Abstract
Seventy-eight isolates of avian infectious bronchitis virus (IBV) were obtained from different field outbreaks in China in 2009 and genotyped with 34 reference strains. Four genotypes of IBV and three new isolates were identified by phylogenetic analysis and BLAST searches of the entire S1 gene. The results showed that most IBV strains that have circulated in China in recent years belong to the genotype of QX-like strains, and that they could be grouped further into two clusters, regardless of the level of genetic variation displayed. A study of pathogenicity that used three QX-like strains—ck/CH/LSD/091003, ck/CH/LDL/091022 and ck/CH/LJL/090330—showed that the isolates caused the most severe lesions in the kidneys and were therefore nephropathogenic strains with various levels of virulence in specific pathogen free chickens. A vaccination–challenge test that was performed using the three QX-like strains showed that the commercially available H120 vaccine did not provide sufficient protection against challenge with the QX-like isolates, as demonstrated by comparison of the clinical signs, pathological lesions and virus recovery from the trachea and kidney of unvaccinated–challenged and vaccinated–challenged birds.
Introduction
Infectious bronchitis (IB) is a highly contagious viral disease of the upper respiratory and urogenital tracts of chickens that is caused by infectious bronchitis virus (IBV). The disease is prevalent in all countries with an intensive poultry industry, affecting the performance of both meat-type and egg-laying birds, thereby causing considerable economic loss within the poultry industry. In addition, high mortality sometimes occurs in young chickens as a result of renal pathology caused by nephropathogenic strains. The disease is often complicated by secondary bacterial infections that cause increased mortality (Cavanagh & Gelb, Citation2008).
Since IBV was first described in 1936, many serotypes and variants that do not confer complete cross-protection against each other have been isolated worldwide (Cavanagh & Gelb, Citation2008). Cross-protection tends to decrease as the degree of amino acid identity of the S1 subunit of the spike (S) glycoprotein decreases between two strains of IBV (Cavanagh et al., Citation1997). Differences of a few amino acid residues within the S1 region may induce a serotype change that is defined as a lack of cross-neutralization with specific sera and results in diminished cross-protection (Cavanagh et al., Citation1992). The number of IBV serotypes that exist throughout the world is unknown; more than 50 different serotypes have been listed (Ignajatovic & Sapats, Citation2000), and new IBV variants continue to emerge (Bochkov et al., Citation2006).
Generally, the serotypes of IBV isolated in particular geographic areas do not spread to other areas. However, some serotypes of IBV have become widespread worldwide and have become predominant over a period of time in the majority of countries that have a significant poultry industry (Terregino et al., Citation2008).
The QXIBV strain was first isolated in September 1997 in chicken flocks in Qingdao, China and was characterized mainly by swelling of the proventriculus of infected chickens (YuDong et al., Citation1998). However, it was not recognized as a novel type of IBV at that time. From 1999 to 2004, we investigated five chicken flocks in four provinces in China and found that the QX-like variant (taking the LX4 strain as representative) is a novel genotype of nephropathogenic IBV (Liu & Kong, Citation2004). Subsequently, we found that this type of IBV was the predominant genotype circulating in chicken flocks in China from 1995 to 2007 (Liu et al., Citation2006, Citation2008, Citation2009). More recently, the prevalence of this type of IBV, designated QX-like IBV, has been reported in Poland (Domanska-Blicharz et al., Citation2006), the UK (Gough et al., Citation2008), Hungary (Benyeda et al., Citation2008, Citation2009), Slovakia and Greece (Benyeda et al., Citation2009), Russia (Bochkov et al., Citation2006), The Netherlands (Landman et al., Citation2005), Italy (Beato et al., Citation2005; Zanella et al., Citation2006), France, Germany and Belgium (Worthington et al., Citation2008), and South Korea (Lee et al., Citation2008). These findings suggest that this novel type of IBV attained a high prevalence within a few years after it reached European countries (Worthington et al., Citation2008). It appears that this virus type tends to spread rapidly among susceptible flocks.
It seems that the QX-like IBV originated in China. However, no information is available currently on the origin and the means of dissemination of this strain from China to other regions (Bochkov et al., Citation2006; Gough et al., Citation2008). The Massachusetts (Mass) strains have been used primarily as live vaccines in China for decades. However, other types of IBV have been isolated consistently from vaccinated chicken flocks, and this implies that the vaccines have provided poor protection against the circulating strains (Liu et al., Citation2006, Citation2008, Citation2009). Thus, the first aim of this study was to monitor constantly, isolate and characterize the newly circulating QX-like IBVs in China and to compare them with those isolated between 1996 and 2008 in China and other countries. The second aim was to evaluate the protection offered by a Mass-type (H120) vaccine against newly isolated QX-like IBVs.
Materials and methods
Field samples and virus isolation
In the course of our continuous surveillance programme for IBV, nasal swabs and tissue samples from the kidney, proventriculus, trachea, caecal tonsil and lung were analysed from 284 flocks of cockerels, broilers, pullets, layers and breeders suspected of IB infection. The samples covered most of the chicken-raising regions of China, and were collected between January and December 2009 (). Nearly all of the chickens showed early signs of respiratory disease, including gasping, coughing, sneezing and tracheal rales. Post-mortem examination was performed and the gross lesions were evaluated. The gross examination showed mild to severe tracheitis, nephritis and proventriculitis. The morbidity ranged from 5 to 70%, and mortality varied between 5 and 30%. In layer flocks, mortality, drops in egg production and deformed eggs were the most prevalent signs documented. The tissue samples were pooled together from chickens from the same flock. Most of the flocks had been vaccinated against IB with commercial live-attenuated vaccines.
Table 1. Epidemiological information for the Chinese field isolates of IBV included in the present study.
For virus isolation, the samples were prepared as 10% w/v tissue suspensions in 0.1% phosphate-buffered saline (PBS), clarified by centrifugation at 1500×g at 4oC for 10 min and filtered through 0.22 µm membrane filters (Millipore Products Division, Bedford, Massachusetts, USA) before inoculation into the allantoic cavities of 9-day-old to 11-day-old specific pathogen free (SPF) embryos (Harbin Veterinary Research Institute, China). Three to five eggs were used for each sample. The inoculated eggs were incubated at 37°C and candled daily. Three to five blind passages were performed until the characteristic embryo changes, such as dwarfing, stunting, or curling of embryos, were observed between 2 and 7 days after inoculation, according to a previous report (Liu & Kong, Citation2004).
Electron microscopy
Samples of allantoic fluids inoculated with each isolate were submitted for electron microscopic examination after the characteristic embryo changes were observed. The supernatant of 1.5 ml allantoic fluid was centrifuged at 12,000×g for 30 min after low-speed centrifugation at 1500×g for 30 min (Allegra™ 21R centrifuge; Beckman). The resulting pellet was resuspended in a minimal volume of deionized water and examined by negative contrast electron microscopy (JEM-1200, EX) as described previously (Liu & Kong, Citation2004).
IBV vaccine and isolates
A commercial Mass-type vaccine, H120, was used for the vaccination–challenge test. The vaccine was administered by the ocular route as recommended by the manufacturer (Liu et al., Citation2008).
Three IBV isolates obtained in the present study—ck/CH/LSD/091003, ck/CH/LDL/091022, and ck/CH/LJL/090330—were used as challenge viruses for the vaccination–challenge study. The titres of the three viruses were determined by inoculation of 10-fold dilutions into groups of five 10-day-old embryonated chicken eggs. The median embryo infectious dose (EID50) was calculated by the method of Reed & Muench (Citation1938). An inoculum containing 104.7 to 104.8 EID50/100 µl was prepared and used to infect chickens experimentally.
Cloning and sequencing of the S1 gene of IBV isolates
A reverse transcriptase (RT)-polymerase chain reaction (PCR) protocol that has been described previously was used for the amplification of the S1 gene (Adzhar et al., Citation1996). Briefly, viral RNA was extracted from 200 µl infected allantoic fluid using TRIzol reagents (Invitrogen, Grand Island, New York, USA) and following the manufacturer's protocol. For the first cDNA strand, a mixture containing 20 µM reverse primer N(–) (5′-ACGCGGAGTACGATCGAGGGTACA-3′), 20 units RNasin (Invitrogen), 0.5 mM each dNTP, 8 µl of 5× buffer and the RNA template was first incubated at 70°C for 5 min, and transferred immediately to ice for another 5 min. Subsequently, 100 units M-MLV Reverse Transcriptase (Invitrogen) was added to make a final volume of 40 µl. The reaction was run at 37°C for 2 h, followed by 72°C for 10 min, and transferred immediately to ice for 5 min. The PCR was performed in a 25 µl reaction containing 2 µl first-strand cDNA; 15 nmol downstream primer and 15 nmol upstream primer (); 5 µl of 10× PCR buffer (Mg2 + Plus; TaKaRa, Japan); 4 µl of 2.5 mmol dNTPs; 2 units Taq polymerase (TaKaRa); and 18 µl water. The reaction was conducted at 95°C for 5 min; 30 cycles of 94°C for 1 min, 50°C for 1 min, 72°C for 2 min; and a final extension step of 72°C for 10 min.
Table 2. Sequences and genome localizations of the primers used in the present study.
A product of about 1700 bp was generated, which was detected by ethidium bromide staining. The DNA generated by PCR amplification was cloned using a T-tailed vector (pMD18-T; TaKaRa), and transformed using JM109 competent cells (TaKaRa) according to the manufacturer′s instructions. Each region of the S1 gene in each IBV isolate was sequenced at least three times and the consensus sequence was determined.
Analysis of the S1 gene
The nucleotide and amino acid sequences of the S1 gene of the IBV isolates were assembled, aligned, and compared with those of other reference IBV strains using the MEGALIGN programme in DNAStar. Phylogenetic analysis of the nucleotide sequences of the S1 gene was performed with the Clustal V method of DNAStar software (Liu et al., Citation2009). A total of 39 IBV reference strains were selected for phylogenetic analysis in the present study. Seven additional IBV strains were used for partial S1 gene comparison with our current IBV strains. These selected IBV strains and their accession numbers are presented in . Most of the strains represented Chinese field isolates of IBV collected in different years from 1997 to 2008, from different regions in China, and which were available in the GenBank database (Yu et al., Citation2001; Bijlenga et al., Citation2004; Liu & Kong, Citation2004; Liu et al., Citation2006; Xu et al., Citation2007). In addition, the S1 genes of three QX-like IBV strains from France (Worthington et al., Citation2008; Benyeda et al., Citation2009), three from The Netherlands (Worthington et al., Citation2008), two from Russia (Bochkov et al., Citation2006), one from Hungary (Benyeda et al., Citation2009), one from Greece (Benyeda et al., Citation2009), one from Slovakia (Benyeda et al., Citation2009) and three from South Korea (Lee et al., Citation2010), as well as several other types of IBV that were circulating in chicken flocks in China were also selected and compared in the present study.
Table 3. IBV strains used in the present study for sequence comparison of the S1 gene.
GenBank accession numbers of IBV sequences
The entire S1 gene nucleotide sequence, including the cleavage site, of each of the 78 IBV isolates used in this study was deposited in GenBank with the accession numbers presented in .
Experimental design
One hundred and forty 1-day-old SPF White Leghorn chicks were housed in different isolators and divided into eight groups. Groups 1 to 6 contained 20 birds each, and Groups 7 and 8 comprised 10 birds. The chickens in Groups 1, 3, 5 and 7 were inoculated with H120 vaccine at 1 day of age according to the manufacturer's instructions. The birds in Groups 2, 4, 6 and 8 were mock-inoculated with sterile allantoic fluid. The birds in Groups 1 and 2 were challenged with IBV isolate ck/CH/LDL/091022, Groups 3 and 4 with ck/CH/LJL/090330 and Groups 5 and 6 with ck/CH/LSD/091003 by oculonasal application at 20 days of age. The chickens in Groups 7 and 8 were mock-inoculated with sterile allantoic fluid.
Sampling
Ten birds from Groups 1 to 6 and five birds from Groups 7 and 8 were killed by intravenous injection with barbiturate 5 days post challenge, and tissue samples were collected from the trachea and kidney. Directly after sampling, each tissue specimen was stored individually in 300 µl virus isolation medium (50% glycerol, 50% PBS) at –20°C until virus recovery was attempted. The rest of the chicks in each group were examined daily for signs of infection for 30 days post inoculation. All the birds were killed humanly at the end of experiment, and then examined post mortem for gross lesions. Sera were collected at 3, 6, 9, 12, 15 and 18 days post vaccination and at 3, 6, 9, 12 and 15 days post challenge and were examined for the presence of IBV antibodies.
Virus recovery and RT-PCR identification
Each tissue sample collected from each of the groups post challenge was used for virus recovery. Individual samples were prepared as 10% w/v tissue suspensions in 0.1% PBS, clarified by centrifugation at 1500×g at 4°C for 10 min, and filtered through 0.22 µm membrane filters (Millipore Products Division) before inoculation into the allantoic cavity of 9-day-old to 11-day-old SPF embryos. Three eggs were used for each sample, and each egg received 0.2 ml filtered sample via the allantoic cavity. The inoculated eggs were incubated at 37°C and were candled daily. Allantoic fluid from two of the inoculated embryos was collected 72 h post inoculation for RT-PCR amplification, and the remaining embryos were examined 1 week later for characteristic lesions of IB, such as dwarfing, stunting, or curling of embryos. One to three blind passages were performed. Dead embryos and embryos that showed characteristic changes of IB were recorded as positive for virus recovery.
Detection of the virus was further confirmed by RT-PCR using the inoculated allantoic fluid. Two hundred microlitres of allantoic fluid from each inoculated embryo was used for RT-PCR amplification. The RNA was extracted, and RT was conducted using IBV primer N(–) (). Primers N(–) and N(+) () were used to amplify most parts of the N gene and a portion of the 3′-untranslated region that was approximately 1600 bp in length (Liu et al., Citation2008). The RT and PCR were carried out under identical reaction conditions to those described previously (Liu et al., Citation2008). The PCR products were analysed on 1.0% agarose gels.
Antibody responses
Serum samples were assayed at a single dilution using a commercial total antibody enzyme-linked immunosorbent assay (IDEXX Corporation, Westbrook, Maine, USA) according to the manufacturer's instructions. Serum-to-positive ratios were calculated as described previously (de Wit et al., Citation1998; Liu et al., Citation2008).
Results
Virus detection and isolation
In the present study, 78 isolates of IBV were obtained from samples collected from 284 chicken flocks suspected of IB infection in China during 2009. The virus was detected in commercial broilers and layers at 3 to 55 weeks old; the birds showed clinical signs of IBV infection and 10 to 30% mortality. On most of the IBV-positive commercial farms, nephritis was observed in both vaccinated and non-vaccinated flocks and was characterized by enlarged, pale kidneys, frequently with urate deposits in the tubules, severe dehydration and weight loss. However, in some cases, slight decreases in productivity were observed but no obvious clinical signs were identified. The diagnosis was based on electron microscope examination of allantoic fluids at different passages, which showed that all isolates had typical coronavirus morphology and that the samples were free of other agents such as Newcastle disease virus (results not shown).
Phylogenetic analysis of IBV isolates
Phylogenetic analysis, based on the nucleotide sequences of the S1 gene of 78 IBV isolates and 39 strains of IBV, showed that the Chinese isolates could mainly be separated into five distinct genetic groups or genotypes (). Genotype I included 57 out of the 78 isolates that were grouped with reference strains LX4 and QX. The strains in this group included field viruses isolated between 1997 and 2009. Phylogenetic analyses comparing the complete S1 gene sequences of the 62 isolates and reference viruses published previously in the GenBank database revealed that the isolates in this group could be further grouped into two separated genetic clusters, identified as cluster I and cluster II, represented by LX4 and QX, respectively. The minimum amino acid divergence observed between isolates of the two clusters was 96.5%. Interestingly, two of the three South Korean QX-like viruses, three QX-like strains from The Netherlands and two from France fell into the same group as QXIBV by S1 gene phylogenetic analysis. Eleven isolates were included in genotype II, which showed maximum nucleotide and amino acid identities with the tl/CH/LDT3/03 strain (Liu et al., Citation2005). Three isolates were grouped in genotype III with IBV strain CK/CH/LSC/99I (Liu et al., Citation2009). Four isolates were grouped with the Mass vaccine strain (H120) in the phylogenetic tree, showing a close relationship with vaccine strains used in the immunization of chicken flocks in China. No strains of the CK/CH/LDL/97I type (Yu et al., Citation2001; Liu et al., Citation2006) were isolated in the present study.
Figure 1. Phylogenetic relationships, based on the sequence of the S1 subunit of the S gene of IB vaccine strains and IBV field strains isolated in China (the first 1697 nucleotides, starting at the AUG translation initiation codon, of the S gene), obtained using the MEGALIGN program in DNAStar with the Clustal V method (Liu et al., Citation2006). The IBV isolates obtained in the present study are in bold and the boxed ones are reference IBV strains. The three IBV isolates used in the vaccination–challenge test are shown in bold. The country, except China, where the reference IBV strains were isolated is shown in parentheses.
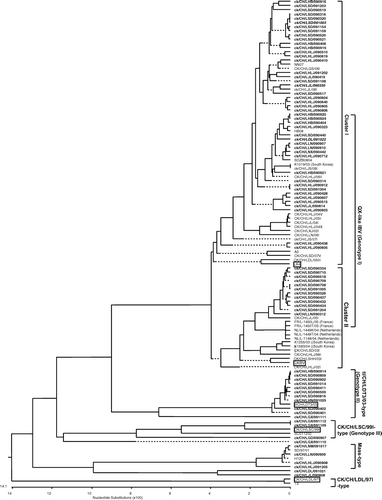
Isolate ck/CH/LGX/091110 was located on a separate branch and showed a close relationship with strain CK/CH/LSC/99I by phylogenetic analysis. The BLAST searches using the S1 gene of CK/CH/LGX/091110 revealed that it shared less than 90% nucleotide identity with other IBV strains, except three strains: CK/CH/Guangxi/Yulin/0904, CK/CH/Guangxi/Luchuan/0906 and CK/CH/Guangdong/Heyuan1/0905 (accession numbers GU938399, GU938394 and GU938401, respectively), which shared more than 97% nucleotide identity with CK/CH/LGX/091110. All these IBV strains had been isolated from Guangxi province, China. Finally, two isolates—ck/CH/LDL/091021 and ck/CH/LJL/090608—clustered into a separate group and shared 93.4% nucleotide identity. The BLAST searches that were conducted using the entire S1 gene of these isolates revealed that the two isolates were most closely related to the IBV isolates isolated in Taiwan, China. Isolate TW2296/95, obtained from a broiler in Taiwan in 1995 (Huang et al., Citation2004), shared the highest nucleotide identity (93%) with ck/CH/LDL/091021 and ck/CH/LJL/090608; the other strains did not share more than 90% nucleotide identity with the two isolates.
Comparison of partial S1 gene encoded amino acid sequences
Owing to the fact that only parts of the S1 genes were available in the GenBank database for the isolates from Russia, France, Slovakia, Greece and Hungary, these partial S1 genes were compared with those of the Chinese QX-like IBVs isolated in this study. Fifteen and seven unique substitutions out of the 143 amino acid residues (positions 46 to 188) compared were observed between Russian RF/11/01 and Chinese QX-like strains, and between Russian RF/17/02 and Chinese QX-like strains, respectively, in hypervariable regions 1 and 2 (a) of the S1 protein. However, comparison of other parts of the S1 protein (positions 257 to 369) showed high similarity between QX-like IBVs isolated from France, Slovakia, Greece and Hungary, and the Chinese strains (b), suggesting a similar origin among those QX-like IBVs circulating in the different countries.
Figure 2. Comparison of partial S1 gene encoded amino acid sequences between European and Chinese QX-like IBV strains included in this study. 2a: Comparison result between the Russian and Chinese QX-like IBV strain. 2b: Comparison between the QX-like strains reported by Benyeda et al. (Citation2009) and the Chinese QX-like IBV strain. The numbers indicate the positions downstream of the Met residue encoded by the ATG start site in the spike gene. X indicates that some strains showed the same amino acid residue and others showed substitutions in this position by comparison with the reference strains. The amino acids in italics are the unique ones that are different from other strains. The origin of the IBV strains is shown in parentheses.

Clinical observations
The birds started developing clinical signs, in the form of ruffled feathers and decreased consumption of food and water, 3 days after challenge with the three IBV isolates. However, none of them caused significant respiratory signs. The control birds were alert and active, whereas infected birds were depressed and huddled; the morbidity rates reached 100% at 5 days post challenge when the clinical signs were most apparent. Two and one of 10 birds died between 5 and 7 days post challenge with isolates ck/CH/LDL/091022 and ck/CH/LJL/090330, respectively, giving mortality rates of 20% and 10%. Both isolates ck/CH/LDL/091022 and ck/CH/LJL/090330 caused obvious swollen pale kidneys, with the tubules and ureters distended with urates, which suggests nephropathogenic potential. Meanwhile, haemorrhagic lesions of the caecal tonsils were observed in all the challenged SPF chickens that died. However, no obvious gross lesions were observed in oviducts and proventriculi of the challenged chickens. The duration of disease was 3 to 10 days after challenge. In addition, no gross lesions were detected either in any of the birds that recovered from infection with the three QX-like strains or in birds of the control groups, and no lesions were observed in the oviducts for the female birds.
Protection provided by Mass H120 vaccine
Although none of the chickens vaccinated with H120 died after challenge with any of the three IBV isolates, some of the vaccinated chickens showed clinical signs, especially those infected with isolates ck/CH/LDL/091022 and ck/CH/LSD/091003 (), which suggests that the H120 vaccine does not provide full protection against challenge with the IB isolates studied here.
Table 4. Results of vaccination–challenge tests using IBV isolates ck/CH/LDL/091022, ck/CH/LJL/090330 and ck/CH/LSD/091003.
The results of the virological examinations are presented in . The challenge virus was recovered from the tracheas and kidneys of all birds in the non-vaccinated groups. Interestingly, in the groups vaccinated with the H120 vaccine, the challenge virus was also re-isolated from the tracheas of all birds. In these same groups, the kidneys of most of the infected chickens were positive for virus re-isolation (). This indicates that vaccination using Mass H120 cannot provide protection against tracheal and renal invasion by IBV isolates ck/CH/LSD/091003, ck/CH/LDL/091022 and ck/CH/LJL/090330.
Serological results
The serological responses induced by the IBV vaccine and challenge viruses are presented in . Only one chicken in each vaccinated group had seroconverted by 6 days post vaccination with H120 vaccine, but by 15 days post vaccination all of the H120-vaccinated chickens had seroconverted. The humoral response induced by the H120 vaccine was detected later than that induced by each of the challenge viruses.
Discussion
In China, IB may be the most severe viral disease that affects the poultry industry, as indicated by our surveillance results for viral diseases in the past decade (data not shown), although the mortality rate for IB is not as high as that of Newcastle disease or highly pathogenic avian influenza. In the present study, we isolated and genotyped 78 IBVs from disease outbreaks in China in 2009 and compared them with Chinese IBV isolates isolated previously and with other reference strains. Although vaccines based on Mass-type strains, such as H120 and H52, have been in use for many years on poultry farms, the regular occurrence of suspected IB and the isolation of the virus from vaccinated flocks (Li & Yang, 2001; Yu et al., 2001; Liu & Kong, 2004; Liu et al., 2005, 2006, 2008, 2009; Xu et al., 2007; Li et al., 2010) implies that poor protection is provided by the Mass-type vaccines. This indicates the importance of continued surveillance of IBV in chicken flocks in China.
Determination of the type of field isolates is important not only for the study of emerging viruses and virus evolution, but also for the selection of an appropriate vaccine against future outbreaks of IB. Genotype classification, based on features of the S1 gene, is commonly used to classify IBV isolates. In the present study, the IBV isolates obtained in China in 2009 clustered into four genotypes. Most of them belonged to genotype I (). This result was consistent with other studies conducted in China (Liu et al., Citation2006, Citation2008, Citation2009; Xu et al., 2009; Li et al., Citation2010), which confirms that this genotype is the predominant IBV type circulating in chicken flocks in China. Interestingly, phylogenetic analysis of large numbers of strains of genotype I isolated from 1997 to 2009 in China showed that this type of virus was split in two major phylogenetic clusters, represented by strains LX4 and QX, respectively (). It is particularly remarkable that one and two of the three Korean IBV strains selected in this study belonged to the LX4 and QX clusters respectively, which indicates the close genetic relationship between Chinese and Korean strains. Furthermore, the viruses isolated in 2009 clustered in the QX cluster closely with strains isolated before 2003. These findings suggest that antigenic subtypes may be present in genotype I, regardless of the level of genetic variation displayed. Remarkably, based on S1 phylogenetic analysis or comparison of the partial S1 genes for amino acid similarity, Chinese QX-like IBVs had a close relationship with strains from The Netherlands, France, Slovakia, Greece and Hungary. However, by the partial S1 gene analysis, Russian QX-like IBVs showed high divergence from Chinese QX-like strains. It seems that the QX-like IBV originated in China. Owing to the geographic location of these countries, this result is hard to explain. It is very likely that the QX-like IBVs in Russia could represent an early introduction from an ancestor that diverged at an early stage in the evolutionary history.
A very challenging aspect of the epidemiology of IBV in China is the emergence of new strains and variants (Li & Yang, Citation2001; Yu et al., Citation2001; Liu & Kong, Citation2004; Liu et al., Citation2005, Citation2006, Citation2008, Citation2009; Xu et al., 2009; Li et al., Citation2010). In the present study, isolate CK/CH/ LGX/091110 was established as a new group based on phylogenetic analysis of the S1 gene. The isolate had a close relationship, shown by the BLAST search, with three other IBV strains deposited in the GenBank database; namely CK/CH/Guangxi/Yulin/0904, CK/CH/Guangxi/Luchuan/0906 and CK/CH/Guangdong/Heyuan1/0905. All four of these IBV isolates were obtained in 2009 and in the same geographic region, which possibly indicates a similar origin and features. Therefore, this finding demonstrates that IBV variants have emerged in Guangxi province in China in recent years, but their pathogenicity and other characteristics remain undetermined. In addition, two isolates from this study—ck/CH/LDL/091021 and ck/CH/LJL/090608—showed less than 93% nucleotide identity of the S1 gene with the known IBV strains in the GenBank database and revealed a distant genetic relationship with known IBV strains. Both of these strains were isolated from the proventriculus of H120-vaccinated broilers in 1999. This provides further evidence for the emergence of new strains and variants of IBV. In the present study, we isolated and analysed three unique strains of IBV by comparison with known IBVs. The results demonstrate the continuing emergence in vaccinated flocks of new IBVs that are not genetically related to the Mass-type vaccines, the only vaccine used in China. The results of IBV surveillance contrast with the conclusion drawn by Li et al. (Citation2010), who reported that no new strains of IBV have circulated in South China in recent years.
In the mid-1990s, transmissible proventriculitis, which was associated with IBV infection, was reported both in layer hens and broilers in China (YuDong et al., Citation1998). Although attempts to reproduce transmissible proventriculitis in SPF chickens have not been successful, at least three types of IBV, including the QXIBV strain of YuDong et al. (Citation1998), the CK/CH/LDL/97I-type T3 strain of Yu et al. (Citation2001) and the Mass-type ZJ971 strain of Xiao et al. (Citation2010), have been reported to cause the condition in chickens under field conditions. In the present study, we selected three QX-like isolates—ck/CH/LDL/091022, ck/CH/LJL/090330 and ck/CH/LSD/091003—which were isolated from H120-vaccinated layers or broilers in different provinces in China, to infect SPF chickens. All the chickens inoculated with the three IBV isolates demonstrated clinical disease in the form of mild respiratory signs with 100% morbidity and various levels of mortality. Infection with ck/CH/LDL/091022 and ck/CH/LJL/090330 caused severe clinical signs and death, in contrast to the milder infection caused by ck/CH/LSD/091003. The gross lesions and the results of virus recovery attempts showed that all three IBV isolates exhibited affinity for the kidney and were nephropathogenic strains. However, gross lesions were not found in the proventriculus of the dead chickens. This finding is inconsistent with a previous study by YuDong et al. (Citation1998), who reported that the QX strain caused proventriculitis in chickens. Recent work, which involved the study of the pathogenicity of a European QX-like strain in SPF chickens, showed that QX-like IBV can replicate in the female reproductive tract and has nephropathogenic potential (Terregino et al., Citation2008). This diversity of pathogenicity is of interest and should be addressed further in future studies.
Pathogenicity for the oviduct of young chickens of various ages caused by four serotypes of IBV has been reported (Crinion et al., Citation1971; Crinion & Hofstad, Citation1972a, Citation1972b) and abnormalities in laying hens following exposure to IBV were observed. Interestingly, contrary to these findings, we did not observe gross lesions in the oviducts of chickens infected with the three QX-like viruses in the relatively short period of our experiment. Although characteristic dilation and serum-like fluid accumulation developed in the oviduct of chickens infected with QX-like viruses, Benyeda et al. (Citation2009) also did not observe microscopic lesions in the oviduct. A study recently carried out in Australia also showed that no macroscopic lesions could be observed in the oviduct of hens inoculated with the nephropathogenic strain N1/88 (Chousalkar et al., Citation2010). The absence of lesions might be due to inappropriate sampling time or to site of sampling (Benyeda et al., Citation2010). Otherwise, it might be due to the different tropism and pathogenicity for the oviduct shown by different QX-like strains.
In general, control of IB usually involves the use of live attenuated vaccines. Although the QXIBV strain was first isolated in 1997 in China, no information is available on the protection against Chinese QX-like IBV conferred by vaccination. The H120 Mass-type vaccine was chosen for this study because it is the officially authorized and most commonly used vaccine in chicken flocks in China. Comparison of the clinical signs, pathological lesions and virus recovery from the trachea and kidney of unvaccinated–challenged and vaccinated–challenged birds indicated that the vaccine provided partial, but not full, protection against challenge with ck/CH/LDL/091022, ck/CH/LJL/090330 and ck/CH/LSD/091003. Interestingly, only small numbe rs of chickens infected with these isolates showed clinical signs after infection. However, not only the tracheas but also the kidneys of chickens challenged with each of the IBV isolates showed similar, high rates of virus recovery, although all of the H120 vaccinated chickens showed seroconversion. The low protection against renal lesions conferred by the H120 vaccine in this study was inconsistent with other reports, which showed that nephritis can be induced following infection via the respiratory tract, presumably as a result of viraemia, and that the humoral immune mechanisms directed against viraemia appear to be of particular importance in protecting the kidney (Holmes, Citation1973; Macdonald et al., Citation1981; Marquardt et al., Citation1982). Our findings demonstrated that the H120 vaccine provided poor protection against Chinese QX-like strains. Our results differ from the conclusion of Terregino et al. (Citation2008), who reported that vaccination at 1 day old and at 14 days old using both the Ma5 (Mass-type) and 4/91 IB vaccines offered good protection against challenge with a QX-like IBV in layer and broiler flocks. The different vaccination protocols used here and by Terregino et al. (Citation2008) could account for the different protection. It has been reported that revaccination using the same or a different serotype of IB vaccine can improve protection against the same or an antigenically different challenge viruses (Cook et al., Citation1999; Worthington et al., Citation2004). Furthermore, differences in the virulence of the QX-like strains used in this study and those used by Terregino et al. (2008) may also partially explain the different findings.
The level of protection provided by the H120 vaccine against Chinese QX-like field strains of IBV in the present study was poor under the experimental conditions used. The level could be even lower in the field when husbandry and management conditions are not optimal, and secondary infection by other viruses and bacteria may also contribute to the severity of disease. Moreover, in this study the vaccine was delivered individually, which is not the routine method of application in large operations when the immunity among chickens may not be uniform. This result is of significance for IB control programmes in China and other countries.
Acknowledgements
The present work was supported by a grant from the National Key Technology R&D Program from Ministry of Science and Technology of the People's Republic of China (No. 2006BAD06A03) and the earmarked fund for Modern Agro-industry Technology Research System.
References
- Adzhar , A. , Shaw , K. , Britton , P. and Cavanagh , D. 1996 . Universal oligonucleotides for the detection of infectious bronchitis virus by the polymerase chain reaction . Avian Pathology , 25 : 817 – 836 .
- Beato , M.S. , De Battisti , C. , Terregino , C. , Drago , A. , Capua , I. and Ortali , G. 2005 . Evidence of circulation of a Chinese strain of infectious bronchitis virus (QXIBV) in Italy . The Veterinary Record , 156 : 720
- Benyeda , Zs. , Mató , T. , Süveges , T. & Palya , V. 2008 . Comparison of the pathogenicity of QX-like IBV strains from different pathological conditions . In Proceedings of the XXIIIrd World's Poultry Congress . Brisbane, Australia .
- Benyeda , Z. , Mató , T. , Süveges , T. , Szabó , E. , Kardi , V. Abonyi-Tóth , Z. 2009 . Comparison of the pathogenicity of QX-like, M41 and 793/B infectious bronchitis strains from different pathological conditions . Avian Pathology , 38 : 449 – 456 .
- Benyeda , Z. , Szeredi , L. , Mató , T. , Süveges , T. , Balka , Gy. , Abonyi-Tóth , Z. , et al. 2010 . Comparative histopathology and immunohistochemistry of QX-like, Massachusetts and 793/B serotypes of infectious bronchitis virus infection in chickens . Journal of Comparative Pathology , 143 , 276 283 .
- Bijlenga , G. , Cook , J.K.A. , Gelb , J. Jr and de Wit , J.J. 2004 . Development and use of the H strain of avian infectious bronchitis virus from the Netherlands as a vaccine: a review . Avian Pathology , 33 : 550 – 557 .
- Bochkov , Y.A. , Batchenko , G.V. , Shcherbakova , L.O. , Borisov , A.V. and Drygin , V.V. 2006 . Molecular epizootiology of avian infectious bronchitis in Russia . Avian Pathology , 35 : 379 – 393 .
- Cavanagh , D. , Davis , P.J. and Cook , J.K.A. 1992 . Infectious bronchitis virus: evidence for recombination within the Massachusetts serotype . Avian Pathology , 21 : 401 – 408 .
- Cavanagh , D. , Ellis , M.M. and Cook , J.K.A. 1997 . Relationship between sequence variation in the S1 spike protein of infectious bronchitis virus and the extent of cross protection in vivo . Avian Pathology , 26 : 63 – 74 .
- Cavanagh , D. & Gelb , J. 2008 . Infectious bronchitis . In Y.M. Saif , A.M. Fadly , J.R. Glisson , L.R. McDougald , L.K. Nolan & D.E. Swayne Diseases of Poultry , 12th edn 117 135 . Wiley-Blackwell Publishing : Iowa .
- Chousalkar , K.K. , Cheetham , B.F. and Roberts , J.R. 2010 . Detection of infectious bronchitis virus strain N1/88 from the oviduct and feces of experimentally infected vaccinated and unvaccinated hens . Poultry Science , 89 : 1603 – 1608 .
- Cook , J.K.A. , Orbell , S.J. , Woods , M.A. and Huggins , M.B. 1999 . Breadth of protection of the respiratory tract provided by different live-attenuated infectious bronchitis vaccines against challenge with infectious bronchitis virus of heterologous serotypes . Avian Pathology , 28 : 477 – 485 .
- Crinion , R.A. , Ball , R.A. and Hofstad , M.S. 1971 . Abnormalities in laying chickens following exposure to infectious bronchitis virus at one day old . Avian Diseases , 15 : 42 – 48 .
- Crinion , R.A.P. and Hosfstad , M.S. 1972a . Pathogenicity of four serotypes of avian infectious bronchitis virus for the oviduct of young chickens of various ages . Avian Diseases , 15 : 351 – 363 .
- Crinion , R.A. and Hofstad , M.S. 1972b . Pathogenicity of two embryo-passage levels of avian infectious bronchitis virus for the oviduct of young chickens of various ages . Avian Diseases , 16 : 967 – 973 .
- de Wit , J.J. , de Jong , M.C.M. , Pijpers , A. and Verheijden , J.H.M. 1998 . Transmission of infectious bronchitis virus within vaccinated and unvaccinated groups of chickens . Avian Pathology , 27 : 464 – 471 .
- Domanska-Blicharz , K. , Minta , Z. & Smietanka , K. 2006 . Molecular studies on infectious bronchitis virus isolated in Poland . In Proceedings of the Vth International Symposium on Avian Corona- and Pneumoviruses 147 153 , Rauischholzhausen, Germany .
- Gough , R.E. , Cox , W.J. , Welchman , D. , De , B. , Worthington , K.J. and Jones , R.C. 2008 . Chinese QX strains of infectious bronchitis virus isolated in the UK . The Veterinary Record , 162 : 99
- Holmes , H.C. 1973 . Neutralizing antibody in nasal secretions of chickens following administration of avian infectious bronchitis virus . Archiv für die gesamte Virusforschung , 43 : 235 – 241 .
- Huang , Y.P. , Lee , H.C. , Cheng , M.C. and Wang , C.H. 2004 . S1 and N gene analysis of avian infectious bronchitis viruses in Taiwan . Avian Diseases , 48 : 581 – 589 .
- Ignjatovic , J. and Sapats , S. 2000 . Avian infectious bronchitis virus . Revue Scientifique et Technique , 19 : 493 – 508 .
- Landman , W.J.M. , Dwars , R.M. & de Wit , J.J. 2005 . High incidence of false layers in (re)production hens supposedly attributed to a juvenile infectious bronchitis virus infection . In Proceedings of the XIVth International Congress of the World Veterinary Poultry Association (p. 369 ). Istanbul, Turkey .
- Lee , E.K. , Jeon , W.J. , Lee , Y.J. , Jeong , O.M. , Choi , J.G. and Kwon , J.H. 2008 . Genetic diversity of avian infectious bronchitis virus isolates in Korea between 2003 and 2006 . Avian Diseases , 52 : 332 – 337 .
- Lee , H.J. , Youn , H.N. , Kwon , J.S. , Lee , Y.J. , Kim , J.H. Lee , J.B. 2010 . Characterization of a novel live attenuated infectious bronchitis virus vaccine candidate derived from a Korean nephropathogenic strain . Vaccine , 28 : 2887 – 2894 .
- Li , H. and Yang , H.C. 2001 . Sequence analysis of nephropathogenic infectious bronchitis virus strains of the Massachusetts genotype in Beijing . Avian Pathology , 30 : 535 – 541 .
- Li , L. , Xue , C. , Chen , F. , Qin , J. , Xie , Q. , Bi , Y. and Cao , Y. 2010 . Isolation and genetic analysis revealed no predominant new strains of avian infectious bronchitis virus circulating in South China during 2004–2008 . Veterinary Microbiology , 143 : 145 – 154 .
- Liu , S. and Kong , X. 2004 . A new genotype of nephropathogenic infectious bronchitis virus circulating in vaccinated and non-vaccinated flocks in China . Avian Pathology , 33 : 321 – 327 .
- Liu , S. , Chen , J. , Chen , J. , Kong , X. , Shao , Y. Han , Z. 2005 . Isolation of avian infectious bronchitis coronavirus from domestic peafowl (Pavo cristatus) and teal (Anas) . Journal of General Virology , 86 : 719 – 725 .
- Liu , S. , Zhang , Q. , Chen , J. , Han , Z. , Liu , X. Feng , L. 2006 . Genetic diversity of avian infectious bronchitis coronavirus strains isolated in China between 1995 and 2004 . Archives of Virology , 151 : 1133 – 1148 .
- Liu , S. , Wang , Y. , Ma , Y. , Han , Z. , Zhang , Q. Shao , Y. 2008 . Identification of a newly isolated avian infectious bronchitis coronavirus variant in China exhibiting affinity for the respiratory tract . Avian Diseases , 52 : 306 – 314 .
- Liu , S. , Zhang , X. , Wang , Y. , Li , C. , Han , Z. Shao , Y. 2009 . Molecular characterization and pathogenicity of infectious bronchitis coronaviruses: complicated evolution and epidemiology in China caused by cocirculation of multiple types of infectious bronchitis coronaviruses . Intervirology , 52 : 223 – 234 .
- Macdonald , J.W. , Randall , C.J. , McMartin , D.A. , Dagless , M.D. and Gazdzinski , P. 1981 . Active and passive immunization against nephritis induced by an avian infectious bronchitis virus . Avian Pathology , 10 : 121 – 129 .
- Marquardt , W.W. , Kadavil , S.K. and Snyder , D.B. 1982 . Comparison of ciliary activity and virus recovery from tracheas of chickens and humoral immunity after inoculation with serotypes of avian infectious bronchitis virus . Avian Diseases , 26 : 828 – 834 .
- Reed , L.J. and Muench , H. 1938 . A simple method of estimating fifty percent endpoints . The American Journal of Hygiene , 27 : 493 – 497 .
- Terregino , C. , Toffan , A. , Beato , M.S. , De Nardi , R. , Vascellari , M. Meini , A. 2008 . Pathogenicity of a QX strain of infectious bronchitis virus in specific pathogen free and commercial broiler chickens, and evaluation of protection induced by a vaccination programme based on the Ma5 and 4/91 serotypes . Avian Pathology , 37 : 487 – 493 .
- Worthington , K.J. , Savage , C. , Naylor , C.J. , Wijmenga , W. & Jones , R.C. 2004 . An RT-PCR survey of infectious bronchitis virus genotypes in the UK and selected European countries between 2002 and 2004 and the results from a vaccine trial . In Proceedings of the IV Symposium on Avian Corona and Pneumovirus Infections 125 133 , Rauischholzhausen, Germany .
- Worthington , K.J. , Currie , R.J.W. and Jones , R.C. 2008 . A reverse transcriptase-polymerase chain reaction survey of infectious bronchitis virus genotypes in Western Europe from 2002 to 2006 . Avian Pathology , 37 : 247 – 257 .
- Xiao , C.T. , Liu , R. , Song , Z.Y. , Liao , M. and Zhou , J.Y. 2010 . Genomic characterization of a proventriculitis-associated infectious bronchitis coronavirus . Virus Genes , 40 : 421 – 422 .
- Xu , C. , Zhao , J. , Hu , X. and Zhang , G. 2007 . Isolation and identification of four infectious bronchitis virus strains in China and analyses of their S1 glycoprotein gene . Veterinary Microbiology , 122 : 61 – 71 .
- Yu , L. , Jiang , Y. , Low , S. , Wang , Z. , Nam , S.J. , Liu , W. and Kwangac , J. 2001 . Characterization of three infectious bronchitis virus isolates from China associated with proventriculus in vaccinated chickens . Avian Diseases , 45 : 416 – 424 .
- YuDong , W. , YongLin , W. , ZiChun , Z. , GenChe , F. , YiHai , J. XiangE , L. 1998 . Isolation and identification of glandular stomach type IBV (QX IBV) in chickens . Chinese Journal of Animal Quarantine , 15 : 1 – 3 .
- Zanella , A. , Barbieri , I. , Gavazzi , L. & Tosi , G. 2006 . Avian infectious bronchitis: isolation of an umpteenth new serotype of virus in Italy . In Proceedings of the Vth International Symposium on Avian Corona and Pneumoviruses 161 168 , Rauischholzhausen, Germany .