Abstract
Aspergillosis is one of the most common causes of death in captive birds. Aspergillosis in birds is mainly caused by Aspergillus fumigatus, a ubiquitous and opportunistic saprophyte. Currently it is not known whether there is a link between the environmental isolates and/or human isolates of A. fumigatus and those responsible for aspergillosis in birds. Microsatellite typing was used to analyse 65 clinical avian isolates and 23 environmental isolates of A. fumigatus. The 78 genotypes that were obtained were compared with a database containing genotypes of 2514 isolates from human clinical samples and from the environment. There appeared to be no specific association between the observed genotypes and the origin of the isolates (environment, human or bird). Eight genotypes obtained from isolates of diseased birds were also found in human clinical samples. These results indicate that avian isolates of A. fumigatus may cause infection in humans.
Introduction
Fungal infections due to Aspergillus species are a major cause of morbidity and mortality among certain species of birds, captive as well as free-ranging, independent of age or status of the immune system (Tell, Citation2005; Beernaert et al., Citation2008). Aspergillus fumigatus, a ubiquitous and saprophytic fungus, is the major aetiological agent responsible for aspergillosis (Tell, Citation2005).
To investigate the genetic and the epidemiological relationship between environmental and clinical isolates, fingerprinting methods with high discriminatory power must be applied. Also, interlaboratory reproducibility and objective interpretation of the fingerprinting data are highly desirable (de Valk et al., 2007a). All of these characteristics can be found in typing methods based on short tandem repeats (STRs), such as microsatellite length polymorphism and STRAf typing (Bart-Delabesse et al., Citation1998; de Valk et al., Citation2005, 2007a; Vanhee et al., Citation2008a). While the discriminatory power is high in pattern-based techniques, such as random amplified polymorphic DNA analysis, restriction fragment length polymorphism analysis, and amplified fragment length polymorphism, STRAf typing proved to be more simple and reproducible (Bart-Delabesse et al., Citation1998; de Valk et al., Citation2005, Citation2007a; Vanhee et al., Citation2008a, Citation2008b). However, any typing method is associated with its specific advantages and disadvantages (de Valk et al., Citation2007b) and any technical complications associated with microsatellite typing (Pasqualotto et al., Citation2007; Alvarez-Perez et al., Citation2009) can be properly addressed (de Valk et al., Citation2009; Klaassen, Citation2010).
Previous molecular typing studies showed that there was a high variability among avian isolates and multiple genotypes recovered from healthy and diseased birds (Lair-Fulleringer et al., Citation2003; Olias et al., Citation2009; Alvarez-Perez et al., Citation2010). However, currently it is not known whether there is a link between environmental isolates and/or human isolates of A. fumigatus and those responsible for aspergillosis in birds.
Therefore, in the present study, STRAf typing was performed on environmental and avian clinical isolates of A. fumigatus and the results were compared with a database containing genotypes from A. fumigatus isolated from clinical human samples and from the environment.
Materials and Methods
Isolates
Sixty-five clinical avian and 23 environmental isolates of A. fumigatus were subjected to STRAf typing. The clinical isolates were collected at six different institutes, four located in Belgium and two in The Netherlands, and were obtained from birds belonging to 13 orders, 18 families and 35 species () that died from an A. fumigatus infection. To collect the environmental isolates, Sabouraud dextrose agar plates were placed at 20 different locations in the vicinity of Ghent, Belgium. After incubation for 3 days at room temperature, the plates were placed in an incubator at 37°C. Fast-growing greenish colonies were purified on Sabouraud dextrose agar.
Table 1. Order, Family and Species of domestic and wild birds from which A. fumigatus isolates were obtained.
The isolates were identified based on the macro-morphology and micro-morphology of the fungus. Determination of partial DNA sequences of the β-tubulin and rodletA genes (Alcazar-Fuoli et al., Citation2008) and the ability to grow at 48°C were used to confirm species identity.
STRAf assay
Fungal DNA was prepared from all isolates as described by Beernaert et al. (Citation2008). Polymerase chain reaction (PCR) primers for the STRAf2, STRAf3 and STRAf4 panels and amplification conditions were as described by de Valk et al. (Citation2005), except that FAM labelling was replaced by VIC labelling, HEX labelling by NED labelling, and TET labelling by PET labelling. Allelic ladders were used with the same fluorescent labels as above for each of the three trinucleotide markers in the STRAf3 panel as described by de Valk et al. (Citation2009). The obtained PCR products were diluted 10-fold with distilled water. Two microlitres of the diluted PCR products were added to 12 µl formamide (Amresco Inc., Ohio, USA) and 1 µl GS 500 LIZ size standard (Applied Biosystems, Halle, Belgium). Following denaturation of the samples for 2 min at 95°C and rapid cooling to 0°C for 30 min, they were injected onto an ABI Prism 3100 Genetic Analyzer (Applied Biosystems) equipped with a 16 capillary array. Genemapper v3.5 (Applied Biosystems) was used to determine the size of each amplified fragment. All results are reported as repeat numbers. Repeat numbers for the markers in the STRAf3 panel were determined by comparison with the allelic ladders. The repeat numbers of the markers in the STRAf2 and STRAf4 panels were determined with the reference size values taken from the original publication and validated using a set of reference isolates with known genotypes (de Valk et al., Citation2005).
Data analysis
Typing data were imported into BioNumerics version 6 software (Applied Maths, St-Martens-Latem, Belgium) and analysed using the categorical multistate similarity coefficient with UPGMA clustering. The obtained genotypes were then compared with a database containing 2514 genotypes from human and environmental A. fumigatus isolates from Europe and the USA.
Results
A total of 88 A. fumigatus isolates were analysed using STRAf typing and a total of 78 genotypes were obtained. Seventy genotypes were found once, six genotypes were found twice and two genotypes were found three times. From the genotypes observed more than once, no relationship could be observed between the species of bird in the clinical isolates and the area of isolation in environmental isolates. There was also no relationship between the geographic area of the clinical isolate K7 and the environmental isolate O7. The Simpson's index of diversity (D) for the clinical avian isolates was calculated to be 0.995.
In the dendrogram (), eight clusters of related genotypes can be identified differing only at a single locus. In one case, an identical genotype was found in a clinical avian isolate and an environmental isolate. Two clusters of genotypes contained clinical and environmental isolates, four clusters only clinical isolates and two clusters only environmental isolates.
Figure 1. Dendrogram generated from genotyping 65 clinical and 23 environmental A. fumigatus isolates. Isolates recovered from clinical samples are denoted K, and strains isolated from the environment are denoted O. The genotypes that show microvariation are framed and to the right are the typing results for the nine markers of the STRAf panel. The scale bars above the dendrogram indicates the percentage identity between the genotypes. CH, Switzerland; D, Germany; N, Norway; NL, Netherlands; SP, Spain.
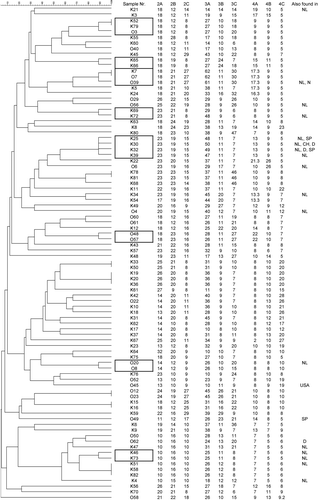
Comparing the results of our analysis with a database containing 2514 genotypes from human and environmental isolates revealed that 58 genotypes in our collection of avian and environmental isolates were not observed before. Twenty genotypes were observed before either in human clinical samples or in environmental samples. Twelve genotypes from avian clinical isolates were identical to genotypes found before in human (n=3) or environmental (n=4) isolates, or both (n=5), from The Netherlands, Switzerland, Germany and Spain. Eight environmental genotypes from Belgium were identical to genotypes found in human and/or environmental isolates from The Netherlands, Spain, Germany, the USA and Norway.
Discussion
Previous epidemiological studies have investigated the origin of avian aspergillosis in limited geographical areas and in one species of bird (Lair-Fulleringer et al., Citation2003; Olias et al., Citation2009; Alvarez-Perez et al., Citation2010). The present study is the first determining the genetic diversity among A. fumigatus isolates obtained from 35 avian species collected at six different institutes and environmental isolates collected at 20 different locations in Belgium.
The results of this study demonstrate that the genetic diversity among avian clinical isolates is extremely high. The 65 isolates, collected from 65 birds affected with aspergillosis, belonged to 57 distinct genotypes. In contrast, Alvarez-Perez et al. (Citation2010) reported 13 distinct genotypes in 33 isolates from five diseased birds, and Lair-Fulleringer et al. (2003) reported 23 distinct genotypes in 114 isolates from 30 healthy and two diseased turkeys. The low number of distinct genotypes in relation to the number of isolates in those studies could be explained firstly by the fact that a limited number of animals with aspergillosis was used. Secondly, the animals belonged to closed collections and multiple isolates from each animal were examined. Finally, the genotyping was performed with a method described by Bart-Delabesse et al. (Citation1998) including only four microsatellite markers, being less discriminatory than the panel of nine microsatellite markers described here. Alternatively, these animals may have been exposed to a common source of material(s) that may have been contaminated with a limited genotypic diversity of A. fumigatus spores such as bird feed.
The environmental and avian isolates are widespread throughout the dendrogram, suggesting that any environmental isolate of A. fumigatus is possibly infectious to birds. This finding is supported by the study of Peden & Rhoades (Citation1992), who inoculated isolates from diverse origins (environmental, mammalian, and avian) in to the air sacs of turkeys. All isolates were able to induce aspergillosis in these birds.
High reproducibility of the STRAf assay and the ease of interlaboratory exchange of the results allowed comparison of the dataset of 88 clinical avian and environmental isolates with a dataset of 2514 genotypes from human and environmental isolates (de Valk et al., 2009). There appeared to be no specific association between the observed genotypes and the origin of the isolates (environment, human or bird). This was expected because of the high degree of genetic diversity among A. fumigatus isolates, independent of the species or geographical region (Debeaupuis et al., Citation1997; Chazalet et al., Citation1998; Rosehart et al., Citation2002; Menotti et al., Citation2005; Klaassen et al., Citation2009). Moreover, eight genotypes derived from diseased birds were also isolated from human clinical samples, indicating that avian isolates of A. fumigatus could be considered infectious to humans.
Acknowledgements
The present work was supported by the Institute for the Promotion of Innovation through Science and Technology in Flanders (IWT Vlaanderen), Brussels, Belgium.
References
- Alcazar-Fuoli , L. , Mellado , E. , Alastruey-Izquierdo , A. , Cuenca-Estrella , J.M. and Rodriguez-Tudela , J.L. 2008 . Aspergillus section Fumigati: antifungal susceptibility patterns and sequence based identification . Antimicrobial Agents and Chemotherapy , 52 : 1244 – 1251 .
- Alvarez-Perez , S. , Garcia , M.E. , Bouza , E. , Pelaez , T. and Blanco , J.L. 2009 . Characterization of multiple isolates of Aspergillus fumigatus from patients: genotype, mating type and invasiveness . Medical Mycology , 47 : 601 – 608 .
- Alvarez-Perez , S. , Mateos , A. , Dominguez , L. , Martinez-Nevado , E. , Blanco , J.L. and Garcia , M.E. 2010 . Polyclonal Aspergillus fumigatus infection in captive penguins . Veterinary Microbiology , 144 : 444 – 449 .
- Bart-Delabesse , E. , Humbert , J.-F. , Delabesse , E. and Bretagne , S. 1998 . Microsatellite markers for typing Aspergillus fumigatus isolates . Journal of Clinical Microbiology , 36 : 2413 – 2418 .
- Beernaert , L.A. , Pasmans , F. , Haesebrouck , F. and Martel , A. 2008 . Modelling Aspergillus fumigatus infections in racing pigeons (Columba livia domestica) . Avian Pathology , 37 : 545 – 549 .
- Chazalet , V. , Debeaupuis , J.P. , Sarfati , J. , Lortholary , J. , Ribaud , P. Shah , P. 1998 . Molecular typing of environmental and patient isolates of Aspergillus fumigatus from various hospital settings . Journal of Clinical Microbiology , 36 : 1494 – 1500 .
- Debeaupuis , J.P. , Sarfati , J. , Chazalet , V. and Latgé , J.-P. 1997 . Genetic diversity among clinical and environmental isolates of Aspergillus fumigatus . Infection and Immunity , 65 : 3080 – 3085 .
- de Valk , H.A. , Meis , J.F.G.M. , Curfs , I.M. , Muehlethaler , K. , Mouton , J.W. and Klaassen , C.H.W. 2005 . Use of a novel panel of nine short tandem repeats for exact and high-resolution fingerprinting of Aspergillus fumigatus isolates . Journal of Clinical Microbiology , 43 : 4112 – 4120 .
- de Valk , H.A. , Meis , J.F.G.M. and Klaassen , C.H.W. 2007a . Microsatellite based typing of Aspergillus fumigatus: strengths, pitfalls and solutions . Journal of Microbiological Methods , 69 : 268 – 272 .
- de Valk , H.A. , Meis , J.F.G.M. and Klaassen , C.H.W. 2007b . Molecular typing of Aspergillus species . Mycoses , 51 : 463 – 476 .
- de Valk , H.A. , Meis , J.F.G.M. , Bretagne , S. , Costa , J.M. , Lasker , B.A. Balajee , S.A. 2009 . Interlaboratory reproducibility of a microsatellite-based typing assay for Aspergillus fumigatus through the use of allelic ladders: proof of concept . Clinical Microbiology and Infection , 15 : 180 – 187 .
- Klaassen , C.H.W. , de Valk , H.A. , Balajee , S.A. and Meis , J.F.G.M. 2009 . Utility of CSP typing to sub-type clinical Aspergillus fumigatus isolates and proposal for a new CSP type nomenclature . Journal of Microbiological Methods , 77 : 292 – 296 .
- Klaassen , C.H.W. 2010 . “ Molecular typing of Aspergillus fumigatus isolates ” . In Aspergillosis: From Diagnosis to Prevention , Edited by: Pasqualotto , A.C. Heidelberg : Springer .
- Lair-Fulleringer , S. , Guillot , J. , Desterke , C. , Seguin , D. , Warin , S. Bezille , A. 2003 . Differentiation between isolates of Aspergillus fumigatus from breeding turkeys and their environment by genotyping with microsatellite markers . Journal of Clinical Microbiology , 41 : 1798 – 1800 .
- Menotti , J. , Waller , J. , Meunier , O. , Letscher-Bru , V. , Herbrecht , R. and Candolfi , E. 2005 . Epidemiological study of invasive pulmonary aspergillosis in a haematology unit by molecular typing of environmental and patient isolates of Aspergillus fumigatus . Journal of Hospital Infection , 60 : 61 – 68 .
- Olias , P. , Lierz , M. , Hafez , H.M. , Jacobsen , I.D. and Gruber , A.D. 2009 . Microsatellite genotyping and virulence assessment of Aspergillus fumigatus isolates from white stork nestlings and their environment . Journal of Comparative Pathology , 141 : 266
- Pasqualotto , A.C. , Denning , D.W. and Anderson , M.J. 2007 . A cautionary tale: lack of consistency in allele sizes between two laboratories for a published multilocus microsatellite typing system . Journal of Clinical Microbiology , 45 : 522 – 528 .
- Peden , W.M. and Rhoades , K.R. 1992 . Pathogenicity differences of multiple isolates of Aspergillus fumigatus in turkeys . Avian Diseases , 36 : 537 – 542 .
- Rosehart , K. , Richards , M.H. and Bidochka , M.J. 2002 . Microsatellite analysis of environmental and clinical isolates of the opportunist fungal pathogen Aspergillus fumigatus . Journal of Medical Microbiology , 51 : 1128 – 1134 .
- Tell , L.A. 2005 . Aspergillosis in mammals and birds: impact on veterinary medicine . Medical Mycology , 43 : S71 – S73 .
- Vanhee , L.M.E. , Symoens , F. , Nelis , H.J. and Coenye , T. 2008a . Microsatellite typing of Aspergillus fumigatus isolates recovered from deep organ samples of patients with invasive aspergillosis . Diagnostic Microbiology and Infectious Disease , 62 : 96 – 98 .
- Vanhee , L.M.E. , Symoens , F. , Nelis , H.J. , Bouchara , J.P. and Coenye , T. 2008b . High-resolution genotyping of Aspergillus fumigatus isolates recovered from chronically colonised patients with cystic fibrosis . European Journal of Clinical Microbiology and Infectious Diseases , 27 : 1005 – 1007 .