Abstract
The major enteric disease (ED) complex in broiler chickens is runting–stunting syndrome and in turkey broilers is poult enteritis mortality syndrome. Viruses from numerous families have been identified in the intestinal tracts of poultry with ED, such as Astroviridae, Coronaviridae, Reoviridae, Rotaviridae, and Parvoviridae. The objective of the present study was to directly demonstrate the presence of the scarcely known chicken parvovirus (ChPV) and turkey parvovirus (TuPV) in Hungarian flocks experiencing clinical signs of ED. ChPV and TuPV infection were demonstrated in 15 chicken flocks and two turkey flocks, in intestinal samples collected between 2008 and 2010. The histopathological investigation revealed enteritis in the duodenum and jejunum, and atrophy of the lymphoid organs. Indirect immunohistochemistry (IHC) suggested the intestinal epithelium of chickens and turkeys as a potential replication site of the virus, similarly to other parvoviruses, while in case of the turkey samples IHC positivity was also observed in the bursa of Fabricius, liver and pancreas. However, no direct connection could be established between the presence of the pathogen in the above-mentioned tissues and the histopathological changes observed in the investigated flocks. The phylogenetic analysis performed on the partial nucleic acid sequence of the NS1 gene revealed an evident clustering tendency of the ChPV and TuPV strains, but also highlighted the potential reciprocal role of these two species in the epidemiology of these viruses. The role of the ChPV and TuPV in the ED is far from understood, but the results of the present study emphasize the fact that in certain, still not fully elucidated conditions, ChPV and TuPV may participate in the emergence of ED in chicken flocks, as suggested by previous experimental infections.
Introduction
Despite the intense research regarding viral enteric disease (ED), a major disease complex still seriously threatening the poultry industry, the causative viral pathogens are not accurately identified, and its pathogenesis is not fully understood. The viral ED complex is a serious economic problem in the poultry industry. In broiler chickens the major enteric disease complex is known as malabsorption or runting–stunting syndrome, and in turkey broilers as poult enteritis mortality syndrome (Page et al., Citation1982; Goodwin et al., Citation1993; Barnes et al., Citation2000; Barnes & Guy, Citation2003; Palade et al., Citation2008). The viral ED is characterized by diarrhoea, depression, ingestion of litter, increased vocalization and huddling. Morbidity and mortality are variable, and the economic impact is primarily due to poor production, failure of affected birds to grow, as well as increased costs of therapy, and poor feed conversion efficiency; but in the severe forms, immune dysfunction and increased mortality have been reported (Day & Zsak, Citation2010). Viruses from numerous families have been identified in the intestinal tracts of poultry with ED: Astroviridae, Coronaviridae, Reoviridae, Rotaviridae (Pass et al., Citation1982; Reynolds et al., Citation1987a, Citationb; Goodwin et al., Citation1993; Guy, Citation1998; Koci et al., Citation2000; Yu et al., Citation2000; Spackman et al., Citation2005; Pantin-Jackwood et al., Citation2007a, Citationb, Citation2008b; Day et al., Citation2007a, Citationb; Jones, Citation2008; Reynolds & Shultz-Cherry, Citation2008), and Parvoviridae (Kisary et al., Citation1984, 1987; Woolcock & Shivaprasad, Citation2008; Zsak et al., Citation2008, Citation2009; Day & Zsak, Citation2010). The role of these viruses in the ED is not fully understood (Zsak et al., Citation2008), but is supported by the syndrome reproducibility with preparations from the intestinal contents of affected birds, which do not contain bacteria or protozoa (Barnes & Guy, Citation2003). The incriminated viruses have been directly demonstrated in both healthy and diseased flocks, suggesting that a certain combination of pathogens and/or factors can lead to the ED (Pantin-Jackwood et al., Citation2008a; Zsak et al., Citation2009). In the 1980s the potential involvement of parvoviruses in the ED was suspected (Trampel et al., Citation1983; Kisary et al., Citation1984). Kisary and his co-workers demonstrated by electron microscopy the presence of parvovirus-like particles in intestinal homogenates of 10-day-old chickens with ED (Kisary et al., Citation1984), and by inoculating 1-day-old chickens with the purified viral particles they obtained the characteristic clinical signs of runting–stunting syndrome (Kisary, Citation1985a). The following molecular biological studies revealed that the virus belongs to the Parvoviridae family (Kisary et al., Citation1985). The full genome of the newly involved virus was recently determined and analysed (Day & Zsak, Citation2010). Parvoviruses have a linear single-stranded DNA, between 4 and 6 kilobase pairs; they are non-enveloped, icosahedral virions of approximately 20 nm in diameter (Tattersall, Citation2006). They encode two major genes: a non-structural gene (NS1), which appears to be conserved within parvoviruses, and is used as a target for polymerase chain reaction (PCR)-based diagnosis; and a structural viral protein (VP1) gene (Cotmore & Tattersall, Citation2006). An enzyme-linked immunosorbent assay-based test was also developed to demonstrate the presence of maternally derived parvovirus-specific antibodies in chicken serum samples and virus-specific antibodies in chicken sera following infection (Strother & Zsak, Citation2009). Positive nuclear staining was obtained by indirect immunoperoxidase staining in the epithelial cells of the small intestine of chickens experimentally infected with the designated ABU strain (Kisary et al. Citation1984; Kisary, Citation2001). Immunofluorescence staining was suggested as a diagnostic tool for parvoviral infection in broiler chicken (Kisary, Citation1985b). A recent survey revealed the presence of parvovirus in chicken and turkey samples from eight different states in the USA. PCR-positive samples were identified in both chicken and turkey enteric samples, between 5 days and 8 weeks of age; however, there was no definite correlation between virus presence and disease (Zsak et al., Citation2009). The phylogenetic analyses comparing NS1 gene segments revealed a strong similarity between the chicken and turkey parvoviruses from the USA, and that the chicken and turkey parvovirus isolates formed distinct phylogenetic groups (Zsak et al., Citation2009).
The objective of the present study was to directly demonstrate the presence of the scarcely known chicken parvovirus (ChPV) and turkey parvovirus (TuPV) in Hungarian flocks experiencing clinical signs of ED. Phylogenetic analysis of the virus strains detected was performed in order to characterize them and to determine the relationships between ChPV and TuPV.
Materials and Methods
Sample collection, histopathology, bacteriology and electron microscopy
The cases considered in the present study were collected between January 2008 and March 2010, from flocks experiencing signs of ED that were directly demonstrated to be positive for ChPV or TuPV. Carcasses of 6-day-old to 3-week-old birds from 17 Hungarian broiler flocks (15 chicken and two turkey), from all over Hungary, experiencing increased mortality, were sent to the Department of Pathology and Forensic Veterinary Medicine (Szent Isván University, Faculty of Veterinary Science, Budapest, Hungary) for diagnostic purposes. Tissue samples from various organs (intestine, pancreas, bursa of Fabricius, liver, spleen and thymus) were stored in 8% neutral buffered formaldehyde solution for histological examination, processed, sectioned and stained with haematoxylin and eosin (Stevens, Citation2007). Aseptically collected fresh liver samples were inoculated into blood agar plates. Cultures were incubated at 37°C and examined after 24 h for microbial growth. Intestinal content samples were suspended 1:3 in distilled water, cleared by low-speed centrifugation, followed by 20 min at 9000 x g. The samples were prepared according to the single-droplet negative staining technique (Harris, Citation2007) and examined on a transmission electron microscope (JEOL, Japan).
To obtain a better insight into the epidemiology and importance of ChPV and TuPV, carcasses from 15 flocks (13 chicken and two turkey), free of ED clinical signs, received for routine assessment of the flock's status were included in this study. These samples were only tested by PCR for the direct demonstration of enteric viruses ().
Table 1. Details of flocks included in the present study and PCR results for viral pathogens known to be involved in ED.
Immunohistochemistry
Paraffin-embedded sections were initially dewaxed in xylene and graded ethanol. After treatment with appropriate antigen retrieval (Target Retrieval Solution, pH 6.0; DAKO, Glostrup, Denmark; 30 min in 880 W microwave oven), the sections were treated with 3% peroxide for 10 min and incubated with unlabelled primary antibody against ChPV (chicken polyclonal antibody 1:500 dilution) for 12 h at 4°C. The primary antibody was obtained on specific pathogen free chickens (Spafas, line 22; Charles River, Ltd, UK), following oral infection at 1 day of age and subcutaneously at 21 days, with the ABU strain. Intestinal samples were collected at 1 day of age, before the oral infection, and at scarification (28 days) were tested by PCR for the viral pathogens known to be involved in ED: avian nephritis virus, avian astrovirus, chicken astrovirus, avian reovirus, avian rotavirus, and avian adenovirus group I, according to previously described protocols (); they were found negative at both times. The designated ABU strain was isolated from chickens in Hungary in 1984 (Kisary et al., Citation1984), and was purified by caesium chloride density gradient centrifugation prior to inoculation of the chickens (Kisary, Citation1985a). Antigen-bound primary antibody was detected using goat anti-chicken IgG (Fc) horseradish-peroxidase conjugate (Alpha Diagnostic Intel. Inc.). 3,3′-Diaminobenzidine tetrahydrochloride was added to the slides for 30 min at room temperature. Sections were counterstained with Mayer's haematoxylin for 10 sec. Positive and negative control intestinal tissue samples were included in each run.
PCR and phylogeny
Fresh pooled intestine samples (5 to 10/flock) were homogenized in 2 ml sterile phosphate-buffered saline. The viral DNA was purified from the supernatants and amplifications were performed according to a previously described protocol (Zsak et al., Citation2009) using a PCR Sprint Thermal Cycler SPRT001 (Hybaid, UK). One primer pair was used in this study, for both diagnosis and phylogeny, designed to amplify a 561 base pair product from the NS1 gene (Zsak et al., Citation2009). Positive (ABU strain) and negative (distilled water) controls were added to each run. Following electrophoresis, the amplicons were cut out from the gel and DNA was extracted with the QiaQuick Gel Extraction Kit (Qiagen, Germany). Fluorescence-based direct sequencings were performed in both directions on the amplicons at Biogon Kft (Budapest, Hungary) employing an ABI 3100 genetic analyser (Applied Biosystems, USA). Nucleotide sequences were identified by BLAST (http://www.ncbi.nlm.nih.gov/BLAST/) search, against GenBank databases. Nucleotide and deduced amino acid sequences were compiled and aligned using the Align Plus 4 software (Scientific & Educational Software, USA). A phylogenetic tree of the nucleotide sequences was established using sequence data of the Hungarian strains (15 ChPV and two TuPV), six strains retrieved from the GenBank, and 20 strains kindly provided by L. Zsak (Zsak et al., Citation2009). Blocks of sequence data leading to 524 nucleotides for ChPV and 527 nucleotides for TuPV were used for the analysis. The phylogenetic tree was constructed by neighbour-joining with a two-parameter distance matrix using the Phylip program. Goose parvovirus strain HG5 was used as the outgroup. Possible recombination events were investigated using the Recombination Detection Program (http://darwin.uvigo.es/rdp/rdp.html; Martin & Rybicki, Citation2000). All pooled intestinal samples were also tested for viral pathogens known to be involved in ED, according to previously described protocols ().
Accession numbers
The Hungarian ChPV and TuPV sequences from the present study were uploaded in the GenBank with the following accession numbers: GQ281291 to GQ281294, GQ281296, and HM208282 to HM208293.
Sequences retrieved from the GenBank were EU304808, EU304809, GU214704, GU214705, GU214706, GQ260159 and AY506546 (goose parvovirus).
Codes for the American origin sequences (no accession numbers available) were Ch367GA05, Ch798MO05, Ch 843AR05, Ch369AR05, Ch804AR05, Ch916CA06, Ch449GA05, Ch812DE05, Ch736CA05, Ch741AR05, Tu294CA04, Tu982MN07, Tu549NC05, Tu1030MN07, Tu41NC03, Tu971MN07, Tu1056MN07, Tu260CA04, Tu974MN07 and Tu1079NC07.
Results
Autopsy, histopathology, electron microscopy and immunohistochemistry
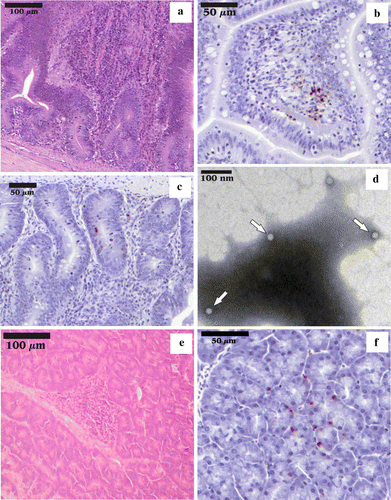
According to the history submitted by the owners and/or treating veterinarians along with the carcasses, the broilers from all the flocks with clinical signs of ED, chickens and turkeys, presented slightly higher than normal daily mortality, stunted growth, and diarrhoea. At necropsy, segments of the small intestine were partially filled with fluid-mucoid content, and a large amount of gas. Dilatation of the intestinal and mesenteric blood vessels and the dark-reddish discolouration of the mucous membrane were present in the duodenum and jejunum, and atrophy of the immune organs (bursa of Fabricius, thymus, spleen) was observed. In the case of all flocks with clinical signs of ED, the histological examination revealed moderate to severe distension of the intestinal crypts and acute catarrhal enteritis with a mixed inflammatory cell population (with an evident lymphohistiocytic dominance) in the jejunum (a) and duodenum, with a low incidence of enterocyte desquamation, but without any signs of villus collapse or atrophy. Nodular lymphohistiocytic pancreatitis (e) was also observed. Routine aerobe bacteriological investigation was negative for all the flocks included in the present study. No correlation was observed between age, intensity of the clinical signs and pathological findings. The electron microscopy examination of the intestinal contents revealed the presence of a large number of icosahedral, non-enveloped viral particles, measuring about 22 to 25 nm in diameter (d) that, based on their ultrastructural morphology, were identified as members of the Parvoviridae family. Positive nuclear staining was detected by indirect immunohistochemistry (IHC) in the epithelial cells and inflammatory cells from the lamina propria of the duodenum and jejunum (b,c) in the case of all samples, chicken and turkey alike, in all examined age groups. In the case of the turkey samples, a positive reaction was also observed in the follicles of the bursa of Fabricius and liver (data not shown), and exocrine pancreas (f). The results summarized above are representative for the changes observed in all 17 flocks presenting clinical signs of ED.
Figure 1. 1a: Enteritis in the jejunum with distension of the crypts, increased number of mononuclear leukocytes in the lamina propria and a few granulocytes; haematoxylin and eosin. 1b: Indirect IHC, counterstained with Mayer's haematoxylin, positive nuclear staining in the inflammatory cells from the lamina propria of jejunum. 1c: Indirect IHC, counterstained with Mayer's haematoxylin, positive nuclear staining in the epithelial cells of the duodenal crypts. 1d: Transmission electron micrograph showing viral particles with size and morphology typical for the Parvoviridae family (arrows). 1e: Nodular pancreatitis with lymphocytes and histiocytes; haematoxylin and eosin. 1f: Indirect IHC, counterstained with Mayer's haematoxylin, positive staining in the exocrine pancreas.
PCR and phylogeny
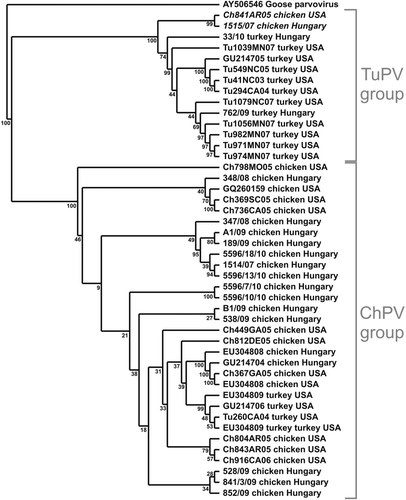
In the case of the samples collected from flocks with clinical signs of ED, the PCR for CHPV and TuPV resulted in the successful amplification of 561 base pair products from all 17 samples. The turkey flocks, as well as eight chicken flocks, were free from any other enteric viral infection as determined by PCR (). In the case of the samples collected from clinically healthy flocks, only two chicken flocks were found positive for ChPV, while six were positive for avian nephritis virus and five for avian reovirus. No positivity was found for haemorrhagic enteritis virus or avian adenovirus group I. The alignment of the nucleic acid sequences of the NS1 gene revealed that the level of identity between the Hungarian strains varied from 89 to 100%, while the overall level of identity between all of the strains used for the phylogenetic analysis varied from 88 to 100%. The phylogenetic tree constructed based on the nucleotide sequence of the analysed NS1 gene segment revealed an evident clustering of the virus strains of different species origin (), as previously reported (Zsak et al., Citation2009). Two ChPV strains (1515/07 from Hungary and Ch841AR05 from the USA) proved to be more closely related to TuPV strains than to ChPV strains (). The analysis of the deduced amino acid sequence of the ChPV strains resulted in sequences 174 amino acids long, and 175 amino acids in the case of the TuPV strains. The same analysis revealed that the two separately clustered ChPV strains presented unique sequence at four amino acid sites: 524, 616, 617 and 651 (positions according to the full length of the NS1 protein of the reference ABU strain [GU214704]). The Recombination Detection Program-based investigation of the analysed gene segment did not reveal any recombination events between the examined strains (data not shown).
Figure 2. Phylogenetic relationship of the investigated strains based on the nucleotide sequence of the examined region of the Hungarian ChPV and TuPV strains from the present study, the sequences retrieved from the GenBank (accession numbers indicated for each strain) and the American-origin sequences (no accession numbers available). Goose parvovirus strain HG5 was used as the outgroup.
Discussion
Our investigations revealed the presence of the scarcely known ChPV/TuPV in 17 Hungarian flocks experiencing ED. The wide distribution of ChPV/TuPV in American commercial flocks was recently reported (Zsak et al., Citation2009); however, there was no definite correlation between virus presence and disease. The mortality in the affected Hungarian flocks was assessed by the owners and/or treating veterinarians to be just above the accepted level, observations consistent with previous reports in cases of ED (Barnes et al., Citation2000; Barnes & Guy, Citation2003).The common pathological finding was the predominantly lymphohistiocytic enteritis in the duodenum and jejunum with dilatation of the crypts without villus atrophy, as usually found in reovirus and rotavirus intestinal viral infection, considered to be widespread in chicken flocks, especially in the first 2 weeks of life (Pantin-Jackwood et al., Citation2008a; Day et al., Citation2007b). IHC-positive nuclear staining was obtained consistently in the epithelial and inflammatory cells of the duodenum and jejunum. However, despite the concomitant presence of the positive IHC staining and the inflammatory reaction, the performed investigations do not allow for any conclusions to be drawn regarding the exact cause of the inflammatory reaction. The positive IHC reaction only indicates enterocytes of the small intestine and the local inflammatory cells as possible multiplication sites for the parvovirus. To our knowledge there is no study in the literature regarding the multiplication site for ChPV. Positive nuclear staining was obtained at IHC in the intestinal epithelium of broiler chickens, after experimental infection with the reference ABU strain (Kisary, Citation2001), but no data are available regarding the natural infection. The positive IHC nuclear staining in the intestinal epithelial cells confirms the intestinal epithelium as a multiplication site for ChPV, as described for most parvoviruses (Hueffer & Parrish, Citation2003). Furthermore, in the two turkey flocks and in six chicken flocks no other viral pathogens were found as determined by PCR (). However, despite the negative PCR results for all other pathogens from , the presence of these viruses cannot be completely ruled out, as classic PCR is a diagnostic technique having its limitations, such as the lack of ability to demonstrate the presence of all circulating strains; therefore, due to their high variability, some strains could remain undetectable, as previously demonstrated (Smyth et al., Citation2009). Multiplex nodular pancreatitis was also observed in all cases; however, positive nuclear staining at IHC for the pancreas (along with the liver and bursa of Fabricius) was observed only in turkeys. Currently there are no data in the literature to explain these findings in the case of TuPV; however, analogies can be made with immunosuppressive parvoviruses, where lymphoid tissue is a known site for virus multiplication (Hueffer & Parrish, Citation2003; Parrish & Hueffer, Citation2003; Bloom & Kerr, Citation2006).
The nucleic acid sequence and phylogenetic analysis of the investigated NS1 gene segment revealed two major clusters: a ChPV group and a TuPV group; this finding is consistent with the characteristics of parvoviruses of other species (e.g. canine/feline parvoviruses). Two ChPV strains (italicized in ) were more closely related to the TuPV group; furthermore, they presented unique sequences at four deduced amino acid sites. This finding could be relevant, as parvoviruses are typically small viruses of approximately 5000 nucleotides long, and minor mutations resulting in only a few key amino acid changes can lead to drastic changes in their infective behaviour (Truyen et al., Citation1995; Parrish & Hueffer, Citation2003). Therefore the possibility that ChPV and TuPV could have evolved from a common ancestor cannot be ruled out. Still the probability of a recombinant virus should not be excluded, as these two strains seem to form a clearly distinctive cluster, but they could have also evolved separately from other ChPVs. On the other hand, in spite of the observed genetic diversity of the analysed strains, due to a number of reasons (including confidentiality issues), no reliable data were available for the production performance (e.g. feed conversion, production index, etc.). Thus, no objective, scientifically substantiated conclusions can be drawn regarding the variation in pathogenicity of the Hungarian ChPV and TuPV strains.
The chicken and turkey samples analysed in the present study were collected from geographically isolated regions of Hungary. However, samples 5596/7/10, 5596/10/10, 5596/13/10 and 5596/18/10 were collected at the same time and originated from a single breeder with different age-group flocks; still, only two of them were 100% identical in the examined region. There are two possible explanations for this finding: either this particular flock became infected with three different strains at the same time; or, in a short period of time, dramatic changes occurred within the viral strains, which is an unlikely situation for parvoviruses. The presented case was not unique, as samples 347/08 and 348/08 also originated from one breeder, and were collected at the same time.
The ChPV and TuPV are relatively newly identified viruses, and the full genome was determined only recently (Day & Zsak, Citation2010). There are few data in the literature regarding the definite correlation between virus presence and disease. One study was made to determine the prevalence of the virus in healthy American broiler flocks (Zsak et al., Citation2008); however, there are no reports currently available directly connecting the natural infection with clinical symptoms and/or specific enteric pathological changes. The role of the ChPV and TuPV in ED is far from understood, but the results of the present study indicate the fact that these viruses are more frequently present in chicken flocks experiencing ED than in flocks free from ED (), suggesting a potential role in the pathogenesis of ED. They have also revealed that the situation is similar in the case of turkey flocks experiencing poult enteritis mortality syndrome, and that the virus strains circulating in Hungary are genetically similar to ChPV and TuPV strains demonstrated in the USA. However, as most of the currently used diagnostic techniques have their specific limitations, and a considerable genetic diversity among the circulating virus strains can be observed in the case of all currently known pathogens, all conclusions regarding the potential implication and role of these viruses in the pathogenesis of ED should be carefully interpreted and confirmed by extensive investigations of naturally occurring ED/poult enteritis mortality syndrome and meticulously planned experimental infections.
Acknowledgements
The authors express their gratitude to Laszlo Zsak for providing the 20 nucleic acid sequences of American origin used in this study. The present work was financially supported by CBA Commercial Ltd, Hungary, the food business network.
References
- Barnes , H.J. and Guy , J.S. 2003 . “ Poult enteritis-mortality syndrome ” . In Diseases of Poultry , 11th edn , Edited by: Saif , Y.M. , Barnes , H.J. , Glisson , J.R. , Fadly , A.M. , McDougald , L.R. and Swayne , D.E. 1171 – 1180 . Ames : Iowa State Press .
- Barnes , H.J. , Guy , J.S. and Vaillancourt , J.P. 2000 . Poult enteritis complex . Scientific and Technical Review , 19 : 565 – 588 .
- Bloom , M.E. and Kerr , J.R. 2006 . “ Pathogenesis of parvovirus infection ” . In Parvoviruses , Edited by: Kerr , J.R. , Cotmore , S.F. , Bloom , M.E. , Linden , R.M. and Parish , C.R. 323 – 341 . London : Hodder Arnold .
- Cotmore , S.F. and Tattersall , P. 2006 . “ Structure and organization of the viral genome ” . In Parvoviruses , Edited by: Kerr , J.R. , Cotmore , S.F. , Bloom , M.E. , Linden , R.M. and Parish , C.R. 73 – 94 . London : Hodder Arnold .
- Day , J.M. and Zsak , L. 2010 . Determination and analysis of the full-length chicken parvovirus genome . Virology , 399 : 59 – 64 .
- Day , J.M. , Pantin-Jackwood , M.J. and Spackman , E. 2007a . Sequence and phylogenetic analysis of the S1 genome segment of turkey-origin reoviruses . Virus Genes , 35 : 235 – 242 .
- Day , J.M. , Spackman , E. and Pantin-Jackwood , M.J. 2007b . A multiplex RT-PCR test for the differential identification of turkey astrovirus type 1, turkey astrovirus type 2, chicken astrovirus, avian nephritis virus, and avian rotavirus . Avian Diseases , 51 : 681 – 684 .
- Goodwin , M.A. , Davis , J.F. , McNulty , M.S. , Brown , J. and Player , E.C. 1993 . Enteritis (so-called runting stunting syndrome) in Georgia broiler chicks . Avian Diseases , 37 : 451 – 458 .
- Guy , J.S. 1998 . Virus infections of the gastrointestinal tract of poultry . Poultry Science , 77 : 1166 – 1175 .
- Harris , J.R. 2007 . “ Negative staining of thinly spread biological samples ” . In Electron Microscopy Methods and Protocols , 2nd edn , Edited by: Kuo , J. 116 – 119 . Totowa : Humana Press .
- Hess , M. , Raue , R. and Hafez , H.M. 1999 . PCR for specific detection of haemorrhagic enteritis of turkeys, an avian adenovirus . Journal of Virological Methods , 81 : 199 – 203 .
- Hueffer , K. and Parrish , C.R. 2003 . Parvovirus host range, cell tropism and evolution . Current Opinion in Microbiology , 6 : 392 – 398 .
- Jones , R.C. 2008 . “ Other reovirus infections ” . In Diseases of Poultry , 12th edn , Edited by: Saif , Y.M. , Fadly , A.M. , Glisson , J.R. , McDougald , L.R. , Nolan , L.K. and Swayne , D.E. 322 – 328 . Ames, IA : Blackwell Publishing .
- Kisary , J. 1985a . Experimental infection of chicken embryos and day-old chickens with parvovirus of chicken origin . Avian Pathology , 14 : 1 – 7 .
- Kisary , J. 1985b . Indirect immunofluorescence as a diagnostic tool for parvovirus infection of broiler chickens . Avian Pathology , 14 : 269 – 273 .
- Kisary , J. 2001 . Detection of chicken parvovirus antigen by immunoperoxidase staining . Magyar Állatorvosok Lapja 123 , 518 521 . (In Hungarian with English abstract)
- Kisary , J. , Nagy , B. and Bitay , Z. 1984 . Presence of parvoviruses in the intestine of chickens showing stunting syndrome . Avian Pathology , 13 : 339 – 343 .
- Kisary , J. , Avalosse , B. , Miller-Faures , A. and Rommelaere , J. 1985 . The genome structure of a new chicken virus identifies it as a parvovirus . Journal of General Virology , 66 : 2259 – 2263 .
- Kisary , J. , Miller-Faures , A. and Rommelaere , J. 1987 . Presence of fowl parvovirus in fibroblast cell culture prepared from uninoculated White Leghorn chicken embryos . Avian Pathology , 16 : 115 – 121 .
- Koci , M.D. , Seal , B.S. and Schultz-Cherry , S. 2000 . Molecular characterization of an avian astrovirus . Journal of Virology , 7 : 6173 – 6177 .
- Mándoki , M. , Bakonyi , T. , Ivanics , É. , Nemes , Cs. , Dobos-Kovács ., M. & Rusvai , M. 2006 . Phylogenetic diversity of avian nephritis virus in Hungarian chicken flocks . Avian Pathology 35 , 224 229 .
- Martin , D. and Rybicki , E. 2000 . RDP: detection of recombination amongst aligned sequences . Bioinformatics , 16 : 562 – 563 .
- Page , R.K. , Fletcher , O.J. , Rowland , G.N. , Gaudry , D. and Villegas , P. 1982 . Malabsorption syndrome in broiler chickens . Avian Diseases , 26 : 618 – 624 .
- Palade , E.A. , Demeter , Z. , Dobos-Kovács , M. , Rusvai , M. & Mándoki , M. 2008 . Detection of infectious bronchitis virus, avian nephritis virus and infectious bursal disease virus by multiplex RT-PCR based diagnostic method . Magyar Állatorvosok Lapja 130 , 559 564 . (In Hungarian with English abstract)
- Pantin-Jackwood , M.J. , Spackman , E. and Day , J.M. 2007a . Pathology and virus tissue distribution of turkey origin reoviruses in experimentally infected turkey poults . Veterinary Pathology , 44 : 185 – 195 .
- Pantin-Jackwood , M.J. , Spackman , E. , Day , J.M. and Rives , D. 2007b . Periodic monitoring of commercial turkeys for enteric viruses indicates continuous presence of astrovirus and rotavirus on the farms . Avian Diseases , 51 : 674 – 680 .
- Pantin-Jackwood , M.J. , Day , J.M. , Jackwood , M.W. and Spackman , E. 2008a . Enteric viruses detected by molecular methods in commercial chicken and turkey flocks in the United States between 2005 and 2006 . Avian Diseases , 52 : 235 – 244 .
- Pantin-Jackwood , M.J. , Spackman , E. and Day , J.M. 2008b . Pathogenesis of type 2 turkey astroviruses with variant capsid genes in 2-day-old specific pathogen free poults . Avian Pathology , 37 : 193 – 201 .
- Parrish , C.R. and Hueffer , K. 2003 . “ Parvovirus host range, cell tropism and evolution—studies of canine and feline parvoviruses, minute virus of mice, porcine parvovirus, and Aleutian mink disease virus ” . In Parvoviruses , Edited by: Kerr , J.R. , Cotmore , S.F. , Bloom , M.E. , Linden , R.M. and Parrish , C.R. 343 – 351 . London : Edward Arnold .
- Pass , D.A. , Robertson , M.D. and Wilcox , G.E. 1982 . Runting syndrome in broiler chickens in Australia . Veterinary Record , 110 : 386 – 387 .
- Reynolds , D.L. and Schultz-Cherry , S.L. 2008 . “ Astrovirus Infections ” . In Diseases of Poultry , 12th edn , Edited by: Saif , Y.M. , Fadly , A.M. , Glisson , J.R. , McDougald , L.R. , Nolan , L.K. and Swayne , D.E. 351 – 356 . Ames, IA : Blackwell Publishing .
- Reynolds , D.L. , Saif , Y.M. and Theil , K.W. 1987a . A survey of enteric viruses of turkey poults . Avian Diseases , 31 : 89 – 98 .
- Reynolds , D.L. , Saif , Y.M. and Theil , K.W. 1987b . Enteric viral infections of turkey poults: incidence of infection . Avian Diseases , 31 : 272 – 276 .
- Smyth , V.J. , Jewhurst , H.L. , Adair , B.M. and Todd , D. 2009 . Detection of chicken astroviruses by reverse transcriptase-polymerase chain reaction . Avian Pathology , 38 : 293 – 299 .
- Spackman , E. , Kapczynski , D. and Sellers , H. 2005 . Multiplex real-time reverse transcription-polymerase chain reaction for the detection of three viruses associated with poult enteritis complex: turkey astrovirus, turkey coronavirus, and turkey reovirus . Avian Diseases , 49 : 86 – 91 .
- Stevens , A. 2007 . “ The haematoxylins ” . In Theory and Practice of Histological Techniques , 6th edn , Edited by: Bancroft , J.D. and Stevens , A. 107 – 118 . Edinburgh : Churchill Livingstone .
- Strother , K.O. and Zsak , L. 2009 . Development of an enzyme-linked immunosorbent assay to detect chicken parvovirus-specific antibodies . Avian Diseases , 53 : 585 – 591 .
- Suresh , M. , & Sharma , M. 1996 . Pathogenesis of type II avian adenovirus infection in turkeys: in vivo immune cell tropism and tissue distribution of the virus . Journal of Virology 70 , 30 36 .
- Tang , Y. , Ismail , M.M. and Saif , Y.M. 2005 . Development of antigen-capture enzyme-linked immunosorbent assay and RT-PCR for detection of turkey astroviruses . Avian Diseases , 49 : 182 – 188 .
- Tattersall , P. 2006 . “ The evolution of parvovirus taxonomy ” . In Parvoviruses , Edited by: Kerr , J.R. , Cotmore , S.F. , Bloom , M.E. , Linden , R.M and Parish , C.R. 5 – 14 . London : Hodder Arnold .
- Trampel , D.W. , Kinden , D.A. , Solorzano , R.F. and Stogsdill , P.L. 1983 . Parvovirus like enteropathy in Missouri turkeys . Avian Diseases , 27 : 49 – 54 .
- Truyen , U. , Gruenberg , A. , Chang , S-F. , Obermaier , B. , Veialainen , P. and Parrish , R. 1995 . Evolution of the feline sub-group parvoviruses and the control of canine host range in vivo . Journal of Virology , 69 : 4702 – 4710 .
- Woolcock , P.A. and Shivaprasad , H.L. 2008 . Electron microscopic identification of viruses associated with poult enteritis in turkeys grown in California 1993–2003 . Avian Diseases , 52 : 209 – 213 .
- Yu , M. , Ismail , M.M. , Qureshi , M.A. , Dearth , R.N. , Barnes , H.J. and Saif , Y.M. 2000 . Viral agents associated with poult enteritis and mortality syndrome: the role of a small round virus and a turkey coronavirus . Avian Diseases , 44 : 297 – 304 .
- Zsak , L. , Strother , K.O. and Kisary , J. 2008 . Partial genome sequence analysis of parvoviruses associated with enteric disease in poultry . Avian Pathology , 37 : 435 – 441 .
- Zsak , L. , Strother , K.O. and Day , J.M. 2009 . Development of a polymerase chain reaction procedure for detection of chicken and turkey parvoviruses . Avian Diseases , 53 : 83 – 88 .