Abstract
Disseminated histoplasmosis caused by Histoplasma capsulatum, a zoonotic fungal organism, is an important disease in animals and humans, particularly those with compromised immune systems. Reports of disseminated histoplasmosis in an avian species are not available within the current literature. Candida albicans, another fungal agent with zoonotic importance, is a commensal of the avian digestive tract that is often associated with opportunistic infections particularly in young or immunocompromised birds. This report describes a case of concomitant histoplasmosis and candidiasis in an Eclectus parrot (Eclectus roratus) characterized by severe granulomatous glossitis, blepharitis and osteomyelitis with numerous intrahistiocytic and extracellular yeasts (H. capsulatum) as well as intralesional hyphae, pseudohyphae and conidia (C. albicans). To our knowledge, co-infection with H. capsulatum and C. albicans has not been reported in an avian species.
Introduction
Disseminated histoplasmosis is a fungal infection caused by Histoplasma capsulatum, a zoonotic, dimorphic soil fungus endemic to the Americas and sub-Saharan Africa (Ajello, Citation1964; Panackal et al., Citation2002; Loulergue et al., Citation2007; Valli, Citation2007; Centers for Disease Control and Prevention [CDC], Citation2008). Outbreaks of histoplasmosis are not known to occur outside the Americas. However, numerous sporadic cases have been reported worldwide, probably due to international travel between endemic and non-endemic areas (Panackal et al., Citation2002; Valli, Citation2007; CDC, 2008). In the United States, the organism thrives in hot and humid environments and is especially prevalent in the Ohio and Mississippi river valleys (Ajello, Citation1964; Panakal et al., Citation2002; Loulergue et al., Citation2007; Valli, Citation2007; CDC, 2008). H. capsulatum has been frequently isolated from soils heavily contaminated with bird faeces, thus prompting public health concerns that birds might serve as a reservoir or carrier for the fungal pathogen (Ajello, Citation1964; Gustin & Kelley, Citation1971; Cermeño et al., Citation2006). However, failure to culture this organism from bird faeces alone negates the notion of birds as carriers for the disease. Contaminated soils simply provide an ideal growth medium and breeding ground for H. capsulatum (Schwarz et al., Citation1957; Ajello, Citation1964; Gustin & Kelley, Citation1971).
Disseminated histoplasmosis is well documented in mammalian species, including humans, with a predilection for immunocompromised individuals such as those infected with the human immunodeficiency virus (Panakal et al., Citation2002; Loulergue et al., Citation2007; CDC, 2008). Among domestic animals, the disease most commonly affects dogs and cats and is not known to be transmitted from animal to human (Valli, Citation2007). Various avian texts report that avian histoplasmosis is common, particularly among captive zoo animals; however, reports of disseminated histoplasmosis in an avian species are not available within the current literature (Bauck, Citation1994; Kunkle, Citation2003).
Candida albicans is a common environmental organism considered a normal inhabitant of the avian digestive tract, with the crop as the most common site of infection. Impacted food, beak abnormalities and tongue necrosis are all predisposing factors for infections within the oral cavity and young birds with crop stasis are especially susceptible (Bauck, Citation1994; Kunkle, Citation2003). Reports of concurrent infection with H. capsulatum and C. albicans in an avian species have not been documented.
Materials and Methods
Case history
A 1.5-year-old, female Eclectus parrot (Eclectus roratus) presented to the Zoological Medicine & Surgery Service at Texas A & M University, College of Veterinary Medicine in December 2008 for evaluation of oral and peri-ocular masses as well as a right wing and leg lameness of several months’ duration. The bird was reportedly only minimally responsive to prolonged antibiotic treatment and was being fed an avian hand-feeding formula since she had difficulty eating seeds.
Results and Discussion
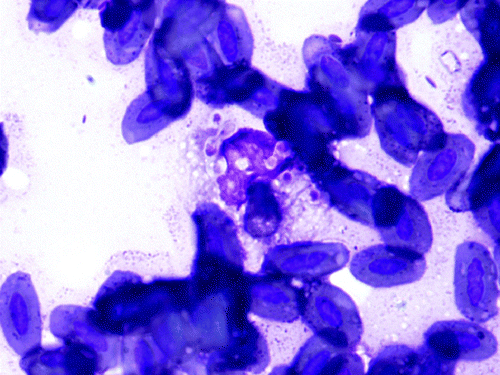
On presentation, the bird's feather quality was poor and unkempt and she was non-weight-bearing lame on the right limb with a firm swelling on the left limb in the region of the tarsometatarsal joint. Pain was elicited on palpation of the right wing, particularly within the area distal to the carpus. A leukocytosis (25.4 × 103 WBC/µl [reference range 5.5 to 25 × 103 WBC/µl]) with mature heterophilia (56%, 14,224/µl [reference range 53.9 to 75%]) and monocytosis (41%, 10,414/µl [reference range 0 to 11%]) was observed on haematologic evaluation, and initial biochemical analysis demonstrated severe elevations in creatine kinase (CK) (2592 u/l [reference range 132 to 1600 u/l]) and AST (716 u/l [reference range 65 to 339 u/l]) (Fudge, Citation2000; Carpenter, Citation2005). Whole body radiographs showed multifocal, irregular expansile lesions affecting all of the extremities with characteristics suggestive of osteomyelitis, and a malunion fracture within the right ulna ( and ). A large, firm, malodorous plaque was on the tongue, and a similar firm mass was within the skin of the right lower eyelid (). Cytologic preparations of the oral and eyelid masses revealed pyogranulomatous inflammation with intracellular yeasts consistent with H. capsulatum noted within macrophages and multi-nucleated giant cells (). The owner elected euthanasia due to poor prognosis.
Figure 1. Ventral–dorsal radiographic image of an Eclectus parrot (E. roratus) right wing. Note the multifocal, expansile lesions causing varying degrees of corticolysis within the extremities (short arrows).
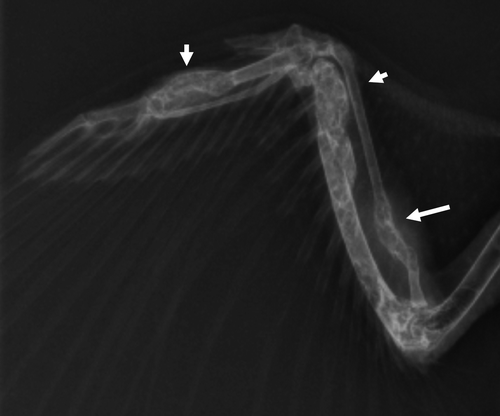
Figure 2. Ventral–dorsal radiographic image of Eclectus parrot (E. roratus) right distal tibia. Note the expansile lesion causing corticolysis within the distal tibia (short arrow).
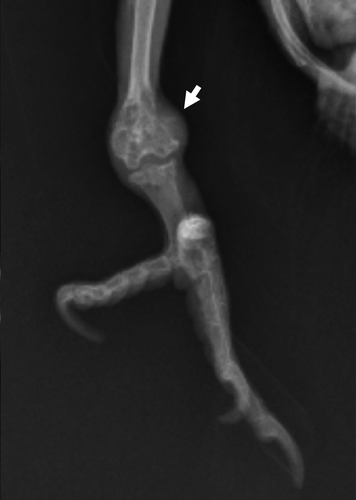
Figure 4. Cytologic preparation of the right lower eyelid, Eclectus parrot (E. roratus). Foamy macrophage with several, 2 to 5 µm, oval thin-walled yeasts exhibiting narrow-based budding (H. capsulatum) surrounded by a few avian erythrocytes. Diff Quick. 1000x magnification.
At necropsy a 1 × 1.5 × 0.1 cm3, pale tan to brown, firm nodular plaque on the dorsal surface of the caudal half of the tongue and a focal, 0.3 × 0.5 × 0.2 cm3, raised, pale pink nodule within the lower lid of the right eye were observed (). In addition, the right tibia and radius and ulna bilaterally exhibited marked, proximal swelling. The rhinotheca and gnathotheca were also markedly overgrown and maloccluded. Representative tissue sections were fixed in 10% buffered formalin, processed routinely, sectioned at 5µm and stained with haemotoxylin and eosin (HE), Periodic-acid Schiff, Grocott's Methenamine Silver and Gridley stains. Using a labelled streptavidin–biotin peroxidase technique previously described, tongue sections were immunostained with a polyclonal antibody to Histoplasma spp. (Nunes et al., Citation2006). Archival tissues infected with H. capsulatum and primary antibody replaced with buffer served as positive and negative controls, respectively.
Histologically, the oral plaque, eyelid mass and expansile lesions within the radii, ulnas and right tibia were similar and characterized by severe granulomatous inflammation with extensive necrosis, haemorrhage and ulceration. The granulomas were predominantly composed of numerous histiocytes and multi-nucleated giant cells with lesser numbers of heterophils, lymphocytes, plasma cells and reactive fibroblasts admixed with oedema and fibrin. Myriad intracytoplasmic and extracellular, round to oval, 2 to 4 µm yeasts consistent with H. capsulatum were observed within histiocytes and multi-nucleated giant cells ( ). These yeasts stained strongly positive for antibody to H. capsulatum (). The inflammatory infiltrate disrupted and effaced the tissue architecture within the tongue, eyelid and long bones, causing focally extensive ulceration of the tongue and eyelid as well as severe cortical lysis of the radius, ulna and tibia. Tongue sections occasionally contained a small number of 2 to 4 µm, basophilic, yeasts with fewer, slender, 4 to 6 µm wide, parallel-walled, irregularly septate hyphae, pseudohyphae and few budding yeasts morphologically consistent with C. albicans ().
Figure 5. Tongue, Eclectus parrot (E. roratus). Large numbers of macrophages and multi-nucleated giant cells both foreign body and Langhans types containing numerous, intrahistiocytic, 2 to 4 µm yeasts with a central, 1 µm basophilic nucleus and surrounding clear zone (H. capsulatum). HE. Bar=25 µm. Inset: Multi-nucleated giant cell, Langhans type, containing numerous intrahistiocytic yeasts. HE. 1000x magnification.
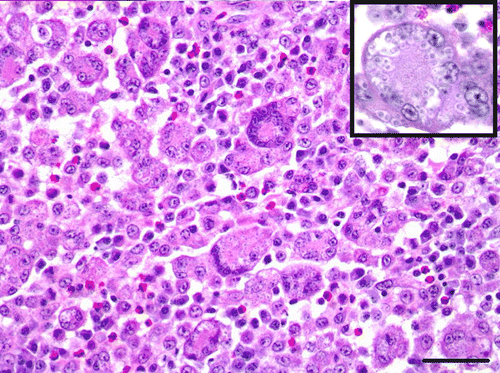
Figure 6. Tongue, Eclectus parrot (E. roratus). Large numbers of macrophages and multinucleated giant cells with positive-staining intrahistiocytic yeasts (H. capsulatum). Grocott's Methenamine Silver. Bar=25 µm.
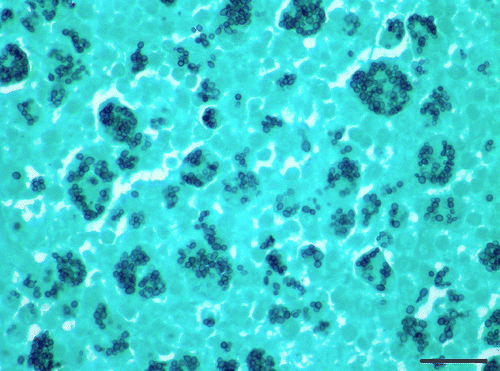
Figure 7. Immunohistochemistry of tongue, Eclectus parrot (E. roratus). Intrahistiocytic yeasts are strongly positive for H. capsulatum markers. 600x magnification.
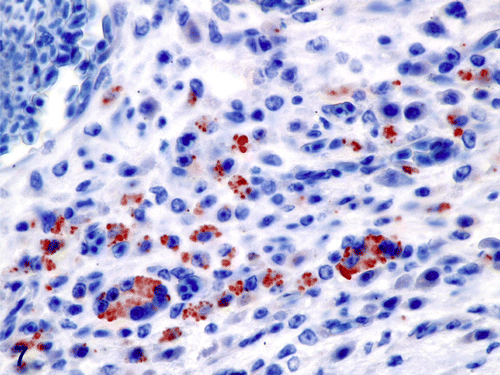
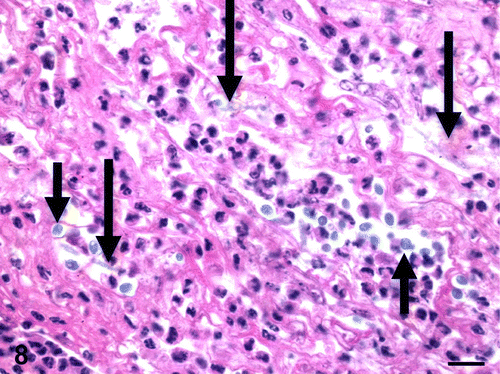
Figure 8. Tongue, Eclectus parrot (E. roratus). Fibrinous and heterophilic inflammation with moderate numbers of 2 to 4 µm, basophilic to amphophilic, extracellular yeasts (short arrows) and occasional, slender, 4 to 6 µm wide, basophilic to amphophilic, parallel-walled, irregularly septated fungal hyphae (long arrows) (C. albicans). HE. Bar=10 µm.
Isolated DNA from paraffin-embedded tongue sections submitted to the Washington Animal Disease Diagnostic Laboratory (WADDL) in Pullman confirmed the presence of C. albicans using universal fungal DNA primers. Additional paraffin-embedded sections of bone and skin were later sent to WADDL and the Veterinary Diagnostic Laboratory at the University of Illinois in Urbana, respectively. However, H. capsulatum DNA could not be isolated from these paraffin-embedded sections, most probably due to suspected formalin degradation of the fungal DNA. Fresh liver samples submitted to the Texas Veterinary Medical Diagnostic Laboratory (TVMDL) in College Station for both psittacine beak and feather disease (circovirus) and polyoma virus polymerase chain reaction (PCR) were negative. Bacterial cultures of the tongue mass also submitted to TVMDL revealed mixed bacterial growth including Pseudomonas aeruginosa, Citrobacter spp., Enterococcus spp., Clostridium spp., and an unidentified, anaerobic Gram-negative rod. Fungal cultures of the tongue mass were negative. However, heavy bacterial growth reported on the fungal plates may have inhibited fungal isolation.
Markedly elevated CK in this case is most probably attributed to the degree of acute tongue necrosis given the relatively short half-life of this muscle leakage enzyme. Struggling while being restrained for venipuncture may have also contributed to its elevation (Lassen, Citation2004). CK, as a specific marker of muscular injury, is reportedly increased in rare cases of fungal myositis associated with disseminated histoplasmosis (Crum-Cianflone, Citation2008). It is possible that the skeletal muscle tissues adjacent to sites of severe osteomyelitis were also affected in this case. However, since additional sections of skeletal muscle were not examined, we are unable to confirm the presence of a more fulminate fungal myositis.
Histoplasmosis is a fungal disease caused by infection with H. capsulatum, a zoonotic soil fungus and intracellular parasite that is endemic to the United States. The disease has been associated with the roosting sites of various avian species including pigeons, grackles, starlings, and chickens (Schwarz et al., Citation1957; Ajello, Citation1964; Gustin & Kelley, Citation1971; Panackal et al., Citation2002; Cermeño et al., Citation2006; Loulergue et al., Citation2007; Valli, Citation2007; CDC, 2008). Since the 1950s, several cases of histoplasmosis have been reported in individuals involved in construction or cleaning activities within endemic areas containing high concentrations of bird faeces, fuelling the public health concern that birds might be carriers for the disease (Ajello, Citation1964; Gustin & Kelley, Citation1971; Panackal et al., Citation2002; Loulergue et al., Citation2007; CDC, 2008). Similar cases have also been reported in conjunction with spelunking activities in caves heavily contaminated with bat guano (Panackal et al., Citation2002; CDC, 2008). Although H. capsulatum has been isolated from organs cultured in experimentally infected birds, the organism has not been successfully isolated from bird faeces, indicating that birds are not reservoir hosts or carriers of H. capsulatum (Schwarz et al., Citation1957; Ajello, Citation1964; Cermeño et al., Citation2006). Alternatively, it is thought that the addition of bird and bat excreta to soil already contaminated with H. capsulatum create the ideal growth medium for the fungi, allowing the organism to thrive and produce macroconidia and microconidia from its highly environmentally resistant mycelial stage (Schwarz et al., Citation1957; Ajello, Citation1964; Gustin & Kelley, Citation1971; Cermeño et al., Citation2006; Greene, Citation2006).
Histoplasmosis is a chronic granulomatous fungal disease of animals, including humans, that generally manifests as a pulmonary infection from inhalation of the organism. Although histoplasma rarely affects the skeleton, it is considered a major aetiology for fungal osteomyelitis, with differentials including coccidioidomycosis and blastomycosis (Hershkovitz et al., Citation1998; Rothschild & Martin, Citation2006). Expansile, lytic lesions within the bone that are not restricted by the cortex, trabeculae or subchondral bone, as observed in this case, are characteristic of fungal infection or multiple myeloma, the latter of which has smooth margins compared to the irregular, proliferative margins of the former (Herschkovitz et al., Citation1998; Rothschild & Martin, Citation2006).
Histoplasmosis is commonly associated with immunosuppression that probably contributes to development of the disseminated form of the disease. In disseminated histoplasmosis, granulomas are detected within many internal organs including the lungs, lymph nodes, spleen, liver, intestine, and brain. Among domestic animals, dogs and cats are the most frequently infected. Clinical signs of disseminated histoplasmosis vary and may include inappetence, weight loss, emaciation, diarrhoea, coughing, dyspnoea, pyrexia and/or lameness (Greene, Citation2006; Valli, Citation2007). Anaemia of chronic disease is common and hypercalcaemia associated with granulomatous disease, specifically the conversion of dietary vitamin D to 1,25-dihydroxycholecalciferol by activated macrophages, is rarely reported (Weller et al., Citation1990; Greene, Citation2006). However, the majority of canine histoplasmosis cases occur without clinical signs or lesions (Valli, Citation2007). Chickens, reptiles and amphibians are resistant to infection (Valli, Citation2007).
Oral granulomas in psittacine birds are often associated with mycobacterial infections, particularly Mycobacterium avium that was not observed in this case (Bauck, Citation1994; Anderson, Citation1997). Similar lesions have been described in birds with Cryptococcus neoformans and Trichomonas spp. infections or seed foreign bodies (Anderson, Citation1997).
C. albicans is a saphrophytic fungus and commensal of the upper avian digestive tract (Bauck, Citation1994). The crop is typically the principal site of infection, but oral candidiasis has been associated with tongue necrosis, impacted food, and beak abnormalities, all of which were evident in this case. This particular bird was also being fed formula, which may have been inadvertently overheated, causing mucosal damage and subsequent bacterial and fungal colonization. The prolonged use of antibiotics in this case is another predisposing factor that probably disrupted the normal oral flora and contributed to the development of oral candidiasis, especially since Candida yeasts are able to adhere to and invade the epithelium when there is any change in the mucosal environment (Brown et al., Citation2007). Endotoxin released by dividing and dying Candida may not only damage the epithelium but may allow deeper penetration of the organism resulting in an increased susceptibility to systemic infection (Brown et al., Citation2007). This is the first reported case of disseminated histoplasmosis with concurrent oral candidiasis in an avian species. However, secondary infections with C. albicans are not uncommon and have been reported in psittacine birds with cutaneous avian pox and systemic staphylococcosis (Tsai et al., Citation1997; Hermans et al., Citation2000).
A diagnosis of disseminated histoplasmosis with concurrent oral candidiasis was made based on the gross, microscopic, immunohistochemical and PCR findings. The severity and extent of systemic disease in this case suggests that the parrot was immunocompromised, most probably due to stress, young age or a combination of several unknown environmental and physiological factors. Two common viral causes of avian immunosuppression, polyoma virus and circovirus were ruled out by PCR. Although we were unable to isolate H. capsulatum DNA from selected tissues due to suspected formalin degradation, both the cellular morphology and immunohistochemical staining of these fungal organisms are highly suggestive of H. capsulatum. In addition, given the generalized skeletal involvement of H. capsulatum and the relatively sparse numbers of Candida within examined tissue sections, we suspect a primary histoplasmosis with secondary oral candidiasis. Candida was not detected in the internal organs by microscopic examination suggesting an absence of systemic involvement by this agent.
Acknowledgements
The authors thank Ms Sarah Jones and group for assistance with histology and Mr John Roths for photographic support.
References
- Ajello , L. 1964 . Relationship of Histoplasma capsulatum to avian habitats: a review . Public Health Reports (1896–1970) , 79 : 266 – 270 .
- Anderson , N.L. 1997 . Recurrent deep foreign body granuloma in the tongue of an African Grey Parrot (Psittacus erithacus timneh) . Journal of Avian Medicine and Surgery , 11 : 105 – 109 .
- Bauck , L. 1994 . “ Mycoses ” . In Avian Medicine: Principles and Application , Edited by: Ritchie , B.W. , Harrison , G.J. and Harrison , L.R. 1005 Lake Worth, FL : Wingers Publishing .
- Brown , C.C. , Baker , D.C. and Barker , I.K. 2007 . “ Alimentary system ” . In Jubb, Kennedy and Palmer's Pathology of Domestic Animals 5th edn , Edited by: Maxie , M.G. Vol. 2 , 230 Philadelphia, PA : Elsevier .
- Carpenter , J.W. 2005 . Exotic Animal Formulary 3rd edn 271 . St Louis, MO : Elsevier Saunders .
- Centers for Disease Control and Prevention 2008 . Outbreak of histoplasmosis among travelers returning from El Salvador-Pennsylvania and Virginia, 2008 . Morbidity and Mortality Weekly Report (MMWR) , 57 , 1348 – 1353 .
- Cermeño , J.R. , Hernández , I. , Cabello , I. , Orellán , Y. , Cermeño , J.J. Albornoz , R. 2006 . Cryptococcus neoformans and Histoplasma capsulatum in dove's (Columbia livia) excreta in Bolívar State, Venezuela . Revista latinoamericana de microbiología , 48 : 6 – 9 .
- Crum-Cianflone , N.F. 2008 . Bacterial, fungal, parasitic, and viral myositis . Clinical Microbiology Reviews , 21 : 473 – 494 .
- Fudge , A.M. 2000 . Laboratory Medicine Avian and Exotic Pets 378 382 . Philadelphia, PA : W.B. Saunders Company .
- Greene , C.E. 2006 . “ Histoplasmosis ” . In Infectious Diseases of the Dog and Cat 3rd edn , Edited by: Greene , C.E. 577 – 583 . St Louis, MO : Elsevier .
- Gustin , P.N. and Kelley , D.C. 1971 . A survey of zoo aviaries for the presence of Histoplasma capsulatum and Cryptococcus neoformans . Mycopathologia et mycologia applicata , 45 : 93 – 102 .
- Hermans , K. , Devriese , L.A. , De Herdt , P. , Godard , C. and Haesebrouck , F. 2000 . Staphylococcus aureus infections in psittacine birds . Avian Pathology , 29 : 411 – 415 .
- Herschkovitz , I. , Rothschild , B.M. and Dutour , O. 1998 . Clues to recognition of fungal origin of lytic skeletal lesions . American Journal of Physical Anthropology , 106 : 47 – 60 .
- Kunkle , R.A. 2003 . “ Fungal Infections ” . In Diseases of Poultry 11th edn , Edited by: Saif , Y.M. , Barnes , H.J. , Glisson , J.R. , Fadly , A.M. , McDougald , L.R. and Swayne , D.E. 901 Ames : Iowa State Press .
- Lassen , E.D. 2004 . “ Laboratory evaluation of muscle injury ” . In Veterinary Hematology and Clinical Chemistry , Edited by: Troy , D.B. 417 Philadelphia, PA : Lippincott Williams & Wilkins .
- Loulergue , P. , Bastides , F. , Baudouin , V. , Chandenier , J. , Mariani-Kurkdjian , P. Dupont , B. 2007 . Literature review and case histories of Histoplasma capsulatum var. duboisii infections in HIV-infected patients . Emerging Infectious Diseases , 13 : 1647 – 1652 .
- Nunes , J. , Mackie , J.T. and Kiupel , M. 2006 . Equine histoplasmosis presenting as a tumor in the abdominal cavity . Journal of Veterinary Diagnostic Investigation , 18 : 508 – 510 .
- Panackal , A.A. , Hajjeh , R.A. , Cetron , M.S. and Warnock , D.W. 2002 . Fungal infections among returning travelers . Clinical Infectious Disease , 35 : 1088 – 1095 .
- Rothschild , B.M. and Martin , L.R. 2006 . Skeletal Impact of Disease , Alberquerque : New Mexico Museum of Natural History .
- Schwarz , J. , Baum , G.L. , Wang , C.J. , Bingham , E.L. and Rubel , H. 1957 . Successful infection of pigeons and chickens with Histoplasma capsulatum . Mycopathologia et mycologia applicata , 8 : 189 – 193 .
- Tsai , S.S. , Chang , T.C. , Yang , S.F. , Chi , Y.C. , Cher , R.S. , Chien , M.S. and Itakura , C. 1997 . Unusual lesions associated with avian poxvirus infection in rosy-faced lovebirds (Agapornis roseicollis) . Avian Pathology , 26 : 75 – 82 .
- Valli , V.E.O. 2007 . “ Hematopoietic system ” . In Jubb, Kennedy and Palmer's Pathology of Domestic Animals 5th edn , Edited by: Maxie , M.G. Vol. 3 , 299 – 301 . Philadelphia, PA : Elsevier .
- Weller , R.E. , Dagle , G.E. , Malaga , C.A. and Baer , J.F. 1990 . Hypercalcemia and disseminated histoplasmosis in an owl monkey . Journal of Medical Primatology , 19 : 675 – 680 .
