Abstract
The first evidence of avian nephritis virus (ANV) in ducks is described. A diagnostic investigation was performed on three duck farms in Croatia. Samples from dead-in-shell ducklings and ducklings aged 30 days were collected and prepared for molecular and histopathological examination. Intestinal and liver samples were tested by polymerase chain reaction (PCR) for the presence of ANV, duck enteritis virus, duck hepatitis virus 1 and Derzsy's disease virus. Multiple tissues were collected for histological examination and lesions were found to be confined to the kidney and intestine. Moderate focal interstitial and periglomerular mononuclear cell infiltrates (mostly lymphocytes and plasma cells) were detected in the kidney. The duodenum showed rather diffuse pericryptal mononuclear cell hyperplasia (lymphocytes) and fibroplasia. ANV was detected by PCR in all the intestinal samples, while no other viruses were found. Sequence comparisons of the portion of the open reading frame 1b encoding the RNA-dependent RNA polymerase gene confirmed that the virus detected and sequenced from ducklings shared high nucleotide and amino acid identities with ANV-1. Additional work is required to determine the clinicopathological significance of ANV infection in ducks.
Introduction
Astroviruses are small (27 to 30 nm) non-enveloped positive-sense, single-stranded RNA viruses with characteristic star-shaped morphology (Cook & Myint, Citation1995; Koci et al., Citation2000). They were first reported in 1975 in the stools of children and since then they have been described in some mammalian species (cattle, sheep, cat, pigs, dogs, mice, deer, minks and bats) as a cause of gastroenteritis (Appleton & Higgins, Citation1975; Wilcocks et al., Citation1992; Tang et al., Citation2005; Chu et al., Citation2008). Besides affecting mostly young mammals, astroviruses became a common finding in poultry after they were first reported in 1980 in turkey faeces (McNulty et al., Citation1980; Koci & Schultz-Cherry, Citation2002). Avian astroviruses are linked to poult enteritis and mortality syndrome (PEMS) in turkeys, runting and stunting syndrome (RSS) in chickens, fatal hepatitis in ducklings and enteritis in guinea fowl (Gough et al., Citation1984; Baxendale & Mebatsion, Citation2004; Catolli et al., Citation2005; Pantin-Jackwood et al., Citation2006; Spackman et al., Citation2010). They are classified as turkey astrovirus, chicken astrovirus, avian nephritis virus (ANV) and duck astrovirus (DAstV) (Wilcocks et al., 1992; Koci & Schultz-Cherry, Citation2002; Baxendale & Mebatsion, Citation2004; Fu et al., Citation2009).
ANV was first isolated from chickens in 1976 and, based on electron microscopy (EM), was initially thought to be a picornavirus (Yamaguchi et al., Citation1979). ANV is the first astrovirus that was not identified by the morphological characteristics (Imada et al., Citation2000). In 2000 the viral genome was completely sequenced and the virus was reclassified as a new member of the family Astroviridae (Imada et al., Citation2000). This virus is associated with diarrhoea, retarded growth, tubulonephrosis, interstitial nephritis, uricosis and death of primarily young chicks (Imada et al., Citation1979; Shirai et al., Citation1991). ANV is known to be one of the aetiological agents of RSS (Goodwin et al., Citation1993; Songserm et al., Citation2000; Pantin-Jackwood et al., Citation2006) and the susceptibility to ANV increases if the chickens are immunosuppressed (Narita et al., Citation1990a).
It was first believed that ANV infects only chickens, but then antibodies against ANV were detected in turkey flocks including specific pathogen-free flocks in some European countries and Japan (Connor et al., Citation1987; McNulty et al., Citation1989; Imada et al., Citation1990; Decaesstecker & Meulemans, Citation1991). Pantin-Jackwood et al. (Citation2006) identified and sequenced ANV for the first time from turkeys affected with PEMS; however, the role of ANV has not yet been clarified.
The ANV genome includes 6927 nucleotides and a polyA tail and has three open reading frames (ORFs) (Imada et al., Citation2000). ANV is genetically and antigenically distinct from other known astroviruses, but turkey astrovirus-1 seems to be most closely related to ANV (Thouvenelle et al., Citation1995; Imada et al., Citation2000). Chicken astrovirus also shows some sequence identity with ANV; however, the isolates of this virus differ antigenically and serologically from ANV (Baxendale & Mebatsion, Citation2004). There are at least two reported serotypes of ANV (ANV-1, ANV-2) (Shirai et al., Citation1991; Pantin-Jackwood et al., Citation2006), and field strains of ANV show different degrees of pathogenicity in chickens varying from subclinical infection to death (Frazier et al., Citation1990; Shirai et al., Citation1991).
Astroviral infection in ducks is caused by DAstV, historically known as duck hepatitis virus type 2 (DHV-2) (Asplin, Citation1965; Gough et al., Citation1984; Koci et al., Citation2000). DAstV is associated with fatal hepatitis in ducklings, which was first described in the UK in 1965 (Asplin, Citation1965). DHV-2 was later characterized as an astrovirus by morphology and renamed DAstV-1 (Monroe et al., Citation2005). DAstV genome possesses typical astrovirus organization and it was completely sequenced by Fu et al. (Citation2009). Duck hepatitis virus type 3 (DHV-3) was previously known as a picornavirus, but is newly identified as an astrovirus (Wang et al., Citation2008; Todd et al., Citation2009).
The aim of the present study is to report and describe the first evidence of ANV detected in ducklings in Croatia. Furthermore, this paper shows comparison of the ANV polymerase gene (ORF 1b) from our isolates with some published ANV sequences.
Materials and Methods
Sample origin and collection
Diagnostic investigation was performed on three duck farms in northern Croatia. Carcasses of ducklings aged 30 days were collected from one farm, while dead-in-shell ducklings were gathered from two other farms. Mortality of ducks from the first farm was slightly increased but without any observed signs. On the second and third farms, death of embryos at the final stage of development was stightly increased. All gathered carcasses were dissected and the organ samples were taken for the purpose of molecular diagnostic testing. Samples from ducklings aged 30 days were also taken for histopathological examination.
Sample preparation
Liver and intestines from 12 30-day-old ducklings from the first farm were collected and stored separately at –70°C. Prior to RNA extraction, samples were pooled; one sample corresponded to a pool of about five organ samples per farm. Samples of liver and intestines of 32 and 30 dead-in-shell ducklings from the second and third farms, respectively, were also collected. Liver and intestine samples were pooled separately; one sample corresponded to a pool of five to 10 organ samples. Altogether, six pooled samples (three from liver and three from intestines) were examined from the first farm, and 12 from the each of the two other farms.
The organ samples (300 µl) were collected in 1.5-ml sterile tubes. DNA and RNA were extracted using High PureViral Nucleic Acid Kit (Roche Diagnostics, Mannheim, Germany) according to the manufacturer's instructions. Prior to extraction, intestinal suspensions were prepared by cutting slices from the ileo-caecal junction, and homogenizing in sterile phosphate-buffered saline; one part of the content was mixed with 10 parts of sterile phosphate-buffered saline (140 mM NaCl, 2.7 mM KCl, 8.0 mM Na2HPO4, 1.5 mM KH2PO4), frozen and thawed three times, and centrifuged for 10 min at 3000 x g. Reverse transcription procedures were implemented using 5 µl RNA in a 50-µl reaction volume containing 20 U RNaseH-M-MLV reverse transcriptase (SuperScript™ III reverse transcriptase; Invitrogen, Carlsbad, California, USA), 5 pmol random hexamer primer, 0.5 mM dNTPs, 10 mM dithiothreitol, 50 mM Tris–HCl, 75 mM KCl and 3 mM MgCl2. Reverse transcription was carried out in the GenAmp PCR System 2400 (Applied Biosystems, Foster City, California, USA) and the mixture was incubated at 50°C for 45 min followed by 72°C for 10 min. The cDNA obtained was then used for amplification in polymerase chain reaction (PCR) assays.
Histopathology
For histopathological analysis, the tissue samples (liver, spleen, lungs, kidney, heart, duodenum, trachea) were fixed in neutral 10% formalin solution, embedded in paraffin and cut into 4-µm-thick sections on a rotary microtome (MICROM HM 325; Zeiss, Austria). After deparaffinization, the sections were stained with haematoxylin and eosin. The slices were examined under the light microscope (LEICA DMLB, Germany) and images were captured with the digital camera PIXERA Pro 150ES.
Primers, PCR and sequencing
The primers for ANV (forward primer: 5′-CTT CTT TGG TGG ACT GGA TAA G-3′, and reverse primer: 5′-CCT TCT TGA CAT GAG TTA CCT C-3′) were designed to amplify a portion of the ORF 1b from nucleotides 3729 to 3980, numbering from Imada et al. (Citation2000). Primers were constructed by aligning published polymerase gene sequences of ANV strains (GenBank accession numbers AB033998 and DQ324832). The expected amplicon length was 255 bp. The PCR reaction (total volume 50 µl) contained 5 µl cDNA, 25 µl JumpStart™ REDTaq®ReadyMix™ (Sigma-Aldrich, Steinheim, Germany) and 0.20 µM each primer. Thermal cycling parameters were: initial denaturation at 94°C for 2 min, then 35 cycles of: denaturation at 94°C for 25 sec, annealing at 53°C for 25 sec, and extension at 72°C for 30 sec, followed by the final extension at 72°C for 5 min. As a positive control sample, we used our previous ANV isolate from chicken (unpublished data). The reaction products were analysed by 2% agarose gel electrophoresis and stained with ethidium bromide.
The PCR products that showed expected amplicon lengths were considered positive and purified by QIAquick purification kit (Qiagen, Hilden, Germany). Sequencing was performed in both directions by Macrogen Inc. (Seoul, Korea).
Samples were also tested for the presence of duck enteritis virus (DEV), duck hepatitis virus type 1 (DHV-1) and parvovirus (DDV). The primers for DEV were designed by Plummer et al. (Citation1998) to amplify a 421 bp fragment of the UL-6 gene. The PCR reaction and primers for DHV-1 were described by Wang et al. (Citation2008). The primers used for detection of Derzsy's disease virus (DDV) were designed by Wozniakowski et al. (Citation2009) to amplify a 1604 bp fragment of the VP3 protein-encoding region of goose and Muscovy duck parvoviruses.
Multiple alignment and phylogenetic analysis
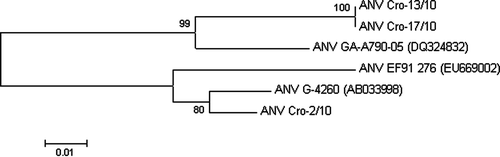
The sequence data were initially aligned to known sequences using the basic BLAST search program. Sequence identities of nucleotides, as well as those of amino acids, were analysed using the ClustalX implemented in Mega4 software (Tamura et al., Citation2007). The same tool was used to perform neighbour-joining analysis with 1000 bootstrap replicates, based on the p-distance. Names and accession numbers of the ANV strains used in the study are can be seen in .
Sequence data
The nucleotide sequences obtained from our ANV strains ANV Cro-2/10, ANV Cro-13/10 and ANV Cro-17/10 were deposited in GenBank with accession numbers HM755445, HM755446 and HM755447, respectively. Accession number HM755445 is associated with the sample from the 30-day-old ducklings; accession numbers HM755446 and HM755447 were associated with the dead-in-shell ducklings.
Results
Virus detection and phylogenetic analysis
All tested pooled intestinal samples from ducklings aged 30 days and dead-in-shell ducklings from two different farms showed positive ANV PCR assay results and were sequenced. All samples were negative for the presence of DEV, DHV-1 and DDV.
The determined nucleotide sequences of the reverse-transcribed part of the ORF 1b region were analysed. All sequences from the same farm were identical, and for that reason only one sequence from each farm was submitted to GenBank and was further analysed. The initial BLAST search revealed significant similarity with three ANV isolates: GA-A790-05 (DQ324832), EF91 276 (EU669002) and G4260 (AB033998). Our sequences ANV Cro-13/10 and ANV Cro-17/10 were 100% identical, showing greatest similarity with isolate ANV GA-A790-05 (DQ324832) (93.5%). These isolates also had four unique nucleotide changes compared with other three aligned ANVs. Sequence ANV Cro-2/10 showed only 85.7% similarity with the other two Croatian isolates, and it was most similar to ANV G-4260 (AB033998) (97.4%). It also had one unique nucleotide change. None of the nucleotide changes in our ANVs affected the amino acid sequence. The amino acid sequence similarity between strains used in the present study was higher than nucleotide sequence similarity (between 88 and 100%).
A neighbour-joining tree based on alignments of 155 nucleotide sequences (corresponding to positions 3828 to 3982 of the G4260 ANV-1 virus) of six analysed ANVs showed that ANV Cro-2/10 was grouped together with G-4260 (AB033998) and EF91-276 (EU669002), separately from other two Croatian viruses that were clustered with ANV GA-A790-05 (DQ324832) ().
Histopathology
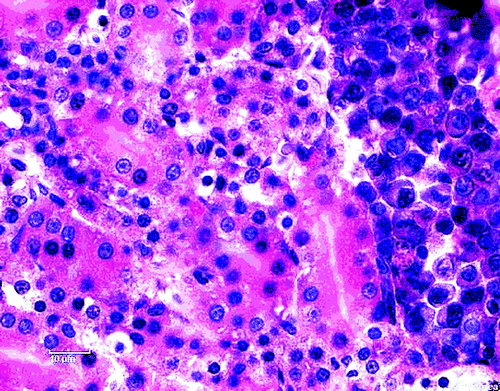
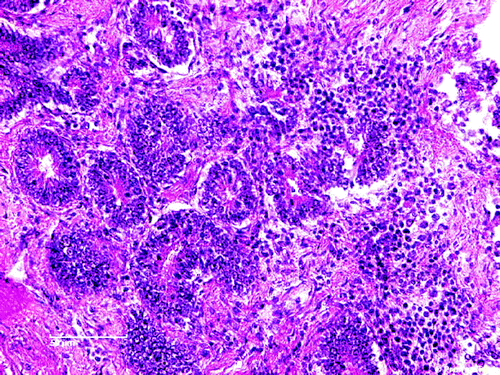
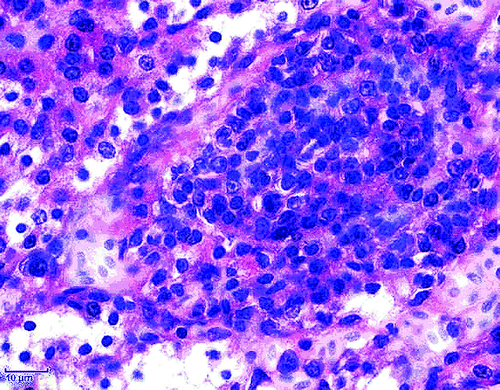
Moderate multifocal periglomerular and interstitial mononuclear cell infiltrates were the most prominent histological lesions in the kidneys. The predominant cells were the lymphocytes (), lymphocytes and plasma cells () and plasma cells (). A rather diffuse pericryptal mononuclear cell (lymphocytes) hyperplasia and fibroplasia were found in the duodenum ().
Figure 2. Focal mononuclear cell hyperplasia (lymphocytes) in the kidney. H & E stain (scale bar= 10 µm).
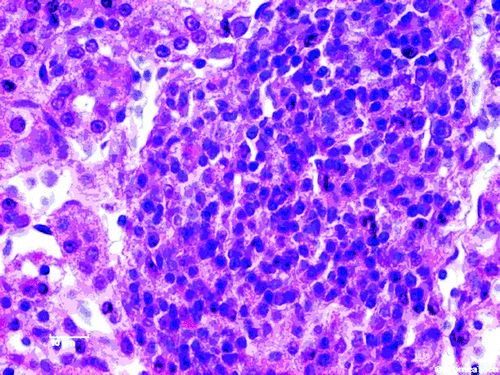
Figure 3. Focal mononuclear cell hyperplasia (lymphocytes and plasma cells) in the kidney. H & E stain (scale bar=10 µm).
Discussion
The present study describes a diagnostic investigation performed on three duck farms in Croatia. Ducklings aged 30 days and dead-in-shell ducklings were tested for the presence of ANV but also for DEV, DHV-1 and DDV. All of these viruses (DEV, DHV-1 and DDV) with the exception of ANV have been known as causative agents of diseases in ducks (Plummer et al., Citation1998; Wang et al., Citation2008; Wozniakowski et al., Citation2009). However, based on partial ORF 1b sequence comparisons, we confirmed the presence of ANV-1 in organ samples of ducklings aged 30 days and dead-in-shell ducklings. ANV was the only virus detected from these ducklings, and PCR reactions for other viruses showed negative results.
One-day-old chicks are thought to be the most susceptible to ANV infection (Imada & Kawamura, Citation2003), although detection of ANV in older chicks and turkey poults is also a common finding (Pantin-Jackwood et al., Citation2006, Citation2007). In addition to the fatal hepatitis caused by DAstV, and infection with DHV-3, no other astroviruses have been described in ducks.
Histopathological examination of the organs from ducklings aged 30 days gave indications for involvement of ANV in this case. Moderate multifocal periglomerular and interstitial mononuclear cell infiltrates (lymphocytes and plasma cells; to 4, respectively) were the most prominent histological lesions in the kidneys. Rather diffuse pericryptal lymphocyte hyperplasia and fibroplasia were found in the duodenum (). In addition to the degeneration and the presence of inclusion bodies in some of degenerated epithelial tubular cells as well as the moderate interstitial infiltration by lymphocytes (Meulemans, Citation2008; Mándoki et al., Citation2006), focal mononuclear cellular hyperplasia has also been identified in later stage of infection with ANV (Narita et al., Citation1990b; Imada & Kawamura, Citation2003; Mándoki et al., Citation2006). Imada & Kawamura (Citation2003) found lymphoid follicles 14 to 28 days post infection in chickens experimentally infected with ANV. With reference to to the examination conducted in this report, ANV infection in ducks showed lesions similar to those described in chickens (Imada & Kawamura, Citation2003; Mándoki et al., Citation2006).
These histopathological findings gave us directions for the confirmation of ANV presence in the intestine samples by molecular methods. Our sequence comparisons of the portion of the ORF 1b encoding the RNA-dependent RNA polymerase (RdRp) gene confirmed that the virus detected and sequenced from ducklings shared high nucleotide and amino acid identities with ANV-1, and it is phylogenetically grouped with these viruses (). This finding indicates that ANV infects ducks as well as chickens and turkeys. In addition to DAstV and DHV-3, ANV is now the third identified virus from the family Astroviridae that infects ducks.
Although all three farms involved in this research share a common breeder and are located in northern Croatia, nucleotide differences between strains isolated from ducklings aged 30 days (ANV Cro-2/10) and dead-in-shell ducklings (ANV Cro-13/10 and ANV Cro-17/10) were detected. While sequences ANV Cro-13/10 and ANV Cro-17/10 are 100% identical, the sequence ANV Cro-2/10 showed only 85.7% similarity with ANV strains detected in embryos. We can presume that two types of ANV circulate among ducks on the tested farms and/or one type of ANV is more prevalent in embryos than in older ducks. Because this is the first detection of ANV infection in ducklings in Croatia, this thesis needs further testing and investigation.
The finding of ANV in ducklings dead in shell indicates naturally occurring vertical infection. Furthermore, we should consider and investigate the possibility that ANV may play a role in the death of duckling embrgos.
In chickens and turkeys, ANV is often found accompanied by other common enteroviruses (Pantin-Jackwood et al., Citation2006) related to RSS and PEMS. ANV infection in ducks could also be associated with other viruses, but in our research we did not find any (DEV, DHV-1, DDV).
Although ANV is associated with retarded growth, uricosis and death of mostly young chickens (Imada et al., Citation1979; Shirai et al., Citation1991), its complete role in the RSS and PEMS is not totally understood (Pantin-Jackwood et al., Citation2006). Obviously, ANV acts as a pathogenic agent in ducks, but the severity and significance of ANV infection in this avian species need to be clarified.
The finding of ANV in ducklings opens a new view on the spreading of this virus among domestic poultry and a questions about its role and pathogenesis in ducks.
Acknowledgements
The present research was supported by grants 053-0531863-1856 and 048-0481186-1183 from the Ministry of Science, Education and Sports, Republic of Croatia.
References
- Appleton , H. and Higgins , H.G. 1975 . Viruses and gastroenteritis in infants . The Lancet , 2 : 124
- Asplin , F D. 1965 . Duck hepatitis: vaccination against two serological types . The Veterinary Record , 77 : 487 – 488 .
- Baxendale , W. and Mebatsion , T. 2004 . The isolation and characterisation of astroviruses from chickens . Avian Pathology , 33 : 364 – 370 .
- Catolli , G. , Toffan , A. , De Battisti , C. , Salviato , A. , Terregino , C. and Capua , I. 2005 . Astroviruses found in the intestinal contents of guinea fowl suffering from enteritis . The Veterinary Record , 156 : 220
- Chu , D.K.W. , Poon , L.L.M. , Guan , Y. and Peiris , J.S.M. 2008 . Novel astroviruses in insectivorous bats . Journal of Virology , 82 : 9107 – 9114 .
- Connor , T.J. , McNeilly , F. , McFerran , J.B. and McNulty , M.S. 1987 . A survey of avian sera from Northern Ireland for antibody to avian nephritis virus . Avian Pathology , 16 : 15 – 20 .
- Cook , N. and Myint , S. 1995 . Astroviruses . Journal of Medical Microbiology , 42 : 1 – 2 .
- Decaesstecker , M. and Meulemans , G. 1991 . An ELISA for the detection of antibodies to avian nephritis virus and related entero-like viruses . Avian Pathology , 20 : 523 – 530 .
- Frazier , J.A. , Howes , K. , Reece , R.L , Kidd , A.W. and Cavanagh , D. 1990 . Isolation of noncytopathic viruses implicated in the aetiology of nephritis and baby chick nephropathy and serologically related to avian nephritis virus . Avian Pathology , 19 : 139 – 160 .
- Fu , Y. , Pan , M. , Wang , X. , Xu , Y. , Xie , X. Knowles , N.J. 2009 . Complete sequence of a duck astrovirus associated with fatal hepatitis in ducklings . Journal of General Virology , 90 : 1104 – 1108 .
- Goodwin , M.A. , Davis , J.F. , McNulty , M.S. , Brown , J. and Player , E.C. 1993 . Enteritis (so-called runting stunting syndrome) in Georgia broiler chicks . Avian Diseases , 37 : 451 – 458 .
- Gough , R.E. , Collins , M.S. , Borland , E. and Keymer , L.F. 1984 . Astrovirus-like particles associated with hepatitis in ducklings . The Veterinary Record , 114 : 279
- Imada , T. and Kawamura , H. 2003 . “ Avian nephritis ” . In Diseases of Poultry , 11th edn , Edited by: Saif , Y.M. , Barnes , H.J. , Glisson , J.R. , Fadly , A.M. , McDougald , L.R. and Swayne , D.E. 379 – 465 . Ames : Iowa State Press .
- Imada , T. , Yamaguchi , S. and Kawamura , K. 1979 . Pathogenicity for baby chicks of the G-4260 strain of picornavirus “avian nephritis virus” . Avian Diseases , 23 : 582 – 588 .
- Imada , T. , Yamaguchi , S. , Mase , M. , Tsukamoto , K. , Kubo , M. and Moorooka , A. 2000 . Avian nephritis virus (ANV) as a new member of the family astroviridae and construction of of infectious ANV cDNA . Journal of Virology , 74 : 8487 – 8493 .
- Imada , T. , Yamaguchi , S. , Miura , N. and Kawamura , H. 1990 . Antibody survey against avian nephritis virus among chickens in Japan . National Institute of Animal Health Quarterly , 20 : 79 – 80 .
- Koci , M.D. and Schultz-Cherry , S. 2002 . Avian astroviruses . Avian Pathology , 31 : 213 – 227 .
- Koci , M.D. , Seal , B.S. and Schultz-Cherry , S. 2000 . Molecular characterization of an avian astrovirus . Journal of Virology , 74 : 6173 – 6177 .
- Mándoki , M. , Bakonyi , T. , Ivanics , E. , Nemes , C. , Dobos-Kovács , M. and Rusvai , M. 2006 . Phylogenetic diversity of avian nephritis virus in Hungarian chicken flocks . Avian Pathology , 35 : 224 – 229 .
- McNulty , M.S. , Curran , W.L. and McFerran , J.B. 1980 . Detection of astroviruses in turkey faeces by direct electron mycroscopy . The Veterinary Record , 106 : 561
- McNulty , M.S. , Connor , T.J. and McNeilly , F. 1989 . A survey of Specific pathogen-free chicken flocks for antibodies to chicken anesmia agent, avian nephritis virus and group A rotavirus . Avian Pathology , 18 : 215 – 220 .
- Meulemans , G. 2008 . Avian nephritis viral infections . The Merck Veterinary Manual . Whitehouse Station, NI, , USA : Merck & Co, Inc . Available online at http://www.merckvetmanual.com/mvm/index.jsp?cfile=htm/bc/201800.htm (accessed 15 June 2010) .
- Monroe , S.S. , Carter , M.J. , Herrmann , J. , Mitchell , D.K. & Sanchez-Fauquier , A. 2005 . Astroviridae . In C.M. Fauquet , A. Mayo , J. Maniloff , U. Desselberger & L.A. Ball VIIIth Virus Taxonomy. Report of the International Committee on Taxonomy of Viruses 859 864 . London : Elsevier Academic Press .
- Narita , M. , Kawamura , H. , Furuta , K. , Shirai , J. & Nakamura , K. 1990a . Effects of cyclophosphamide in newly hatched chickens after inoculation with avian nephritis virus . American Journal of Veterinary Research 51 , 1623 1628 .
- Narita , M. , Kawamura , H. , Nakamura , K. , Shirai , J. , Furuta , K. & Abe , F. 1990b . An immunohistological study on the nephritis in chicks experimentally produced with avian nephritis virus . Avian Pathology , 19 , 497 509 .
- Pantin-Jackwood , M.J. , Spackman , E. and Woolcock , P.R. 2006 . Molecular characterization and typing of chicken and turkey astroviruses circulating in the United States: implication for diagnostics . Avian Diseases , 50 : 397 – 404 .
- Pantin-Jackwood , M.J. , Spackman , E. , Day , J.M. and Rivers , D. 2007 . Periodic monitoring of commercial turkeys for enteric viruses indicates continuous presence of astrovirus and rotavirus on the farms . Avian Diseases , 51 : 674 – 680 .
- Plummer , P.J. , Alefantis , T. , Kaplan , S. , O'Connell , P. , Shawky , S. and Schat , K.A. 1998 . Detection of duck enteritis virus by polymerase chain reaction . Avian Diseases , 42 : 554 – 564 .
- Shirai , J. , Nakamura , K. , Shinohara , K. and Kawamura , H. 1991 . Pathogenicity and antigenicity of avian nephritis isolates . Avian Diseases , 35 : 49 – 54 .
- Songserm , T. , Pol , J.M. , Van Roozerlaar , D. , Kok , G.L. , Wagenaar , F. and Ter Hurne , A.A. 2000 . A comparative study of the pathogenesis of malabsorption syndrome in broilers . Avian Diseases , 44 : 556 – 567 .
- Spackman , E. , Day , J.M. and Pantin-Jackwood , M.J. 2010 . Astrovirus, reovirus, and rotavirus concomitent infection causes decreased weight gain in broad-breasted white poults . Avian Diseases , 54 : 16 – 21 .
- Tamura , K. , Dudley , J. , Nei , M. and Kumar , S. 2007 . MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0 . Molecular Biology and Evolution , 24 : 1596 – 1599 .
- Tang , Y. , Murgia , M.V. and Saif , Y.M. 2005 . Molecular characterisation of the capsid gene of two serotypes of turkey astroviruses . Avian Diseases , 49 : 514 – 519 .
- Thouvenelle , M.L. , Haynes , J.S. and Reynolds , D.L. 1995 . Astrovirus infection in hatchling turkeys: histologic, morphometric, and ultrastructural findings . Avian Diseases , 39 : 328 – 336 .
- Todd , D. , Smyth , V.J. , Ball , N.W. , Donnelly , B.M , Wylie , M. , Knowles , N.J. and Adair , B.M. 2009 . Identification of chicken enterovirus-like viruses, duck hepatitis virus type 2 and duck hepatitis virus type 3 as astroviruses . Avian Pathology , 38 : 21 – 29 .
- Wang , L. , Pan , M. , Fu , Y. and Zhang , D. 2008 . Classification of duck hepatitis virus into three genotypes based on molecular evolutionary analysis . Virus Genes , 37 : 52 – 59 .
- Wilcocks , M.M. , Carter M.J. & Madeley , C.R. 1992 . Astroviruses . Reviews in Medical Virology , 2 , 97 106 .
- Wozniakowski , G. , Kozdrun , W. , Samorek-Salamanowicz , E. and Krol , K. 2009 . Touchdown PCR for the detection of waterfowl parvoviruses . Bulletin of the Veterinary Institute in Pulawy , 53 : 3 – 7 .
- Yamaguchi , S. , Imada , T. and Kawamura , H. 1979 . Characterisation of a picornavirus isolated from broiler chicks . Avian Diseases , 23 : 571 – 581 .