Abstract
Few data are available on the molecular characterization of Cryptosporidium spp. in chickens and ducks in China. In this study, 2579 faecal samples from 46 chicken farms and eight Pekin duck farms in 21 prefectures in Henan Province were examined. The overall infection rate of Cryptosporidium was 10.6% (163/1542) in layer chickens (10 out of 17 farms), 3.4% (16/473) in broilers (five out of 29 farms), and 16.3% (92/564) in Pekin ducks (four out of eight farms), respectively. The highest infection rates were observed in 31-day-old to 60-day-old layer chickens (24.6%) and 11-day-old to 30-day-old Pekin ducks (40.3%). The season of highest prevalence in chickens was spring (15.6%) and the lowest was winter (P<0.01). One hundred and eighty-seven Cryptosporidium-positive samples were analysed by polymerase chain reaction (PCR)–restriction fragment length polymorphism analysis of the small subunit rRNA gene, and 55 were further analysed by DNA sequencing of the PCR products. Two Cryptosporidium species were identified: Cryptosporidium baileyi (184/187) on 15 chicken farms and four duck farms, and Cryptosporidium meleagridis (3/187) on three layer chicken farms. C. baileyi was the predominant Cryptosporidium species, found in all age groups of chickens and all Cryptosporidium-positive ducks examined, whereas C. meleagridis was only identified in 31-day-old to 120-day-old layer chickens. Considering the large size of the chicken industry and the close contact between chickens and humans, and that C. meleagridis is the third most common Cryptosporidium parasite in humans, then C. meleagridis could potentially become an emerging zoonosis in some areas in China.
Introduction
Cryptosporidium has been reported in more than 30 avian species worldwide (Ryan, Citation2010). However, few studies have examined the genetic diversity of Cryptosporidium spp. in avian hosts. So far, only three avian Cryptosporidium spp. are recognized: Cryptosporidium meleagridis, Cryptosporidium baileyi, and Cryptosporidium galli (Slavin, Citation1955; Current et al., Citation1986; Ryan et al., Citation2003). In addition, a few genotypes have been also described in recent years, for example avian genotypes (I to V), goose genotypes (I to IV), duck genotype, and Eurasian woodcock genotype (Morgan et al., Citation2001; Xiao et al., Citation2002; Jellison et al., Citation2004; Zhou et al., Citation2004; Meireles et al., Citation2006; Ng et al., Citation2006; Abe & Makino, Citation2010).
The chicken was the first avian species in which Cryptosporidium infection was described (Tyzzer, Citation1929). However, most reports on the prevalence in chickens were published in the 1980s and 1990s, with infection rates ranging from 4.5 to 50% depending on the location and the methods used in detection (de Graaf et al., Citation1999). In contrast, in the past 10 years there have been only about six reports (Trampel et al., Citation2000; Kimura et al., Citation2004; Huber et al., Citation2007; Soltane et al., Citation2007; Shemshadi et al., Citation2010; Smith et al., Citation2010). Likewise, there are only a few reports (about seven) concerning Cryptosporidium infections in ducks (Mason, Citation1986; Richter et al., Citation1994; O'Donoghue, Citation1995; Morgan et al., Citation2001; Kuhn et al., Citation2002; Huber et al., Citation2007; Amer et al., Citation2010).
Poultry play a critical role in the agricultural economy of China. In 2007 the total layer chicken and duck populations were 2.3 billion and 74 million, respectively, accounting for 39.2% and 68.2%, respectively, of the total number of these birds in the world (http://kids.fao.org/glipha/). In China, although a few studies have reported Cryptosporidium infections in chickens and domestic ducks, almost all of the diagnoses were based on morphological identification of oocysts in faeces (Zhang et al., Citation1997; Li et al., Citation1998). Few studies have genetically characterized avian-derived Cryptosporidiumisolates and all have identified C. baileyi infections in chickens (Zhang et al., Citation2004; Wang et al., Citation2007). In a more recent study, five C. baileyi isolates from Ruddy Shelduck (Tadorna ferruginea) were identified using microscopic and molecular analyses (Amer et al., Citation2010). In the present study, a large number of avian-derived Cryptosporidium isolates were genetically characterized to gain a better understanding of the distribution of Cryptosporidium spp. in chickens and domestic ducks in China.
Materials and Methods
Sample collection and examination
Fresh faecal samples were collected between June 2006 and July 2007 from different age groups of chickens and ducks on 46 chicken farms and eight Pekin duck (Anas platyrhynchos) farms in 21 prefectures in Henan Province. In total 2579 samples were examined in this study, including 1542 samples from layer chickens, 473 samples from broiler chickens, and 564 samples from Pekin ducks (). The samples were examined for Cryptosporidium oocysts by bright field microscopy at 400x magnification after the oocysts had been concentrated by the Sheather's sugar flotation technique. Cryptosporidium-positive samples were stored in 2.5% potassium dichromate at 4°C prior to molecular biological characterizations.
Table 1. Number of faecal samples examined for Cryptosporidium oocysts by microscopy on each farm and the Cryptosporidium genotypes determined by PCR-RFLP analysis of the SSU rRNA gene.
DNA extraction
Cryptosporidium oocysts were isolated from positive faecal samples using discontinuous density sucrose gradient centrifugation. Genomic DNA was extracted from the purified oocysts using the Mag Extractor-Genome kit (Toyobo Co. Ltd, Osaka, Japan), based on chaotropic extraction followed by absorption of DNA onto silica-coated magnetic beads, using the previously described procedures (Wang et al., Citation2008). The supernatant containing DNA was kept at –20°C before it was used in molecular analysis.
Cryptosporidium genotyping
Ninety-five Cryptosporidium-positive samples from chickens on different farms and age groups and all 92 Cryptosporidium-positive samples from Pekin ducks were characterized genetically. Cryptosporidium spp. were genotyped by amplifying an ~830 base pair fragment of the small subunit (SSU) rRNA gene by nested polymerase chain reaction (PCR) and restriction fragment length polymorphism (RFLP) analysis of the PCR products using restriction enzymes SspI and VspI (Xiao et al., Citation2000). Fifty-five samples (31 from chickens and 24 from Pekin ducks) were further sequenced directly on an ABI 3730 DNA Analyzer (Applied Biosystems), using the secondary PCR primers and the Big Dye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems). Sequence accuracy was confirmed by two-directional sequencing and by sequencing a new PCR product if necessary.
Sequence data
Sequence analysis was performed by alignment of SSU rRNA sequences obtained in the present study and reference sequences downloaded from GenBank using the program ClustalX 1.83 (Thompson et al., Citation1997). Sequences of the partial SSU rRNA gene of C. baileyi and C. meleagridis from chickens and Pekin ducks were deposited in GenBank under accession numbers EU814408 to EU814431 and HM002494 to HM002495.
Statistical analysis
The chi-square test was used to compare cryptosporidiosis prevalence rates, and differences were considered significant when P<0.01.
Results
Prevalence of Cryptosporidium spp
The overall prevalence of Cryptosporidium was 10.6% (163/1542) for layer chickens (on 10 out of 17 farms), 3.4% (16/473) for broiler chickens (on five out of 29 farms), and 16.3% (92/564) for Pekin ducks (on four out of eight farms), respectively (). In layer chickens, the highest infection rate (24.6%) was observed in birds aged from 31 to 60 days, and the lowest was seen in 61-day-old to 120-day-old chickens (P<0.01) (). In broiler chickens, however, 4.9% and 3.0% of infection rates were noted in birds aged from 1 to 20 days and from 21 to 60 days, respectively (P>0.05) (). In Pekin ducks, Cryptosporidium infections were only identified in birds between 11 and 50 days of age, with the highest infection rate (40.3%) being seen in 11-day-old to 30-day-old ducks (P<0.01) (). In chickens, the highest-prevalence season was spring (119/764, or 15.6%) and the lowest (8/407, or 2.0%) was winter (P<0.01) ().
Figure 1. Prevalence of Cryptosporidium-positive specimens detected by microscopy in chickens in different seasons.
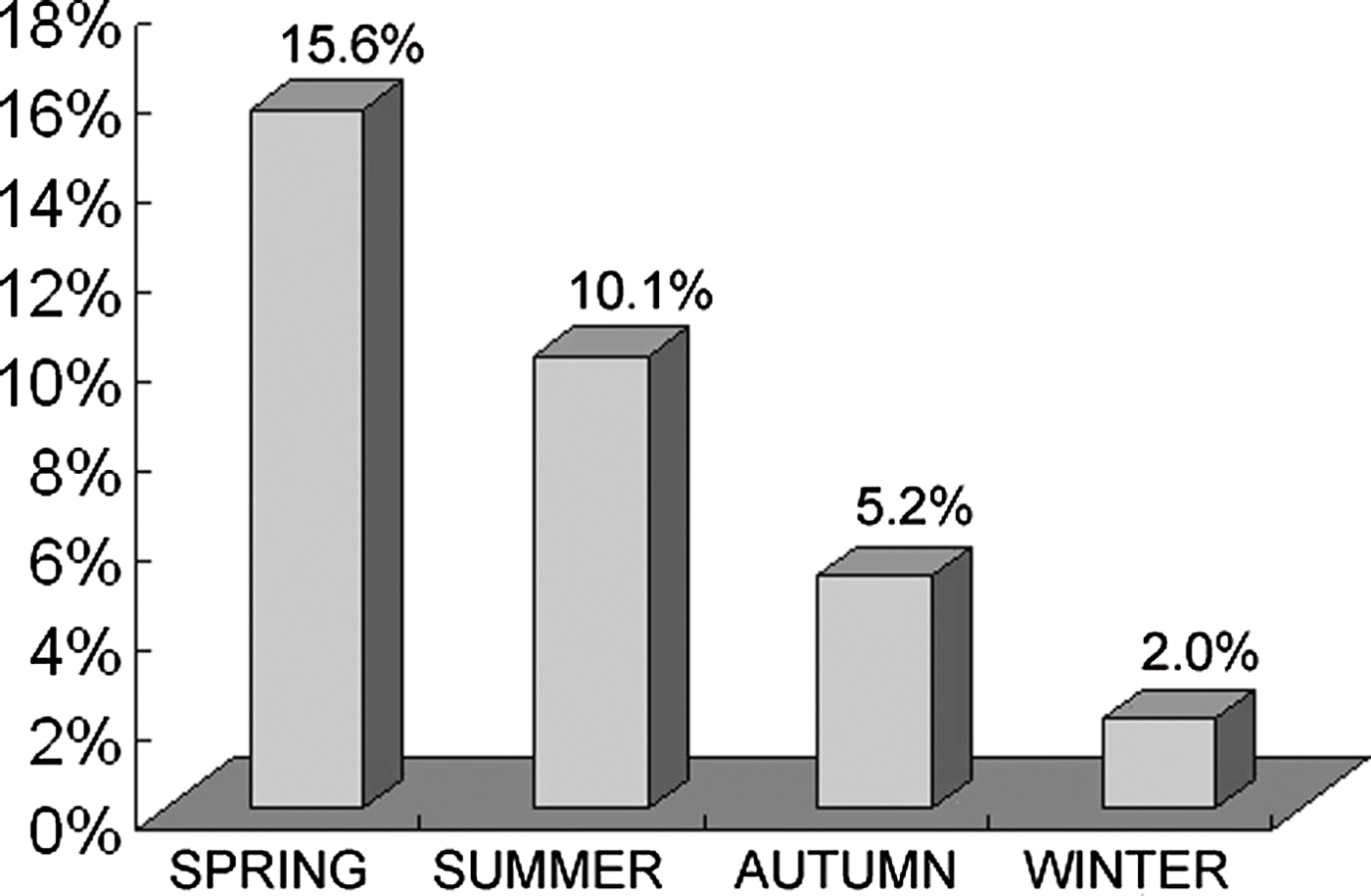
Table 2. Prevalence and distribution of Cryptosporidium species in different age groups of birds.
Distribution of Cryptosporidium species
One hundred and eighty-seven Cryptosporidium-positive samples from chickens and Pekin ducks were amplified by the nested PCR. RFLP analysis of the SSU rRNA gene products revealed the presence of two Cryptosporidium species, including C. baileyi (184/187) on nine layer chicken farms, five broiler farms and four Pekin duck farms and C. meleagridis (3/187) on three layer chicken farms (). DNA sequencing of the SSU rRNA PCR products from 52 C. baileyi-positive and three C. meleagridis-positive samples confirmed the identification of the species. There was complete agreement between RFLP and DNA sequencing results.
Age patterns of Cryptosporidium species
C. baileyi was the predominant species (92/95, or 96.8%) and was found in all age groups of chickens. In contrast, C. meleagridis (n=3) was only identified in 31-day-old to 120-day-old layer chickens (). In Pekin ducks, Cryptosporidium infection was found only in the age group of 11 to 50 days ().
Discussion
Previously, most investigations of avian-derived Cryptosporidium were carried out in broiler chickens and the prevalence rate varied in different countries. In the USA, 23.7% (118/498) of broiler flocks and 29.4% (35/119) of broiler chickens had Cryptosporidium infections (Gorham et al., Citation1987; Ley et al., Citation1988; Snyder et al., Citation1988; Goodwin et al., Citation1996). In European countries such as Scotland and Greece, the infection rates in broilers were 18.7% (26/139) and 24.3% (17/70), respectively (Randall, Citation1982; Papadopoulou et al., Citation1988). In Africa, 24% (54/225) and 4.5% (9/200) prevalence rates were reported in Morocco and Tunisia, respectively (Kichou et al., Citation1996; Soltane et al., Citation2007). In Japan and Iran, rates of 33.3% (4/12) and 23.8% (57/152) were observed, respectively, in broiler chickens (Itakura et al., Citation1984; Shemshadi et al., Citation2010). In the present study, a 3.4% (16/473) infection rate was found in broiler chickens. The difference in prevalence rates observed might be attributed to the use of different detection methods (such as histology, faecal examination, serology, and other methods). On the other hand, differences in hygiene and animal management may also be responsible (de Graaf et al., Citation1999).
In comparison with broilers, there are few studies concerning Cryptosporidium infection in layer chickens. Two previous studies showed the infection rates were 5.9% (1/17) and 36.8% (25/68) in the USA and Japan, respectively (Itakura et al., Citation1984; Ley et al., Citation1988). In the present study, a 10.6% (163/1542) infection rate was observed in layer chickens. Moreover, the prevalence of Cryptosporidium in layer chickens differed in different age groups, with the highest infection rate (24.6%) being seen in the age group between 31 and 60 days old (P<0.01) (). This finding was similar to the previous observation that younger chickens are more susceptible to Cryptosporidium infections (Fayer & Ungar, Citation1986; Shi et al., Citation1995; de Graaf et al., Citation1999).
In addition, Cryptosporidium infection in chickens appears to be seasonal. In the present study, the highest infection rate (15.6%) was seen in spring and decreased significantly in summer and autumn, reaching the lowest levels in winter (2.0%) (P<0.01) (). Previously, a few reports also observed a significantly lower prevalence of cryptosporidiosis cases in winter (Goodwin & Brown, Citation1986, Citation1994).
The prevalence of cryptosporidiosis in ducks also varied among countries in the world. In the USA, an investigation indicated that 49% of ducks were carriers of Cryptosporidium (Kuhn et al., Citation2002). A study conducted in Germany showed a 57% (73/128) prevalence rate in ducks (Richter et al., Citation1994), whereas in Australia only one of 97 ducks examined was positive (Mason, Citation1986). In a more recent study, 3.4% (5/148) of Ruddy Shelduck (Tadorna ferruginea) examined in China were Cryptosporidium-positive (Amer et al., Citation2010). In this study, a 16.3% of infection rate was seen in Pekin ducks. The present data suggested cryptosporidiosis was prevalent in younger ducks, with the highest infection rate (40.3%) being seen in 11-day-old to 30-day-old ducks (P<0.01) ().
Thus far, four Cryptosporidium species/genotypes have been identified in chickens and ducks, including C. meleagridis, C. baileyi, C. galli, and the duck genotype (Slavin, Citation1955; Current et al., Citation1986; Morgan et al., Citation2001; Ryan et al., Citation2003; Jellison et al., Citation2004; Zhou et al., Citation2004; Amer et al., Citation2010). Among the species/genotypes, C. baileyi was originally isolated from commercial broiler chickens and is probably the most common avian Cryptosporidium species. In addition to the chickens and ducks, it has been reported in more than 17 other avian hosts (Huber et al., Citation2007; Amer et al., Citation2010; Ryan, Citation2010). C. meleagridis also appears to have a broad host range and has been found in various avian species including chickens, turkeys, parrots, and cockatiels (O'Donoghue, Citation1995; Morgan et al., Citation2000; Sreter & Varga, Citation2000; Darabus & Olariu, Citation2003; Abe & Iseki, Citation2004; Huber et al., Citation2007). C. galli was first described by Pavlásek in hens, and later this species was re-described (Ryan et al., Citation2003). In contrast, the duck genotype has a limited host range, having only been found in a black duck (Anas rubripes) and Canada geese (Branta canadensis) (Jellison et al. Citation2004; Zhou et al., Citation2004).
Unlike previous observations in cattle, pigs, and recently in sheep (Santín et al., Citation2004; Kvác et al., Citation2009; Wang et al., Citation2010), there was no obvious age association in the distribution of Cryptosporidium species/genotypes in chickens and ducks. C. baileyi was the predominant species and was indentified in all age groups of chickens and was responsible for all infections in ducks, although C. meleagridis was only seen in 31-day-old to 120-day-old layer chickens (). Thus, further studies are needed to gain a better understanding of any association with age.
In conclusion, C. baileyi was found to be the predominant Cryptosporidium species in chickens and Pekin ducks in Henan, China. Only a small number of cases of C. meleagridis were identified in young layer chickens, but the poultry industry is big in China and the contact between chickens and humans is close. C. meleagridis is the third most common Cryptosporidium parasite in humans (Xiao, Citation2010), and it is possible that C. meleagridis could be an emerging zoonotic infection in some areas in China.
Acknowledgements
The present study was supported in part by the National Natural Science Foundation of China (No. 30871863, and 30928019), the Ph.D. Programs Foundation of Ministry of Education of China (No. 20094105110003), Henan Province Special Fund of Public Welfare (No. 81100912300), and the Ministry of Health Special Funds of Public Sector Research (No. 200808012).
References
- Abe N. Iseki M. 2004 Identification of Cryptosporidium isolates from cockatiels by direct sequencing of the PCR-amplified small subunit ribosomal RNA gene Parasitology Research 92 523 526
- Abe N. Makino I. 2010 Multilocus genotypic analysis of Cryptosporidium isolates from cockatiels, Japan Parasitology Research 106 1491 1497
- Amer S. Wang C. He H. 2010 First detection of Cryptosporidium baileyi in Ruddy Shelduck (Tadorna ferruginea) in China The Journal of Veterinary Medical Science 72 935 938
- Current W.L. Upton S.J. Haynes T.B. 1986 The life cycle of Cryptosporidium baileyi n. sp. (Apicomplexa, Cryptosporidiidae) infecting chickens Journal of Protozoology 33 289 296
- Darabus , G. and Olariu , R. 2003 . The homologous and interspecies transmission of Cryptosporidium parvum and Cryptosporidium meleagridis . Polish Journal of Veterinary Sciences , 6 : 225 – 228 .
- de Graaf D.C. Vanopdenbosch E. Ortega-Mora L.M. Abbassi H. Peeters J.E. 1999 A review of the importance of cryptosporidiosis in farm animals International Journal for Parasitology 29 1269 1287
- Fayer , R. and Ungar , B.L. 1986 . Cryptosporidium spp. and cryptosporidiosis . Microbiological Reviews , 50 : 458 – 483 .
- Goodwin , M.A. and Brown , J. 1986 . Histologic incidence and distribution of Cryptosporidium sp. infection in chickens: 68 cases in 1986 . Avian Diseases , 32 : 365 – 369 .
- Goodwin , M.A. and Brown , J. 1994 . Incidence of respiratory cryptosporidiosis in Georgia broilers: 1987–1992 . Avian Diseases , 38 : 358 – 360 .
- Goodwin , M.A. , Brown , J. , Resurreccion , R.S. and Smith , J.A. 1996 . Respiratory coccidiosis (Cryptosporidium baileyi) among Northern Georgia broilers in one company . Avian Diseases , 40 : 572 – 575 .
- Gorham S.L. Mallinson E.T. Synder D.B. Odor E.M. 1987 Cryptosporidia in the bursa of Fabricius—a correlation with mortality rates in broiler chickens Avian Pathology 16 205 211
- Huber F. da Silva S. Bomfim T.C. Teixeira K.R. Bello A.R. 2007 Genotypic characterization and phylogenetic analysis of Cryptosporidium sp. from domestic animals in Brazil Veterinary Parasitology 150 65 74
- Itakura C. Goryo M. Umemura T. 1984 Cryptosporidial infection in chickens Avian Pathology 13 487 499
- Jellison K.L. Distel D.L. Hemond H.F. Schauer D.B. 2004 Phylogenetic analysis of the hypervariable region of the 18S rRNA gene of Cryptosporidium oocysts in feces of Canada geese (Branta canadensis): evidence for five novel genotypes Applied and Environmental Microbiology 70 452 458
- Kichou F. Saghir F. El Hamidi M. 1996 Infection naturelle à Cryptosporidium sp. chez le poulet de chair au Maroc Avian Pathology 25 103 111
- Kimura A. Suzuki Y. Matsui T. 2004 Identification of the Cryptosporidium isolate from chickens in Japan by sequence analyses The Journal of Veterinary Medical Science 66 879 881
- Kuhn R.C. Rock C.M. Oshima K.H. 2002 Occurrence of Cryptosporidium and Giardia in wild ducks along the Rio Grande River valley in southern New Mexico Applied and Environmental Microbiology 68 161 165
- Kvác M. Hanzlíková D. Sak B. Kvetonová D. 2009 Prevalence and age-related infection of Cryptosporidium suis, C. muris and Cryptosporidium pig genotype II in pigs on a farm complex in the Czech Republic Veterinary Parasitology 160 319 322
- Ley , D.H. , Levy , M.G. , Hunter , L. and Barnes , H.J. 1988 . Cryptosporidia-positive rates of avian necropsy accessions determined by examination of auramine O-stained faecal smears . Avian Diseases , 32 : 108 – 113 .
- Li P.Y. Liao S.F. Lu F.L. Peng K.S. Wang G.J. Chen S.H. Ma F.L. 1998 Study on infection of chicken Cryptosporidium and its seasonal variations in Hefei Area Journal of Anhui Agricultural University 25 14 17 (in Chinese)
- Mason , R.W. 1986 . Conjunctival cryptosporidiosis in a duck . Avian Diseases , 30 : 598 – 600 .
- Meireles M.V. Soares R.M. dos Santos M.M.A.B. Gennari S.M. 2006 Biological studies and molecular characterization of a Cryptosporidium isolate from ostriches (Struthio camelus) Journal of Parasitology 92 623 626
- Morgan U.M. Monis P.T. Xiao L. Limor J. Sulaiman I. Raidal S. et al. 2001 Molecular and phylogenetic characterisation of Cryptosporidium from birds International Journal for Parasitology 31 289 296
- Morgan U.M. Xiao L. Limor J. Gelis S. Raidal S.R. Fayer R. et al. 2000 Cryptosporidium meleagridis in an Indian ring-necked parrot (Psittacula krameri) Australian Veterinary Journal 78 182 183
- Ng J. Pavlasek I. Ryan U. 2006 Identification of novel Cryptosporidium genotypes from avian hosts Applied and Environmental Microbiology 72 7548 7553
- O'Donoghue P.J. 1995 Cryptosporidium and cryptosporidiosis in man and animals International Journal for Parasitology 25 139 195
- Papadopoulou C. Xylouri E. Zisides N. 1988 Cryptosporidial infection in broiler chickens in Greece Avian Diseases 32 842 843
- Randall C.J. 1982 Cryptosporidiosis of the bursa of Fabricius and trachea in broilers Avian Pathology 11 95 102
- Richter D. Wiegand-Tripp G. Bruckhardt E. Kaleta E.F. 1994 Natural infections by Cryptosporidium sp. in farm-raised ducks and geese Avian Pathology 23 277 286
- Ryan U. 2010 Cryptosporidium in birds, fish and amphibians Experimental Parasitology 124 113 120
- Ryan U.M. Xiao L. Read C. Sulaiman I.M. Monis P. Lal A.A. et al. 2003 A redescription of Cryptosporidium galli Pavlasek, 1999 (Apicomplexa: Cryptosporidiidae) from birds Journal of Parasitology 89 809 813
- Santín M. Trout J.M. Xiao L. Zhou L. Greiner E. Fayer R. 2004 Prevalence and age-related variation of Cryptosporidium species and genotypes in dairy calves Veterinary Parasitology 122 103 117
- Shemshadi B. Bahadori S.R. Mozafari A. 2010 Study on cryptosporidiosis incidence in broilers in Garmsar region, Iran Comparative Clinical Pathology
- Shi T.W. Wu X.Z. Cheng Z.G. Huang B. Zhao Q.P. 1995 Study on the prevalence of Cryptosporidium spp. in chickens Shanghai Journal of Animal Husbandry Veterinary Medicine 4 2 3 (in Chinese)
- Slavin , D. 1955 . Cryptosporidium meleagridis (sp. nov.) . Journal of Comparative Pathology , 65 : 262 – 266 .
- Smith R.P. Chalmers R.M. Mueller-Doblies D. Clifton-Hadley F.A. Elwin K. Watkins J. et al. 2010 Investigation of farms linked to human patients with cryptosporidiosis in England and Wales Preventive Veterinary Medicine 94 9 17
- Snyder , D.B. , Current , W.L. and Russek-Cohen , E. 1988 . Serologic incidence of Cryptosporidium in Delmarva broiler flocks . Poultry Science , 67 : 730 – 735 .
- Soltane , R. , Guyot , K. , Dei-Cas , E. and Ayadi , A. 2007 . Prevalence of Cryptosporidium spp. (Eucoccidiorida: Cryptosporiidae) in seven species of farm animals in Tunisia . Parasite , 14 : 335 – 338 .
- Sreter T. Varga I. 2000 Cryptosporidiosis in birds—a review Veterinary Parasitology 87 261 279
- Thompson J.D. Gibson T.J. Plewniak F. Jeanmougin F. Higgins D.G. 1997 The ClustalX windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools Nucleic Acids Research 24 4876 4882
- Trampel D.W. Pepper T.M. Blagburn B.L. 2000 Urinary tract cryptosporidiosis in commercial laying hens Avian Diseases 44 479 484
- Tyzzer , E.E. 1929 . Coccidiosis in gallinaceous birds . American Journal of Hygiene , 10 : 269
- Wang J.C. Jian F.C. Zhang L.X. Ning C.S. Yan W.C. Sun M.F. et al. 2007 Phylogenetic analysis of the Cryptosporidium based on 18S rRNA gene and HSP70 gene sequences Acta Veterinaria et Zootechnica Sinica 38 947 953 (in Chinese)
- Wang R.J. Zhang L.X. Ning C.S. Feng Y.Y. Jian F.C. Xiao L.H. et al. 2008 Multilocus phylogenetic analysis of Cryptosporidium andersoni (Apicomplexa) isolated from a bactrian camel (Camelus bactrianus) in China Parasitology Research 102 915 920
- Wang Y. Feng Y. Cui B. Jian F. Ning C. Wang R. et al. 2010 Cervine genotype is the major Cryptosporidium genotype in sheep in China Parasitology Research 106 341 347 . doi: 10.1007/s00436-009-1664-x
- Xiao L. 2010 Molecular epidemiology of cryptosporidiosis: an update Experimental Parasitology 124 80 89
- Xiao L. Alderisio K. Limor J. Royer M. Lal A.A. 2000 Identification of species and sources of Cryptosporidium oocysts in storm waters with a small-subunit rRNA-based diagnostic and genotyping tool Applied and Environmental Microbiology 66 5492 5498
- Xiao L. Sulaiman I.M. Ryan U.M. Zhou L. Atwill E.R. Tischler M.L. et al. 2002 Host adaptation and host-parasite co-evolution in Cryptosporidium: implications for taxonomy and public health International Journal for Parasitology 32 1773 1785
- Zhang L.X. Jiang J.S. Liu Q. Ning C.S. Zhao J.F. Liu H.Y. 2004 Cryptosporidium species from dairy cow and poultry identified by PCR-restriction fragment length polymorphism Acta Veterinaria et Zootechnica Sinica 35 555 559 (in Chinese)
- Zhang L.X. Ning C.S. Wang X.B. Xu Z.M. Li J.Z. 1997 Investigation on cryptosporidiosis in ducks and experimental infection to chickens China Poultry 6 7 8 (in Chinese)
- Zhou L. Kassa H. Tischler M.L. Xiao L. 2004 Host-adapted Cryptosporidium spp. in Canada geese (Branta canadensis) Applied and Environmental Microbiology 70 4211 4215