Abstract
Distribution, character, and severity of lesions were evaluated in tissues from the central nervous system of chickens inoculated with 10 different Newcastle disease virus (NDV) isolates: CA 1083, Korea 97-147, Australia (all velogenic viscerotropic), Texas GB and Turkey North Dakota (both velogenic neurotropic), Nevada cormorant, Anhinga and Roakin (all mesogenic), and B1 and QV4 (lentogenic). Tissues for the present study included archived formalin-fixed, paraffin-embedded brain (all strains) plus spinal cord (two strains). Encephalitis was observed in all velogenic viscerotropic and velogenic neurotropic strains, and in some mesogenic strains. In general, the encephalitic lesions began at 5 days post infection, with more severe lesions occurring around 10 days post infection. At this time point, especially in the grey matter of the brain, cerebellum and spinal cord, there were neuronal necrosis, neuronal phagocytosis, and clusters of cells with microglial morphology. Axonal degeneration and demyelination was also observed. Immunohistochemistry (IHC) for viral nucleoprotein confirmed the presence of virus. In the areas of encephalomyelitis, IHC for CD3 revealed that many of the inflammatory cells were T lymphocytes. IHC using an antibody for glial fibrillar acid protein showed reactive astrogliosis, which was most pronounced at the later time points.
Introduction
Newcastle disease (ND) causes considerable impact on the poultry industry worldwide, with significant morbidity and mortality, and high economic losses. Occurrence of the disease requires reporting to the World Organization for Animal Health (Office International des Epizooties) and subsequent trade restrictions (Alexander & Senne, Citation2008). ND is caused by different strains of avian paramyxovirus-1 (APMV-1), Avulavirus genus, Paramyxoviridae family. Newcastle disease virus (NDV) has a negative-sense, single-stranded, filamentous RNA genome and a glycoprotein/lipid membrane (Phillips et al., Citation1998; Wise et al., Citation2004).
Classification of ND strains has been implemented through the use of pathogenicity indices, mainly the mean death time in embryonated eggs and the intracerebral pathogenicity index (ICPI). The mean death time consists of inoculation via the chorioallantoic route of 10-day-old embryonated chicken eggs, and subsequent scoring of the time to embryo death (Alexander & Senne, Citation2008; Office International des Epizooties, Citation2008). The ICPI involves intracerebral inoculation of 1-day-old chicks, followed by numerical scoring of the clinical signs over an 8-day period. The ICPI is the internationally recognized method for the classification of the different NDV isolates, and strains that score >0.7 are considered reportable to the international community. Another internationally recognized method for classification is the amino acid sequence of the fusion protein cleavage site. Strains with at least three arginine or lysine residues between positions 113 and 116 and a phenylalanine residue at position 117 are considered virulent and are therefore notifiable (Alexander & Senne, Citation2008).
The virulence of NDV varies greatly among the different isolates. The clinical signs, lesions and severity depend on the virulence of the virus (Bhaiyat et al. Citation1995; Alexander & Senne, Citation2008), species, immune status, age and concurrent diseases (Alexander & Senne, Citation2008; Farkas et al., Citation2009). Because of the wide span of virulence, NDV isolates have been divided into three major groups, from the most to the least virulent: velogenic, mesogenic and lentogenic. The velogens have been further divided into viscerotropic (VVND) and neurotropic (VNND) based on behaviour in adult chickens subsequent to cloacal inoculation. Velogenic viscerotropic NDV strains inoculated in this manner produce a high mortality rate (approaching 100%) and haemorrhagic intestinal lesions centred over lymphoid areas; neurotropic velogenic NDV have a lower mortality rate (50%) and cause mainly nervous signs. The mesogenic viruses in general are associated with a low mortality rate, and cause mild respiratory or nervous signs; lentogenic viruses cause minimal or subclinical infection (Alexander & Senne, Citation2008).
In the present study, we describe and compare the lesions and the reaction of the neuropil and glial cells in the central nervous system (CNS) in chickens inoculated with 10 different NDV isolates. Also, 12 regions of the brain using the new anatomical nomenclature (Reiner et al., Citation2004) were analysed, considering the intensity and spatial distribution of the lesions. Using an antibody directed against the viral nucleoprotein, the distribution and relative amounts of the virus were determined. In addition, the character of the inflammation and glial reaction was partially assessed through immunohistochemical identification of lymphocytes and astrocytes.
Materials and Methods
Tissue samples
Tissues for the present study included archived formalin-fixed, paraffin-embedded brain without (eight cases) or with (two strains; Turkey ND and Anhinga) spinal cord from experimentally infected male and female 4-week-old White Leghorn specific pathogen free chickens. All of the tissues had been harvested in an identical manner from several different pathogenesis experiments (Brown et al., Citation1999; Susta et al., Citation2011). Every experiment was approved by the Institutional Animal Care and Use Committee. In all cases, birds were infected with approximately 10 5 TCID50 units of virus via eye-drop instillation. Birds were sampled at 2, 5, and 10 days post infection (d.p.i.) or if severely ill. For each time period and strain, two chickens were euthanized and tissues harvested. Birds were euthanized with intravenous injection of pentobarbital and tissues collected into 10% neutral buffered formalin immediately post mortem. After 52 to 54 h in formalin, tissues were processed for histology. Archived paraffin blocks were stored at room temperature. All chickens for all these studies were from the same source flock kept at the Southeast Poultry Research Laboratory. All the tissues in this study were from these experiments. A list of the viruses used, their pathotype and the initial publication for each study is presented in .
Table 1. Viruses in archived tissue used in the present study.
Histopathology
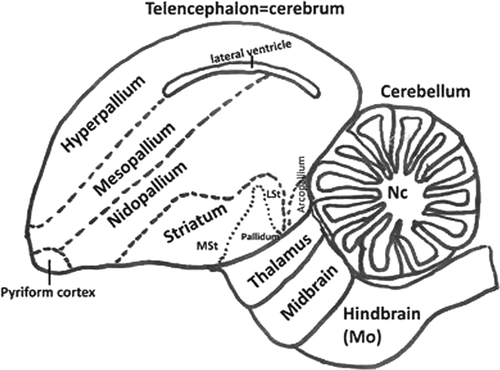
For the present study, brain tissues were examined histologically de novo. All the brains consisted of sagittal sections (taken from the median plane). These sections allowed analysis of the telencephalon (cerebrum), cerebellum, thalamus, midbrain and hindbrain (medulla oblongata). illustrates the location of these brain regions. For those strains having spinal cord available (two strains: Turkey ND and Anhinga), sections were longitudinal (cervical, thoracic and lumbar) from two strains. A list of all lesions seen by light microscopy in these sections was prepared. The lesions observed in the brains and spinal cords were graded according to the lesion severity and character. Specific lesions included: inflammatory cell infiltration, endothelial hypertrophy, vasculitis, increase of the cellularity of the neuropil (microgliosis and astrogliosis), chromatolysis, neuronal necrosis and neuronophagy, axonal degeneration and demyelinization. Each anatomic region of the brain was examined for the presence of these lesions.
Immunohistochemistry
For Newcastle disease virus
Brains from all of the birds—and in addition, for two of the strains, spinal cords—were available, and were processed via immunohistochemistry (IHC) to detect viral nucleoprotein . Unstained sections were subjected to heat-mediated antigen retrieval by microwaving for 20 min at minimum power (approximately 600 W) in unmasking solution (Vector Laboratories, Burlingame, California, USA). Following antigen retrieval, blocking was done with universal blocking reagent (Biogenex, San Ramon, California, USA) as recommended by the manufacturer. The primary antibody, made in rabbits, was an antipeptide, against the nucleoprotein, used at 1:8000 dilution. The slides were incubated with biotinylated goat anti-rabbit antibody and then with avidin–biotin alkaline phosphatase (Vector Laboratories) at room temperature. The reaction was revealed using a naphthol-based dye (Fast Red; DAKO, Carpinteria, California, USA). Negative controls consisted of the brains of age-matched, non-infected chickens.
For T lymphocytes and astrocytes
Selected sections of nervous system showing inflammation were processed via IHC with polyclonal rabbit anti-human CD3 (A 0452-DAKO) and monoclonal mouse anti-glial fibrillar acid protein (GFAP) (MU020-UC; Biogenex).
For CD3, unstained sections were subjected to antigen retrieval by microwaving for 20 min at minimum power in unmasking solution (Vector Laboratories). Following antigen retrieval, blocking was done with universal blocking reagent (Biogenex) and then incubated with the primary anti-CD3 antibody (polyclonal rabbit) at a 1:100 dilution, at 4°C overnight. After incubation, the slides were washed and incubated with an alkaline phosphatase-linked polymer system (Labvision Corporation, Fremont, California, USA) for 30 min at room temperature. As a positive control, the spleen of a specific pathogen free chicken was used. The reaction was revealed using Vector Red (Vector Laboratories).
Selected sections of the brain that had inflammation and increased cellularity in the neuropil were examined by IHC for the presence of GFAP using an automated method (DAKO autostainer). Slides were subjected to antigen retrieval using citrate buffer with pH 6.0 (HK086-9K; Biogenex) followed by heat. Endogenous peroxidase was blocked using 3% hydrogen peroxide (H312-500; Fischer Scientific, Fair Lawn, New Jersey, USA). The slides were incubated with the primary antibody (monoclonal mouse anti-GFAP, MU020-UC; Biogenex) for 60 min at a 1:600 dilution. The secondary antibody used was a biotinylated horse anti-mouse IgG (BA-2001; Vector Laboratories). The detection system used was the labelled streptavidin–biotin streptavidin–horseradish peroxidase (K1016; DAKO) . The reaction was revealed by 3,3’-diaminobenzidine tetrahydrochloride (K3466; DAKO) chromogen to yield a brown staining. The cerebellum of a non-infected specific pathogen free chicken was used as a negative control.
In all procedures, tissue sections were counterstained with Gills II haematoxylin, dehydrated through graded alcohol, cleared in xylene, and cover slipped with Permount.
Results
Clinical disease
The clinical findings have been reported in previous publications for each strain. For purposes of clarity, some are repeated here. As described in previous publications, birds infected with a viscerotropic velogenic strain (CA 1083) were severely depressed at 2 d.p.i. and died or were euthanized when moribund between 4 and 5 d.p.i. (Brown et al., Citation1999). With another viscerotropic velogen, Korea 97-147, birds died between 4 and 5 d.p.i. with mild neurologic signs consisting of head twitch and severe depression (data not published). Birds infected with a third viscerotropic velogenic strain (Australia) demonstrated depression between 3 and 4 d.p.i., and mild neurologic signs including depression and head tremors between 4 and 9 d.p.i. but at 10 d.p.i., they were clinically normal and were euthanized (Susta et al., Citation2011). With the Texas GB strain (neurotropic velogen), birds were bright and alert at 2 d.p.i. but were in lateral recumbency and unable to right themselves by 5 d.p.i. Chickens inoculated with the second velogenic neurotropic strain, Turkey ND, were clinically normal at 5 d.p.i. but by 10 d.p.i. were depressed, with unilateral leg paresis. Birds inoculated with mesogenic (Roakin and Anhinga) and lentogenic (B1 and QV4) isolates demonstrated no signs of clinical disease (Brown et al., Citation1999). However, birds inoculated with another mesogenic strain (Nevada cormorant) had severe neurologic signs (recumbency and paralysis) between 4 and 10 d.p.i. (Susta et al., Citation2011).
Histopathology
Histologic changes were seen in the brains from birds in all strains associated with neurologic signs, which includes the VVND strains (CA 1083, Korea 97-147, Australia) the VNND strains (Turkey ND, Texas GB), and the Nevada cormorant strain (mesogenic). In addition, histologic changes were seen in the brains of birds infected with Anhinga virus (mesogenic strain), even though no overt neurologic signs were recorded for these birds. The brains of birds from chickens infected with Roakin (mesogen) and the two lentogens (B1, QV4) were histologically unremarkable.
For those strains that produced encephalitis and for which there was more than one sampling period in which the encephalitis was present, inevitably the lesion severity was more severe at the later time point. The degree of pathologic changes in the various regions of the brain from the one or two time periods available for each of the strains is presented in . In general, when multiple brain sections were available from one strain at a certain time point, there was good similarity of lesion distribution and severity.
Table 2. Distribution and intensity of histological lesions and stain for virus in different areas of the nervous systema in chickens experimentally infected with different NDV isolates.
In all cases where lesions were observed, it was possible to observe some degree of encephalitis (or encephalomyelitis) and microgliosis. Microglial cells occurred predominantly around vessels or surrounding necrotic neurons. Additional alterations such as vasculitis, vascular proliferation, axonal degeneration, demyelination and astrogliosis were present in some strains. Findings for each strain are described in more detail below.
For the VVND strains (Korea 97-147 and Australia), the inflammatory lesions consisted of one to two layers of lymphocytes in and around vascular walls. Encephalitis was observed in the nuclei cerebellaris, mesopallium, nidopallium, hyperpallium and meninges corresponding to these regions. In these areas there was mild microgliosis. The inflammatory lesions had moderate intensity and multifocal distribution. Chromatolysis and some necrotic neurons were also observed. In birds infected with VVND CA 1083 strain, in the midbrain, nuclei cerebellaris and medulla oblongata there was mild encephalitis, moderate multifocal haemorrhage and oedema around vessels.
For the VNND strains (Turkey ND and Texas GB), inflammatory lesions were initially observed at 5 d.p.i. Inflammatory lesions in general were more pronounced, especially with Turkey ND, and in addition to the perivascular cuffs of lymphocytes there was widespread chromatolysis, axonal degeneration and necrotic neurons. In birds infected with the Texas GB strain, moderate microgliosis was also observed at 10 d.p.i. At this time point, all these changes were most prominent in the striatum and medulla oblongata. In the birds infected with Turkey ND, lesions became progressively more severe at around 10 d.p.i. and were distributed extensively throughout all regions of brain, including telencephalon. The inflammation was characterized as three to four layers of lymphocytes and fewer plasma cells in the walls and around vessels (). In some venules, numerous endothelial cells were hypertrophic, and had large nuclei with vesiculated chromatin protruding into the vascular lumen. Multifocal vascular proliferation was observed in the molecular layer of the cerebellum and medulla oblongata. Multifocal and mild to moderate inflammation was observed in the subarachnoid spaces in the midbrain and cerebellum, and occasionally in the cerebrum. Neuronal necrosis associated with neuronophagia and microgliosis (), occasional mitosis, vacuolization of neuropil and astrocytosis were observed in the grey matter. Axonal degeneration () was present in the white matter and grey matter of the medulla oblongata and spinal cord. In the cerebellar folia (junction between granular and molecular layers) there was multifocal loss of Purkinje cells associated with areas of inflammation and vascular proliferation (). All the lesions had a multifocal to coalescing distribution.
Figure 2. Cerebrum (mesopallium), 4-week-old chicken, experimentally infected with Turkey ND strain. 10 d.p.i. Marked infiltration of lymphocytes and plasma cells in and around the vascular wall. Haematoxylin and eosin. Bar=50 µm.
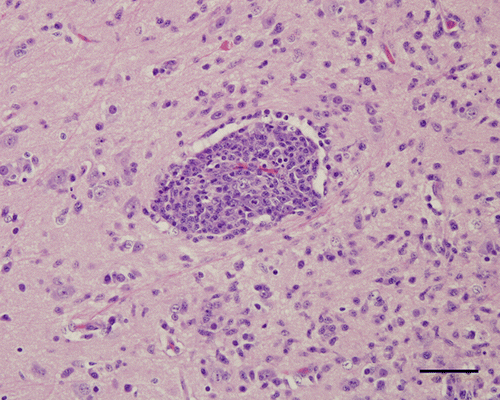
Figure 3. Medulla oblongata, 4-week-old chicken, experimentally infected with Nevada cormorant strain. 9 d.p.i. Necrotic neurons undergoing phagocytosis (arrow) by many microglial cells. Haematoxylin and eosin. Bar=50 µm.
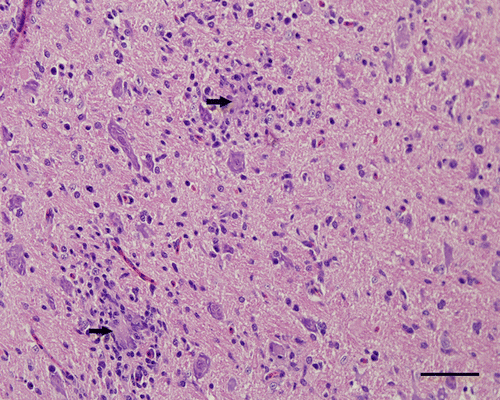
Figure 4. Cerebellum, 4-week-old chicken, experimentally infected with Turkey ND strain. 10 d.p.i. The molecular layer (M) has focal encephalitis and vascular proliferation. Some Purkinje cells in the subjacent Purkinje cell layer (P) are missing. In the granular layer (G), there are no changes. Haematoxylin and eosin. Bar=50 µm.
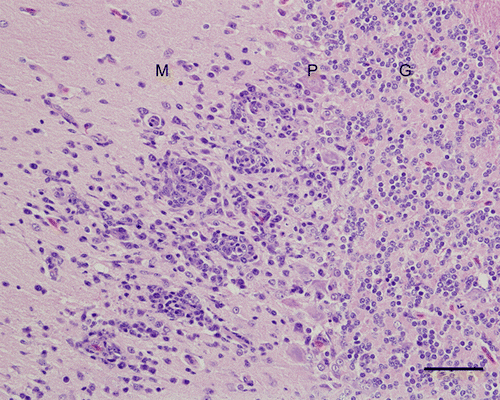
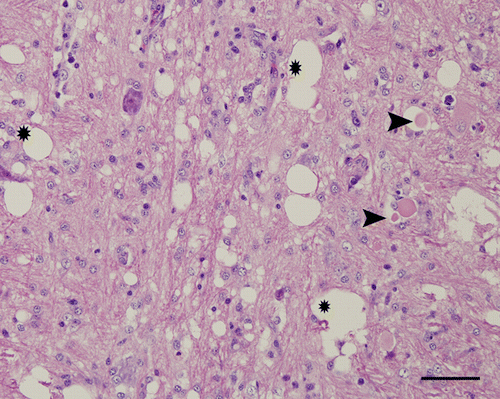
Figure 5. Medulla oblongata, 4-week-old chicken, experimentally infected with Turkey ND strain. Spongy changes (*), axonal spheroids (arrow head) and hypertrophy of the nuclei of astrocytes. Haematoxylin and eosin. Bar=50 µm.
In birds infected with one of the mesogenic strains, Nevada cormorant, less severe lesions were observed at 5 d.p.i., becoming progressively more severe at 6 and 9 d.p.i. The lesions were similar to those described for Turkey ND strain. However, the intensity was less in the cerebrum and there was slightly less neuronal necrosis. The regions affected most intensely were the medulla oblongata, midbrain, thalamus and molecular layer of cerebellum. In these areas, axonal spheroids were present.
For another mesogenic strain (Anhinga) evaluated at 10 d.p.i., the inflammatory lesions were moderate and multifocal, and distributed in the striatum, midbrain, medulla oblongata, molecular layer of cerebellum, nuclei cerebellaris, and grey matter of the spinal cord. The lesions were most intense in the spinal cord. The inflammatory lesions were characterized by one to three layers of lymphocytes and some plasma cells in and around vascular walls. These lesions were associated with mild to moderate microgliosis, axonal degeneration and mild demyelinization (particularly in the medulla oblongata and spinal cord). Lesions were not observed in the brain and spinal cord of the birds at 5 d.p.i.
In chickens infected with the Roakin (mesogen) strain and the two lentogenic strains (B1 and QV4), all of the brains at 2, 5, and 10 d.p.i were examined. There was never any inflammation detected in the nervous tissue.
Immunohistochemistry
For Newcastle disease virus
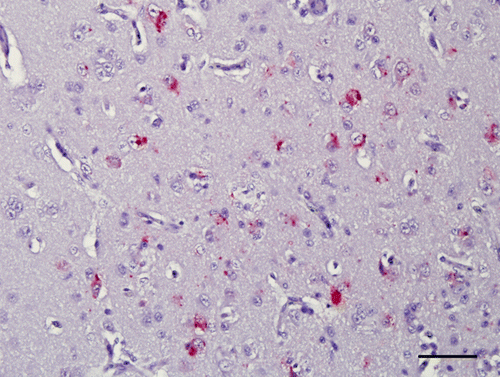
Results are presented in . In general, immunohistochemical signals were seen in multiple regions of the brain. They were always present as multiple granules identifiable in the cytoplasm of neurons, some glial cells and rarely endothelial cells. In general, the viral nucleoprotein signal was more intense and distributed in multifocal areas at 5 d.p.i. In those birds with lesions evaluated around 10 d.p.i, virus was identifiable by IHC in lesser amounts in the brain, often associated with lesions. However, in many cases, there would be no association between degree or location of inflammation and presence of moderate IHC signal (). Viral nucleoprotein was not detected in the brains of chickens infected with the Australia strain (VVND virus). Similarly, virus was not detected in any of the birds infected with strains without encephalitis (Roakin, a mesogen, and B1, QV4, lentogens).
For T lymphocytes and astrocytes
Results are presented in . Many inflammatory cells in the wall and around vessels were positive by IHC-CD3 (T lymphocytes) in the brains of all chickens that presented encephalitis. In the brains with marked inflammation examined at 10 d.p.i. (Turkey ND) and 9 d.p.i. (Nevada cormorant), some CD3-positive cells were also present in the neuropil around vessels. For GFAP, the signal revealed an increased number of astrocytes, as well as increased thickness of the astrocyte processes (astrogliosis), in the parenchyma of the CNS in chickens with encephalitis that survived to 9 or 10 d.p.i. An exception was the chickens infected with the Australia strain (VVND) in which birds were sampled until 10 d.p.i., but still there was no increase in GFAP noted. In chickens infected with Anhinga strain (mesogen), the reaction was minimal. Astrogliosis was strongest in those chickens infected with Turkey ND and Nevada cormorant, and was especially prominent in the medulla oblongata, hyperpallium () and molecular layer of cerebellum. These are also the two strains for which spinal cord was also available, and it was apparent that this reaction extended into this region as well (). The astrocytic reaction was more prominent surrounding areas of intense perivascular cuffs.
Figure 7. Cerebrum (hyperpallium), 4-week-old chicken, experimentally infected with Turkey ND strain. 10 d.p.i. Immunohistochemistry for GFAP. Many GFAP-positive astrocytes can be observed in the areas of inflammation. Bar=50 µm.
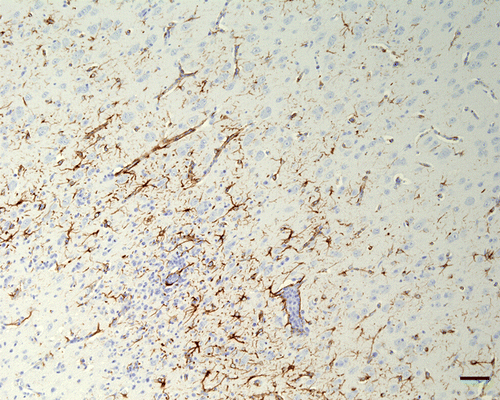
Figure 8. Spinal cord, 4-week-old chicken, experimentally infected with Turkey ND strain. 10 d.p.i. Immunohistochemistry for GFAP. Grey matter and a focal area under meninges with inflammatory changes and a moderate increase of GFAP expression around. Bar=50 µm.
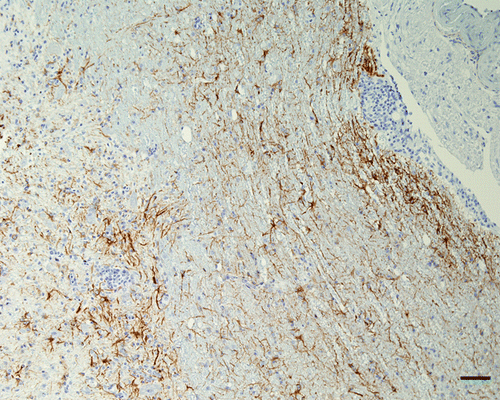
Table 3. Greatest histologic severity lesions, degree of inflammation, signal for CD3 and intensity of signal for GFAP.
Discussion
The present study showed the distribution and extent of neurologic lesions in chickens infected with different strains of NDV. With the lentogens, there was never any evidence of encephalitis or presence of viral nucleoprotein in the brain as detected by IHC. Similarly, with one of the mesogens, Roakin, encephalitis was not a feature and there was no virus present in the brain. For all of the other strains, distinct encephalitic lesions in birds infected with Texas GB, Turkey ND and Nevada cormorant strains became increasingly severe over time . In all these brains, the predominance of lesions was observed especially in the grey matter. The inflammatory lesions were similar in character but differed somewhat in severity in all strains that had encephalitis.
For those two strains that caused death by 5 d.p.i. (CA 1083 and Korea 97-147, both VVND viruses), the inflammation in the brain was mild or moderate, respectively, and multifocal. The amount of virus seen in the brain, as determined by IHC, was mild and multifocal for CA 1083, suggesting that the virus had not replicated extensively in the nervous tissue. For the Korea strain, a moderate to marked amount of viral protein IHC signal indicated a more extensive presence of virus in the brain. Disease progression with VVND virus is rapidly fatal, with birds often succumbing within 4 to 5 d.p.i. (Kommers et al., Citation2002 ; Alexander & Senne, Citation2008). The other VVND strain, Australia, behaved in a very different manner, in that birds survived until 10 d.p.i., which is unusual for a strain in this category. Although these birds had encephalitic lesions similar to the other groups, there was no evidence of viral protein as detected by IHC. The inability to detect virus in the brains of birds infected with the Australia virus could indicate a resolution of the lesions, with disappearance of the antigen, as has been reported previously using fluorescent antibody techniques (Wilczynski, Citation1977). Alternatively, a small amount of virus below the limits of the detection could be generating inflammatory lesions.
For VNND virus (Turkey ND and Texas GB) and a mesogen Nevada cormorant, the amount of virus present in brain was minimal, but lesions were in general more severe. The neurotropic velogenic form is more usually characterized as slower in onset, with birds in general remaining bright and alert but later becoming depressed to variable degrees, with paresis or paralysis most prominent at 5 days or later (Brown et al., Citation1999).
In general, mesogens are not thought to be so virulent, but some strains behave more like the neurotropic strains than the more classical mesogens such as Roakin. In our study, Nevada cormorant and Anhinga, both mesogens, created encephalitis and especially with Nevada cormorant strain, all lesions were remarkably similar to those seen with the VNND strains. There was involvement of all regions of the brain. Mild malacia, axonal degeneration and demyelinization were observed especially in chickens infected with Turkey ND and Nevada cormorant strains. These lesions were found particularly in the medulla oblongata, molecular layer of cerebellum and spinal cord (the latter only available for Turkey ND) and were also observed in other studies. Kuiken et al. (Citation1999) observed axonal degeneration, characterized by swelling and fragmentation of axons in the brachial and lumbosacral plexus of double-crested cormorants (infected naturally with APMV-1/cormorant/Saskatchewan-Canada/2035/95). Nakamura et al. (Citation2008) detected NDV by immunohistochemistry in the peripheral nerves in chickens infected experimentally with VVND virus (APMV1/chicken/Japan/Fukuoka-11/2004).
The main inciting factor for neurologic problems appears to be viral infection of neurons with associated neuronal necrosis. Other investigators have reported on neuronal damage by NDV (Stevens et al., Citation1976; Wilczyinski et al. 1977). In the present study we detected viral nucleoprotein within neuronal cytoplasm immunohistochemically, as have other authors (Kuiken et al., Citation1999; Kommers et al., Citation2001 ; Nakamura et al., Citation2008), suggesting association between virus and neuronal damage. However, viral replication might not be the only mechanism of neuroparenchymal damage. Lesions characterized by endothelial hypertrophy, and mild to moderate proliferative vasculitis, were observed in chickens evaluated at 9 and 10 d.p.i. in our study, especially in the molecular layer of cerebellum. It may be that some of the damage is due to vascular compromise, in addition to the direct neuronal necrosis caused by the virus.
The principal protagonists of CNS inflammation are microglia, blood-borne macrophages and astrocytes (Leme & Chadi, Citation2001). Astrocytes become activated, undergoing remarkable changes in cell shape and functionality in response to diverse CNS injuries such as trauma, infectious diseases or chemical insults (Stichel & Müller, Citation1998; Leme & Chadi, Citation2001; Pozner et al. Citation2008 ). These cells proliferate and increase in size, and also produce a dense network of interdigitated processes (astrogliosis) that surround the lesion, and fill the space vacated by the dead or dying cells (Stichel & Müller, Citation1998; Leme & Chadi, Citation2001). Response of microglial cells occurs within the CNS parenchyma associated with injured neurons. These neurons produce chemokines that attract the microglial cells to the site of the lesion (Streit, Citation2000).
Microglial infiltration and astrocyte activation characterized the reaction observed in many areas of the parenchyma (especially grey matter) of the brain and spinal cord in this study. In those birds that died or were euthanized at the later time points (10 d.p.i.), numerous GFAP-positive astrocytes and processes were detected, especially around vessels with inflammatory changes. Reactive astrogliosis is a feature of many viral encephalitides in which viral infection of astrocytes is probably the prime stimulus. This reaction is expected in axonal degeneration and neuronal necrosis. After injury, proliferation of both cells and processes occurs. Astrocytes are the cells primarily responsible for repair and scar formation in the brain (Maxie & Youssef, Citation2007). Astrogliosis was also observed in the molecular layer of the cerebellum (Bergmann-glia). Ajtai & Kálmán (Citation1998) reported marked immunopositivity for GFAP around the lesion site 1 week after traumatic injury to the cerebellum of chickens. Our results indicated that avian Bergmann-glia also express GFAP in viral injury.
In summary, although the strains typically classified as “neurotropic” ND are known to cause neurologic impairment and damage in the CNS, it seems that all but the least virulent strains of NDV are capable of invading the CNS and causing pathologic impairment. The underlying mechanism in all cases seems to be infection of neurons leading to neuronal necrosis, and subsequent inflammatory response. The primary component of the inflammatory reaction is T lymphocytes, and astrogliosis increases with the time of the lesion. In addition, there may be some vascular compromise that can enhance the lesion extent.
Acknowledgements
R.E. was supported by the Brazilian government sponsoring agency Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Universidade Federal de Minas Gerais (UFMG). The present work was funded by USDA CRIS project number 6612-32000-049-00D. Mention of trade names or commercial products in this manuscript is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the US Department of Agriculture.
References
- Ajtai , B.M. and Kálmán , M. 1998 . Glial fibrillary acidic protein expression but no glial demarcation follows the lesion in the molecular layer of cerebellum . Brain Research , 802 : 285 – 288 .
- Alexander , D.J. & Senne , D.A. 2008 Newcastle disease . In Y.M. Saif , A.M. Fadly , J.R. Glisson , L.R. McDougald , L.K. Nolan & D.E. Swayne , Diseases of Poultry , 12th edn 75 115 Ames, , Iowa : Blackwell Publishing .
- Bhaiyat , M.I. , Kobayashi , Y. , Itakura , C. , Islam , M.A. and Kida , H. 1995 . Brain lesions in chickens experimentally infected with a neuroadapted strain of mesogenic Newcastle disease virus . Journal of Veterinary Medical Science , 57 : 237 – 244 .
- Brown , C. , King , D.J. and Seal , B.S. 1999 . Pathogenesis of Newcastle disease in chickens experimentally infected with viruses of different virulence . Veterinary Pathology , 36 : 125 – 132 .
- Farkas , T. , Székely , É. , Belák , S. and Kiss , I. 2009 . Real-time PCR-based pathotyping of Newcastle disease virus by use of TaqMan minor groove binder probes . Journal Clinical Microbiology , 47 : 2114 – 2123 .
- Kommers , G.D. , King , D.J. , Seal , B.S. & Brown , C.C. 2001 . Virulence of pigeon-origin Newcastle disease virus isolates for domestic chickens . Avian Diseases , 45 , 906 921
- Kommers , G.D. , King , D.J. , Seal , B.S. , Carmichael , K.P. & Brown , C.C. 2002 . Pathogenesis of six pigeon-origin of Newcastle disease virus for domestic chickens . Veterinary Pathology , 39 , 353 362
- Kuiken , T. , Wobeser , G. , Leighton , F.A. , Haines , D.M. , Chelack , B. Bogdan , J. 1999 . Pathology of Newcastle disease in double-crested cormorants from Saskatchewan, with comparison of diagnostic methods . Journal Wildlife Diseases , 35 : 8 – 23 .
- Lee , Y.J. , Sung , H.W. , Choi , J.G. , Kim , J.H. and Song , C.S. 2004 . Molecular epidemiology of Newcastle disease viruses isolated in South Korea using sequencing of the fusion protein cleavage site region and phylogenetic relationships . Avian Pathology , 33 : 482 – 491 .
- Leme , R.J. and Chadi , G. 2001 . Distant microglial and astroglial activation secondary to experimental spinal cord lesion . Arquivos de Neuro-psiquiatria , 59 : 483 – 492 .
- Maxie , M.G. & Youssef , S. 2007 Nervous system . In M.G. Maxie , Jubb, Kennedy, and Palmer's Pathology of Domestic Animals Vol. 1, , 5th edn 281 457 Toronto : Elsevier
- Nakamura , K. , Ohtsu , N. , Nakamura , T. , Yamamoto , Y. , Yamada , M. , Mase , M. and Imai , K. 2008 . Pathologic and immunohistochemical studies of Newcastle disease (ND) in broiler chickens vaccinated with ND: severe nonpurulent encephalitis and necrotizing pancreatitis . Veterinary Pathology , 45 : 928 – 933 .
- Office International des Epizootes . 2008 Manual of Diagnostic Tests and Vaccines for Terrestrial Animals . Paris : World Organization for Animal Health .
- Phillips , R.J. , Samson , A.C.R. & Emmerson , P.T. 1998 . Nucleotide sequence of the 5′-terminus of Newcastle disease virus and assembly of the complete genomic sequence: agreement with the “rule of six” . Archives of Virology Journal , 143 , 1993 2002 .
- Pozner , R.G. , Collado , S. , de Giusti , C.J. , Ure , A.E. , Biedma , M.E. Romanowski , V. 2008 . Astrocyte response to Junin virus infection . Neuroscience Letters , 445 : 31 – 35 .
- Reiner , A. , Perkel , D.J. , Mello , C.V. and Jarvis , E.D. 2004 . Songbirds and the revised avian brain nomenclature . Annals of the New York Academy of Sciences , 1016 : 77 – 108 .
- Stevens , J.G. , Nakamura , R.M. , Cook , M.L. and Wilczynski , S.P. 1976 . Newcastle disease as a model for paramyxovirus-induced neurological syndromes: pathogenesis of the respiratory disease and preliminary characterization of the ensuing encephalitis . Infection and Immunity , 13 : 590 – 599 .
- Stichel , C.C. and Müller , H.W. 1998 . The CNS lesion scar: new vistas on an old regeneration barrier . Cell and Tissue Research , 294 : 1 – 9 .
- Streit , W.J. 2000 . Microglial response to brain injury: a brief synopsis . Toxicologic Pathology , 28 : 28 – 30 .
- Susta , L. , Miller , P.J. , Afonso , C.L. , Brown , C.C. 2011 Clinicopathological characterization in poultry of three strains of Newcastle disease virus isolated from recent outbreaks . Veterinary Pathology 48
- Wilczynski , S.P. , Cook , M.L. and Stevens , J.G. 1977 . Newcastle disease as a model for paramyxovirus-induced neurologic syndromes. II. Detailed characterization of the encephalitis . American Journal of Pathology , 89 : 649 – 666 .
- Wise , M.G. , Sellers , H.S. , Alvarez , R. and Seal , B.S. 2004 . RNA-dependent RNA polymerase gene analysis of worldwide Newcastle disease virus isolates representing different virulence types and their phylogenetic relationship with other members of the paramyxoviridae . Virus Research , 104 : 71 – 80 .