Abstract
The most effective approaches to control the spread of Mycoplasma gallisepticum include strict biosecurity measures, continuous surveillance and eradication of infected flocks. The rapid expansion of the poultry industry worldwide in restricted geographical areas and severe economic losses due to M. gallisepticum outbreaks make it crucial to identify and better control the vectors responsible for the transmission of the disease. In the present study we evaluated the susceptibility of sparrows and pigeons to M. gallisepticum and the tissue distribution of M. gallisepticum in these species as compared with chickens. This information will further define the role of these common avian species in M. gallisepticum transmission. Twenty-six chickens, pigeons, and sparrows were experimentally inoculated with a field strain of M. gallisepticum and were monitored for the development of clinical signs, seroconversion, productive infection by culture, and M. gallisepticum distribution in their tissues by immunohistochemistry. All M. gallisepticum-inoculated chickens showed mild respiratory signs, seroconverted (haemagglutination inhibition geometric mean titre = 494) and shed M. gallisepticum in their tracheas. M. gallisepticum antigens were observed at high levels by immunohistochemistry in their tracheas, conjunctivas, nasal turbinates, and air sacs. The pigeons and sparrows did not show clinical signs or seroconvert but M. gallisepticum was reisolated up to 7 days post inoculation from pigeons and intermittently from sparrows. M. gallisepticum antigens were observed at low level in the conjunctiva of some pigeons and sparrows, as well as in the trachea of some sparrows. We conclude that pigeons and sparrows are partially susceptible to M. gallisepticum infection but do not seroconvert or maintain a steady carrier state similar to chickens and that these species may play a role in M. gallisepticum transmission between poultry farms as mechanical vectors.
Introduction
Avian mycoplasmosis causes considerable economical losses to the poultry industry, especially in chickens and turkeys, all over the world. Mycoplasma gallisepticum is responsible for chronic respiratory disease in chickens and for infectious sinusitis in turkeys. In broilers, it causes a reduction in weight gain, a decrease in feed conversion efficiency, an increased mortality rate, and increased condemnations at slaughter. In breeders and layers, the disease may cause a drop in egg production and an increase in embryo mortality (Ley, Citation2008).
Even with the monitoring and control programmes in place, many chicken flocks become infected. Vertical transmission of M. gallisepticum has been documented and may result in infected progeny flocks (Bradbury, Citation2001). M. gallisepticum can also be transmitted horizontally by contaminated fomites and workers (Ley, Citation2008). Wild birds may also play a role in M. gallisepticum transmission. In recent years, certain strains of M. gallisepticum have caused disease in house finches (Carpodacus mexicanus) and infected similar songbirds in the United States (Ley et al., Citation1996; Luttrell et al., Citation1996, Citation2001; Mikaelian et al., Citation2001; Farmer et al., Citation2005).
There have been conflicting reports in the literature regarding the ability of sparrows to act as carriers of M. gallisepticum. Kleven & Fletcher (Citation1983) found that M. gallisepticum was isolated from less than 50% of house sparrows (Passer domesticus) for up to 10 days after experimental infection. The sparrows had no haemagglutination inhibition (HI) titres and were considered temporary biological carriers (Kleven & Fletcher, Citation1983). Farmer et al. (Citation2005) reported that sparrows infected with a house finch isolate of M. gallisepticum seroconverted by serum plate agglutination (SPA) test but M. gallisepticum could not be cultured. In a different study with a house finch M. gallisepticum isolate, sparrows remained infectious to house finches for 3 days (Dhondt et al., Citation2008). Other reports have indicated that house sparrows may only serve as mechanical vectors (Stallknecht et al., Citation1982).
There are few reports regarding M. gallisepticum infection in pigeons. M. gallisepticum was isolated from pigeons kept 20 m from an infected chicken house (Bencina et al., Citation1987). A serological survey of racing pigeons in Taiwan revealed 1.3% positive results using SPA (Tsai & Lee, Citation2006); however, this test is not very specific and false positive results are commonly encountered (Kleven, Citation2008). In additional reports, other Mycoplasma species were isolated from pigeons but not M. gallisepticum (Sinclair, Citation1980; Keymer et al., Citation1984; Reece et al., Citation1986; Loria et al., Citation2005).
The role of free-ranging pigeons and sparrows in M. gallisepticum transmission between chicken farms is not clearly understood. In the present study we attempted to experimentally infect sparrows and pigeons with a field strain of M. gallisepticum to evaluate their susceptibility and hence their possible role in M. gallisepticum transmission.
Materials and Methods
M. gallisepticum inoculum
The M. gallisepticum isolate A-12 was recovered from broiler chickens in Jordan with respiratory disease in 2004. This isolate was passed six times in Frey's media before being used in this experiment (Gharaibeh & Al Roussan, Citation2008). The inoculum contained approximately 109 colour-changing units/ml.
Birds and housing
One-day-old broiler chicks (Hubbard) were obtained from a local commercial breeder farm known to be M. gallisepticum free and reared until 7 weeks of age. Young adult pigeons (Columba livia) were purchased from a local bird market. Adult house sparrows (P. domesticus) were captured from local areas using mist nets in accordance with recommendations of the Royal Society for the Conservation of Nature. All birds were raised in environmentally controlled rooms in the Animal House Facility at Jordan University of Science and Technology (JUST). Housing, care, experimental procedures, and euthanasia procedures were approved by the Animal Care and Use Committee at JUST.
Experimental design
Two days prior to challenge, 15 blood samples from each bird species were collected and tested by HI. At this time, 10 tracheal swabs from chickens and pigeons and 10 oropharyngeal swabs from sparrows were collected and tested by culture and polymerase chain reaction (PCR) to ensure that they were negative for M. gallisepticum. Two chickens, two sparrows, and two pigeons were also humanely euthanized and their tissues were used as negative controls for immunohistochemical (IHC) staining. Chickens, pigeons, and sparrows were separated into challenge (n=26) and control (n=17) groups and housed in two separate rooms. Chickens were weighed to ensure that the groups had similar average body weights; the groups also had a similar male to female ratio. The challenged group was inoculated by intraocular/intranasal administration of 100 µl challenge M. gallisepticum inoculum. Chickens and pigeons received an additional 100 µl inoculum intratracheally. Sparrows were additionally exposed to aerosolized challenge by coarse spray. Control groups were not inoculated. The birds were then observed daily for clinical signs. At 3, 7, 14 and 21 days post inoculation (d.p.i.), 10 tracheal swabs from chickens, 10 tracheal swabs from pigeons, and 10 oropharyngeal swabs from sparrows were collected for M. gallisepticum isolation. At 7 and 14 d.p.i., three birds from each of the inoculated species were randomly selected and humanely euthanized. A complete set of tissues (nasal turbinate, trachea, conjunctiva, lung, air sac, brain, heart, liver, spleen, kidney, duodenum and pancreas, proventriculus, gizzard, thymus, caecal tonsil, bursa of Fabricius, testis and ovary) were collected for IHC testing from these birds. At 21 d.p.i., 15 blood samples from each group were collected for serological testing. Body weights of negative and inoculated chicken groups at 21 d.p.i. were compared by analysis of variance test using the SAS program (SAS, Citation1996).
Serology
Blood samples were collected from each species and tested by HI test as described previously by Kleven (Citation2008). Briefly, haemagglutination (HA) antigen was prepared by harvesting an actively growing culture of the same inoculum by centrifugation for 30 min at 15,700× g, and then the packed cell were suspended in a small amount of phosphate-buffered saline and mixed with equal volume of glycerol. Aliquots were preserved by freezing at −70°C until use. The mycoplasma HI test was conducted in a microtitre plate using 4 HA units of antigen per test using the β procedure (constant antigen-diluted serum). A HI titre of 1:40 or greater was considered positive.
Isolation of M. gallisepticum and detection by PCR
Dry tracheal or oropharyngeal swabs were dipped several times in Frey's media and then discarded. Inoculated media were incubated at 37°C for at least 10 days or until a colour change was evident (Kleven, Citation2008). PCR was used to test for M. gallisepticum growth in the Frey's broth. DNA was extracted as previously described (Liu et al., Citation2001) and PCR was performed using M. gallisepticum-specific primers as previously described (Nascimento et al., Citation1991).
Differentiation of M. gallisepticum from other Mycoplasma spp. by PCR
PCR to detect the presence of Mycoplasma species other than M. gallisepticum in the inoculated Frey's broth was performed using primers designed for detection of nine different Mycoplasma species (Lauerman et al., Citation1995) by amplification of the 16s/23s ribosomal RNA intergenic spacer region This PCR results in different PCR product sizes for different avian mycoplasmas (M. gallisepticum gives a PCR product size ranging from 888 to 938 base pairs).
Antiserum (primary antibody) production for immunohistochemistry
Two young adult female rabbits weighing about 2.7 to 3 kg (Animal House Facility, Yarmouk University, Irbid, Jordan) were housed in the Animal House Facility at JUST and allowed 7 days for acclimatization. Prior to immunization, blood samples were collected from the marginal ear vein, and the resultant serum was used as a negative control antibody. The rabbits were injected subcutaneously with 4 ml commercial M. gallisepticum bacterin (Intervet Int. B.V., Boxmer, The Netherlands) into four different sites along the left and right sides of the dorsal midline followed by 2 ml injection every 4 weeks for two times. Two weeks after the third injection, rabbit's blood was collected from the jugular vein. Rabbits’ housing, care, experimental procedures, and euthanasia procedure were all approved by Animal Care and Use Committee at JUST. One rabbit serum had a 1/320 titre in the HI test and was stored at −20°C to be used as primary antibody at 1/4000 dilution in the IHC procedure.
Immunohistochemistry procedure
After necropsy, tissues were immediately fixed in 4% phosphate-buffered formaldehyde for 24 h. Formalin-fixed tissues were trimmed, dehydrated and infiltrated with paraffin using a Histokinette processor. Tissue samples were embedded in paraffin blocks. Four-micrometre-thick sections were cut and picked onto poly-l-lysine-coated slides (Sigma-Aldrich Chemie, Steinhem, Germany) for IHC stain, and the sections were allowed to dry overnight at room temperature. The presence of M. gallisepticum in tissues was detected using an IHC technique. The Dako EnVision™+ Dual Link System–HRP system (DAKO Inc., Carpinteria, California, USA) was used according to the manufacturer's instructions with some optimization (Gharaibeh et al., Citation2001). Primary antibody (rabbit antiserum) was used at a dilution of 1:4000 (phosphate-buffered saline containing 3% bovine serum albumin). After IHC staining, sections were then counterstained using haematoxylin, air-dried, cover-slipped, and examined under a light microscope. Negative controls were performed using negative control tissues and negative control serum. Tissues with multifocal staining in less than 30% of epithelial cells were defined as having mild staining (+). Tissues with multifocal staining in 30 to 80% of epithelial cells were defined as having moderate staining (++). Tissues with multifocal staining involving more than 80% of epithelial cells were defined as having intense staining (+++).
Results
Clinical and pathological observations
At 6 d.p.i., tracheal rales became evident in the inoculated chicken group and gradually increased in severity until the last day of the experiment (21 d.p.i.). None of the other groups of birds or any of the control groups showed respiratory signs. One male chicken in the inoculated group died at 13 d.p.i. due to airsacculitis. At necropsy at 7 and 14 d.p.i. for IHC, the chickens inoculated with M. gallisepticum showed moderately to severely thickened air sacs with occasional foamy exudate. No obvious differences were observed in severity or presence of lesions between necropsy performed at 7 and 14 d.p.i. There were no gross lesions observed in inoculated pigeons or sparrows.
Body weights
At 21 d.p.i., the average body weight of the inoculated chicken group (n=19) was 3337.6 g and of the control group (n=17) was 3569.4 g. The inoculated group had a 231.8 g decrease in average body weight compared with the control group; however, this difference was not statistically significant (P=0.25).
Serology
The presence of antibodies against M. gallisepticum was assessed by the HI test at 21 d.p.i. The control groups remained negative for M. gallisepticum antibodies. The inoculated chicken group had evident seroconversion with a geometric mean titre of 494. Inoculated pigeon and sparrow groups remained serologically negative ().
Table 1. HI results for chickens, pigeons and sparrows 21 d.p.i. with M. gallisepticum.
M. gallisepticum isolation and detection
M. gallisepticum was detected in all tested inoculated chickens throughout the experiment with the exception of one negative sample at 21 d.p.i. M. gallisepticum was detected in two of 10 and one of 10 samples from the pigeons at 3 and 7 d.p.i., respectively. From 14 d.p.i. the pigeons were negative for M. gallisepticum isolation. M. gallisepticum was isolated from six of 10 and three of 10 sparrows at 3 and 7 d.p.i., respectively. The sparrows were negative at 14 d.p.i., but three of 10 samples were positive for M. gallisepticum at 21 d.p.i. ().
Table 2. Number of birds positive for M. gallisepticum by culture of 10 birds tested at 3, 7, 14, and 21 d.p.i.
Immunohistochemistry
M. gallisepticum antigens were detected by localization of granular brown pigment with different intensities on the apical portion of the respiratory epithelium of the respiratory organs (). Chicken tissues had the most intense staining in the ciliated border of tracheal epithelium at 7 and 14 d.p.i. The tracheal mucosa was thickened by lymphocytic infiltrates. Moderate staining with similar lymphocytic infiltrates were present in the conjunctiva and respiratory epithelium of the nasal cavity at 7 and 14 d.p.i. Air sacs were thickened with lymphocytic infiltrates at 7 and 14 d.p.i. but had moderate staining for M. gallisepticum antigens only at 14 d.p.i. Sparrows had moderate staining in the conjunctiva with no inflammation at 7 d.p.i. One of three tracheas had mild staining with no inflammation at 14 d.p.i. One of three pigeon conjunctivas had mild staining for M. gallisepticum antigens with no inflammation at 7 d.p.i. ( and ). Major airways in the chicken lungs had similar staining as described for their tracheas while the pulmonary parenchyma did not have any detectable antigen. Non-respiratory tissues examined (brain, heart, liver, spleen, kidney, duodenum, pancreas, proventriculus, gizzard, thymus, caecal tonsil, bursa of Fabricius, testes and ovary) did not have any detectable antigen.
Figure 1. Photomicrographs of IHC staining for M. gallisepticum antigens in tissues from experimentally infected chickens, sparrows, and pigeons. Brown stain indicates presence of M. gallisepticum antigens. Tissues are counterstained using haematoxylin (blue). 1a: Moderate staining (++ ) in a chicken's conjunctiva. 1b: Moderate staining (++) in a chicken's nasal cavity. 1c: Intense staining (+++) in a chicken's trachea. 1d: Moderate staining (++) in a chicken's air sac. 1e: Moderate staining (++) in a sparrow's conjunctiva. 1f: Mild staining (+) in a sparrow's trachea. 1g: Mild staining (+) in a pigeon's conjunctiva. All scale bars = 50 µm.
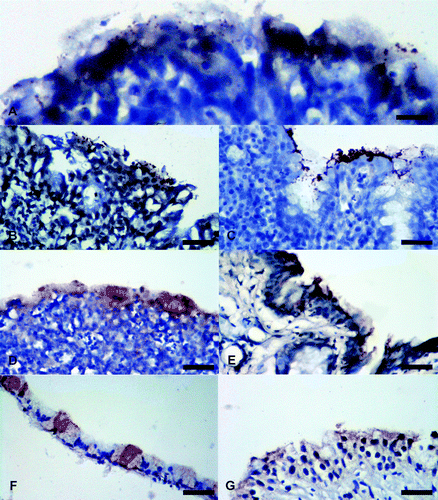
Table 3. Tissues from chickens, sparrows, and pigeons with positive staining for M. gallisepticum antigens using IHC and stain intensity.
Discussion
The clinical signs, gross lesions, consistent shedding, and IHC intense staining in the inoculated chicken group compared with sparrows and pigeons demonstrates the difference in susceptibility between these species. The decrease in the average body weight of the inoculated chicken group (231.8 g) compared with the control group was not statistically significant. Perhaps this difference would have been statistically significant if a larger number of chickens had been included in each group or if chickens had been inoculated at an earlier age or during their peak growth period, as opposed to at 7 weeks of age in the present study.
Where tissues were positive by IHC, the pattern mainly involved the presence of granular staining at the luminal surface of the respiratory epithelium and conjunctiva. This granular pattern has been previously described as microcolonies of M. gallisepticum (Nunoya et al., Citation1995). Chicken tissues had more intense staining that involved more organs than pigeons and sparrows. For pigeons and sparrows, IHC staining was weak, inconsistent, and limited to the conjunctiva at 7 d.p.i. in addition to one sparrow trachea at 14 d.p.i. The difference in staining pattern and intensity between pigeons/sparrows and chickens leads to speculation that the M. gallisepticum antigen detected in pigeons and sparrows might have been retained after inoculation and not sourced from replicating organisms ( and ). To the best of our knowledge, the present study is the first to report on IHC tissue localization of M. gallisepticum antigens in sparrows and pigeons and the first to describe localization in chicken tissues other than the conjunctiva, eyeball, and larynx (Nunoya et al., Citation1995).
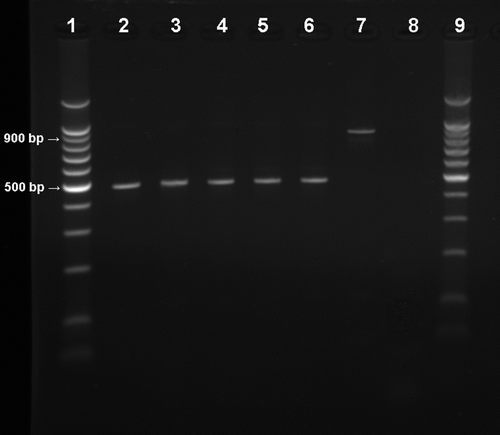
During our attempts to reisolate M. gallisepticum from pigeons, the inoculated Frey's broth frequently changed colour to yellow, indicating growth of an organism in 24 h. PCR specific for M. gallisepticum was negative for most of these fast-colour-changing cultures, as shown in shedding results. The presence of other actively growing mycoplasmas was suspected. Primers previously designed for detection of nine different Mycoplasma species (Lauerman et al., Citation1995) were used to test these cultures by PCR. A band that was clearly a different size from that of M. gallisepticum (around 500 base pairs) was detected (). The band detected was consistent with Mycoplasma gallinarum or Mycoplasma synoviae (Lauerman et al., Citation1995) that have been previously isolated from pigeons (Reece et al., Citation1986), although Lauerman et al. did not include pigeon Mycoplasma spp. in the development of their assay. These unknown Mycoplasma spp. were not tested further in our study for identification. It is possible that a fast-growing Mycoplasma sp. may have overgrown M. gallisepticum in the cultures from pigeons; however, other assays performed on pigeon specimens (IHC, serology) correlated with the low rate detection of M. gallisepticum by culture.
Figure 2. Electrophoresis analysis (1.5% agarose gel) of PCR products of M. gallisepticum shedding amplified by Mycoplasma universal primers (Lauerman et al., 1995). Lanes 1 and 9, 100 base pair (bp) DNA marker (Promega Corp, Madison, Wisconsin, USA); lanes 2 to 6, unidentified Mycoplasma sp. (band size around 500 bp) from inoculated pigeon cultures; lane 7, positive M. gallisepticum sample (band size around 900 bp) from inoculated chicken culture; lane 8 = negative control.
The reisolation of M. gallisepticum from pigeons only during the first week, weak and limited IHC staining of only the conjunctiva, together with failure to seroconvert may indicate the inability of M. gallisepticum to establish an infection in this species. These results are similar to previous studies indicating the isolation of M. gallisepticum from only a small group of pigeons exposed to an infected chicken flock (Bencina et al., Citation1987) and the failure of detection of M. gallisepticum antibodies by HI from racing pigeons (Keymer et al., Citation1984).
In contrast to pigeons, M. gallisepticum was isolated at a higher rate from sparrows 3 and 7 d.p.i. (). No M. gallisepticum could be detected in the sparrows tested at 14 d.p.i., although M. gallisepticum was detected in three sparrows at 21 d.p.i. It is possible that the 10 randomly selected sparrows at 14 d.p.i. may not have been infected. It is also possible that the sparrows were reinfected from the inoculated chickens housed at the same group. However, this was not the case in the pigeon group that was also housed in the same room. Although the sparrows did not show as strong and wide distribution of M. gallisepticum in their tissues when compared with the chickens, there was more intense staining of the conjunctiva compared with the pigeons. Also, one of the sparrow tracheas was mildly positive at 14 d.p.i. whereas none of the pigeon tissues were positive after 7 d.p.i. It appeared that there was more M. gallisepticum in sparrow tissues when compared with pigeons, although, like the pigeons, the sparrows failed to seroconvert. In previous studies, M. gallisepticum was isolated from less than 50% of sparrows up to 10 days after experimentally infecting them. Infection appeared temporary and they had no HI titres (Kleven & Fletcher, Citation1983). Other reports indicated that they may have developed short-lived transient infection in the wild that made them seroconvert by SPA (Stallknecht et al., Citation1982); however, no M. gallisepticum was cultured and serology was not confirmed by HI. M. gallisepticum isolated from house finches can be transmitted from infected sparrows to finches up to 3 d.p.i. (Dhondt et al., Citation2008). Other studies showed that M. gallisepticum from house finches caused seroconversion in sparrows (by SPA) but it could not be cultured (Farmer et al., Citation2005).
Several of the previous reports on M. gallisepticum infection in sparrows and pigeons have studied wild bird populations or captive flocks (Sinclair, Citation1980; Stallknecht et al., Citation1982; Reece et al., Citation1986; Bencina et al., Citation1987; Tsai & Lee, Citation2006) and do not provide evidence on the extent and length of infection after exposure to M. gallisepticum.
There are also experimental infections described in the literature using a virulent laboratory strain of M. gallisepticum (Kleven & Fletcher, Citation1983) or a house finch strain of M. gallisepticum (Farmer et al., Citation2005; Dhondt et al., Citation2008), with the latter described as mildly pathogenic to chickens (O'Connor et al., Citation1999). M. gallisepticum strains vary in virulence and infectivity as well as in their ability to persist in respiratory tissues (Ley, Citation2008). It is conceivable that different strains of M. gallisepticum behave differently in wild birds. In the present study we used a recently isolated M. gallisepticum strain (A-12) from clinically affected broiler chickens.
In conclusion, these results concur with earlier studies that pigeons and sparrows were partially susceptible to M. gallisepticum infection and did not maintain a steady carrier state or seroconvert similar to chickens. Sparrows and pigeons may play a role in M. gallisepticum transmission between poultry farms as mechanical vectors or temporary biological carriers.
Acknowledgements
The authors would like to thank the Deanship of Research at Jordan University of Science and Technology and The Scientific Research Fund of the Ministry of Higher Education and Scientific Research for supporting the present work. They also thank Dr Kamel Mahmoud for his comments and help with the statistical analysis and Dr Naola Ferguson-Noel for her help in revising this manuscript.
References
- Bencina , D. , Dorrer , D. and Tadina , T. 1987 . Mycoplasma species isolated from six avian species . Avian Pathology , 16 : 653 – 664 .
- Bradbury , J.M. 2001 . “ Avian mycoplasmas ” . In Poultry Diseases5th edn , Edited by: Jordan , F. , Pattison , M. , Alexander , D. and Faragher , T. 178 – 193 . London : W.B. Saunders .
- Dhondt , A.A. , Dhondt , K.V. and McCleery , B.V. 2008 . Comparative infectiousness of three passerine bird species after experimental inoculation with Mycoplasma gallisepticum . Avian Pathology , 37 : 635 – 640 .
- Farmer , K.L. , Hill , G.E. and Roberts , S.R. 2005 . Susceptibility of wild songbirds to the house finch strain of Mycoplasma gallisepticum . Journal of Wildlife Diseases , 41 : 317 – 325 .
- Gharaibeh , S. and Al Roussan , D. 2008 . The use of molecular techniques in isolation and characterization of Mycoplasma gallisepticum from commercial chickens in Jordan . International Journal of Poultry Science , 7 : 28 – 35 .
- Gharaibeh , S. , Brown , T. , Stedman , N. and Pantin , M. 2001 . Immunohistochemical localization of avian leukosis virus subgroup J in tissues from naturally infected chickens . Avian Diseases , 45 : 992 – 998 .
- Keymer , I.F. , Leach , R.H. , Clarke , R.A. , Bardsley , M.E. and McIntyre , R.R. 1984 . Isolation of mycoplasma spp. from racing pigeons (Columba livia) . Avian Pathology , 13 : 65 – 74 .
- Kleven , S.H. 2008 . “ Mycoplasmosis ” . In A Laboratory Manual for the Isolation and Identification of Avian Pathogens5th edn , Edited by: Dufour-Zavala , L. , Swayne , D.E. , Glisson , J.R. , Pearson , J.E. , Reed , W.M. , Jackwood , M.W. and Woolcock , P.R. 59 – 64 . Athens, GA : American Association of Avian Pathologists .
- Kleven , S.H. and Fletcher , W.O. 1983 . Laboratory infection of house sparrows (Passer domesticus) with Mycoplasma gallisepticum and Mycoplasma synoviae . Avian Diseases , 27 : 308 – 311 .
- Lauerman , L.H. , Chilina , A.R. , Closser , J.A. and Johansen , D. 1995 . Avian mycoplasma identification using polymerase chain reaction amplicon and restriction fragment length polymorphism analysis . Avian Diseases , 39 : 804 – 811 .
- Ley , D.H. 2008 . “ Mycoplasma gallisepticum infection ” . In Diseases of Poultry , 12th edn , Edited by: Saif , Y.M. , Fadly , A.M. , Glission , J.R. , McDougald , L.R. , Nolan , L.K. and Swayne , D.E. 807 – 834 . Ames, IA : Blackwell Publishing .
- Ley , D.H. , Berkhoff , J.E. and McLaren , J.M. 1996 . Mycoplasma gallisepticum isolated from house finches (Carpodacus mexicanus) with conjunctivitis . Avian Diseases , 40 : 480 – 483 .
- Liu , T. , Garcia , M. , Levisohn , S. , Yogev , D. and Kleven , S.H. 2001 . Molecular variability of the adhesin-encoding gene pvpA among Mycoplasma gallisepticum strains and its application in diagnosis . Journal of Clinical Microbiology , 39 : 1882 – 1888 .
- Loria , G.R. , Tamburello , A. , Liga , F. , Lawes , J. and Nicholas , R.A. 2005 . Isolation of mycoplasmas from pigeons suffering eye lesions and respiratory disease . The Veterinary Record , 157 : 664 – 665 .
- Luttrell , M.P. , Fischer , J.R. , Stallknecht , D.E. and Kleven , S.H. 1996 . Field investigation of Mycoplasma gallisepticum infections in house finches (Carpodacus mexicanus) from Maryland and Georgia . Avian Diseases , 40 : 335 – 341 .
- Luttrell , M.P. , Stallknecht , D.E. , Kleven , S.H. , Kavanaugh , D.M. , Corn , J.L. and Fischer , J.R. 2001 . Mycoplasma gallisepticum in house finches (Carpodacus mexicanus) and other wild birds associated with poultry production facilities . Avian Diseases , 45 : 321 – 329 .
- Mikaelian , I. , Ley , D.H. , Claveau , R. , Lemieux , M. and Berube , J.P. 2001 . Mycoplasmosis in evening and pine grosbeaks with conjunctivitis in Quebec . Journal of Wildlife Diseases , 37 : 826 – 830 .
- Nascimento , E.R. , Yamamoto , R. , Herrick , K.R. and Tait , R.C. 1991 . Polymerase chain reaction for detection of Mycoplasma gallisepticum . Avian Diseases , 35 : 62 – 69 .
- Nunoya , T. , Yagihashi , T. , Tajima , M. and Nagasawa , Y. 1995 . Occurrence of keratoconjunctivitis apparently caused by Mycoplasma gallisepticum in layer chickens . Veterinary Pathology , 32 : 11 – 18 .
- O'Connor , R.J. , Turner , K.S. , Sander , J.E. , Kleven , S.H. , Brown , T.P. , Gomez , L. Jr and Cline , J.L. 1999 . Pathogenic effects on domestic poultry of a Mycoplasma gallisepticum strain isolated from a wild house finch . Avian Diseases , 43 : 640 – 648 .
- Reece , R.L. , Ireland , L. and Scott , P.C. 1986 . Mycoplasmosis in racing pigeons . Australian Veterinary Journal , 63 : 166 – 167 .
- SAS , I. 1996 . SAS/STAT User's Guide: Statistics , Cary, NC : SAS Institute .
- Sinclair , D.V. 1980 . Respiratory disease in pigeons . The Veterinary Record , 106 : 466 – 467 .
- Stallknecht , D.E. , Johnson , D.C. , Emory , W.H. and Kleven , S.H. 1982 . Wildlife surveillance during a Mycoplasma gallisepticum epornitic in domestic turkeys . Avian Diseases , 26 : 883 – 890 .
- Tsai , H.J. and Lee , C.Y. 2006 . Serological survey of racing pigeons for selected pathogens in Taiwan . Acta Veterinaria Hungarica , 54 : 179 – 189 .