood, Citation1999), and according to Wang et al. (Citation2010) pigs are the most important reservoir of this bacterium. Therefore, in this particular case, one possible source of the bacteria was the pigs that were housed on the farm some 15 years before the outbreak. It is, however, noteworthy that laying hens had been kept in the house for several years without any outbreak of erysipelas. An introduction of bacteria surviving in the environment outside the house through a breach in the biosecurity on the farm could be the infection route.
Once the infection was established in Unit 2 (), there was a rapid spread of the bacteria to Unit 3 (followed by Units 1 and 4), which was observed by the progressive mortality of the hens (). Physical contact between hens in different units and the possible vector D. gallinae moving between units are two transmission routes that may be partly responsible for the spread of the disease. These contacts may have been especially important between Units 2 and 3, which in the litter area were separated only by wire netting. However, the farmer's shoes are the most likely route for transmission of the bacteria between units, as they were not changed between units.
Alternative housing systems for laying hens have been introduced since the ban on conventional battery cages in Sweden and the number of flocks diagnosed with erysipelas has increased. According to current European Union legislation, laying hens are not to be held in traditional battery cages as of 1 January 2012 (European Council Directive 1999/74/EC). Depending on the housing systems that are introduced, a rise in the number of outbreaks of erysipelas is also to be expected in other European countries in the future. Similarly, the poultry red mite is thought to be an increasing problem due to the same legislation (Sparagano et al., Citation2009). More research on this potentially zoonotic disease and the importance of D. gallinae in the transmission of this pathogen is therefore needed.
Acknowledgements
Helena Ljung is acknowledged for excellent technical assistance, Anna Aspán, Désirée S. Jansson and David Morrison for valuable comments on the manuscript, and the National Veterinary Institute, Sweden for financial support of this study. Sara Brännström was funded by the Swedish Research Council for Environment, Agricultural Sciences and Spatial Planning (FORMAS). The authors are grateful to the farmer for providing the data and helping with the sampling.
References
- Brännström , S. , Hansson , I. and Chirico , J. 2010 . Experimental study on possible transmission of the bacterium Erysipelothrix rhusiopathiae to chickens by the poultry red mite, Dermanyssus gallinae . Experimental and Applied Acarology , 50 : 299 – 307 .
- Bricker J.M. Saif Y.M 2008 Erysipelas In Y.M. Saif A.M. Fadly J.R. Glisson L.R. McDougald L.K. Nolan D.E. Swayne . Diseases of Poultry , 12th edn 909 922 Ames , IA Blackwell Publishing Professional
- Brooke , C.J. and Riley , T.V. 1999 . Erysipelothrix rhusiopathiae: bacteriology, epidemiology and clinical manifestations of an occupational pathogen . Journal of Medical Microbiology , 48 : 789 – 799 .
- Chirico , J. , Eriksson , H. , Fossum , O. and Jansson , D. 2003 . The poultry red mite, Dermanyssus gallinae, a potential vector of Erysipelothrix rhusiopathiae causing erysipelas in hens . Medical and Veterinary Entomology , 17 : 232 – 234 .
- Cross , G.M.J. and Claxton , P.D. 1979 . Serological classification of Australian strains of Erysipelothrix rhusiopathiae isolated from pigs, sheep, turkeys and man . Australian Veterinary Journal , 55 : 77 – 81 .
- Eriksson H. Jansson D. Fossum O. Chirico J. Gunnarsson A. 2003 Erysipelas in layers—a growing problem in aviary and organic housing systems In XIII Congress of the World Veterinary Poultry Association. Program and Abstracts 130 Denver, CO , , USA
- Eriksson , H. , Jansson , D.S. , Johansson , K.E. , Båverud , V. , Chirico , J. and Aspán , A. 2009 . Characterization of Erysipelothrix rhusiopathiae isolates from poultry, pigs, emus, the poultry red mite and other animals . Veterinary Microbiology , 137 : 98 – 104 .
- Hazeleger , W.C. , Bolder , N.M. , Beumer , R.R. and Jacobs-Reitsma , W.F. 2008 . Darkling beetles (Alphitobius diaperinus) and their larvae as potential vectors for the transfer of Campylobacter jejuni and Salmonella enterica serovar Paratyphi B Variant Java between successive broiler flocks . Applied and Environmental Microbiology , 74 : 6887 – 6891 .
- Købke B. Eigaard N.M. Christensen J.P. Bisgaard M. 2005 Investigations on clonality and stability of clones of Erysipelothrix rhusiopathiae in turkeys and free range layer flocks affected by erysipelas In 14th World Veterinary Poultry Congress Final Program & Abstract Book 313 Istanbul , , Turkey
- Mazaheri , A. , Lierz , M. and Hafez , H.M. 2005 . Investigations on the pathogenicity of Erysipelothrix rhusiopathiae in laying hens . Avian Diseases , 49 : 574 – 576 .
- Nordenfors , H. and Chirico , J. 2001 . Evaluation of a sampling trap for Dermanyssus gallinae (Acari: Dermanyssidae) . Journal of Economic Entomology , 94 : 1617 – 1621 .
- Nordenfors , H. and Höglund , J. 2000 . Long term dynamics of Dermanyssus gallinae in relation to mite control measures in aviary systems for layers . British Poultry Science , 41 : 533 – 540 .
- Øines Ø. Brännström S. 2010 Paper submitted to Medical and Veterinary Entomology
- Opriessnig , T. , Hoffman , L.J. , Harris , D.L. , Gaul , S.B. and Halbur , P.G. 2004 . Erysipelothrix rhusiopathiae: genetic characterization of midwest US isolates and live commercial vaccines using pulsed-field gel electrophoresis . Journal of Veterinary Diagnostic Investigation , 16 : 101 – 107 .
- Quinn P.J. Carter M.E. Markey B.K. Carter G.R. 1994 Erysipelothrix rhusiopathiae In Clinical Veterinary Microbiology 175 177 London Wolfe Publishing, Mosby-Year Book Europe Limited
- Sparagano , O. , Pavlićević , A. , Murano , T. , Camarda , A. , Sahibi , H. Kilpinen , O. 2009 . Prevalence and key figures for the poultry red mite Dermanyssus gallinae infections in poultry farm systems . Experimental and Applied Acarology , 48 : 3 – 10 .
- Tenover , F.C. , Arbeit , R.D. , Goering , R.V. , Mickelsen , P.A. , Murray , B.E. , Persing , D.H. and Swaminathan , B. 1995 . Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing . Journal of Clinical Microbiology , 33 : 2233 – 2239 .
- Valiente Moro , C. , Chauve , C. and Zenner , L. 2005 . Vectorial role of some Dermanyssoid mites (Acari, Mesostigmata, Dermanyssoidea) . Parasite , 12 : 99 – 109 .
- Valiente Moro , C. , Fravalo , P. , Amelot , M. , Chauve , C. , Zenner , L. and Salvat , G. 2007 . Colonization and organ invasion in chicks experimentally infected with Dermanyssus gallinae contaminated by Salmonella Enteritidis . Avian Pathology , 36 : 307 – 311 .
- Wang , Q. , Chang , B.J. and Riley , T.V. 2010 . Erysipelothrix rhusiopathiae . Veterinary Microbiology , 140 : 405 – 417 .
- Wellmann , G. 1955 . Die übertragung der Schweinrotlaufinfektion durch die Staubenfliege (Musca domestica) . Zentralblatt für Bakteriologie, Parasitenkunde, Infektionskrankheit und Hygiene , 162 : 261 – 264 .
- Wood R.L. 1999 Erysipelas In B.E. Straw S. D'Allaire W.L. Mengeling D.J. Taylors . Diseases of Swine , 8th edn 419 430 Ames Iowa State University Press