Abstract
A juvenile, male, yellow-eyed penguin (Megadyptes antipodes) with abnormal stance and decreased mobility was captured, held in captivity for approximately 6 weeks, and euthanized due to continued clinical signs. Radiographically, there was bilateral degenerative joint disease with coxofemoral periarticular osteophyte formation. Grossly, the bird had bilaterally distended, thickened coxofemoral joints with increased laxity, and small, roughened and angular femoral heads. Histologically, the left femoral articular cartilage and subchondral bone were absent, and the remaining femoral head consisted of trabecular bone overlain by fibrin and granulation tissue. There was no gross or histological evidence of infection. The historic, gross, radiographic, and histopathologic findings were most consistent with bilateral aseptic femoral head degeneration resulting in degenerative joint disease. Although the chronicity of the lesions masked the initiating cause, the probable underlying causes of aseptic bilateral femoral head degeneration in a young animal are osteonecrosis and osteochondrosis of the femoral head. To our knowledge, this is the first reported case of bilateral coxofemoral degenerative joint disease in a penguin.
Introduction
Degenerative hip disease is unreported in wild birds. Osteoarthritis, when present, most commonly involves the femorotibial and tibiotarsal–tarsometatarsal joints (Rothschild & Panza, Citation2005, Citation2006). The underlying causes of bilateral hip degenerative joint disease include conditions leading to compromise of the physeal cartilage or the femoral head bone. In humans and small dogs, a congenital type of femoral head osteonecrosis is well described as Legg–Calvé–Perthes disease, and bilateral occurrence is common (Ljunggren, Citation1967; Lee & Fry, Citation1969; Piek et al., Citation1996). In birds, femoral head osteonecrosis and epiphysiolysis occur commonly in fast-growing poultry breeds (McNamee & Smyth, Citation2000), but have not been reported in other domestic avian species or in any species of wild bird. Coxofemoral luxation in birds has been reported to occur when there is traumatic rupture of the capital ligament, but does not lead to coxofemoral degenerative joint disease (Helmer & Reding, Citation2006; Stauber et al ., Citation2008).
Yellow-eyed penguins (Megadyptes antipodes) (YEPs) are large, long-lived, penguins inhabiting temperate regions of New Zealand. They spend much of their time at sea but may walk up to several kilometres inland each day during the nesting season (Darby & Seddon, Citation1990). Non-infectious bony abnormalities have not been reported in YEPs to date (Darby & Dawson, Citation2000; Hocken, Citation2005).
Materials and Methods
History. A free-living male juvenile YEP found in the Catlins, South Otago region of New Zealand, was brought into rehabilitation in January 2009 after it was observed to have difficulty in walking. It was estimated to be approximately 8 weeks of age at the time of intake. On clinical examination it was in poor body condition (2/9, weight 3.2 kg), and had an abnormal stance with bilateral abduction of the pelvic limbs. The YEP was held in rehabilitation for approximately 8 weeks and received a single dose of oral ivermectin (Noromectin® Oral, Norbrook NZ, Ltd., Auckland, New Zealand) on day 37 followed by 18.75 mg/kg enrofloxacin (Baytril® Injectable, Bayer Animal Health New Zealand, North Shore City, New Zealand) daily on days 38 to 52. The bird recovered body condition but its mobility did not improve, and it was euthanized on day 52 by phenobarbital injection.
Diagnostic procedures. The carcass was shipped on ice to the Wildlife Health Centre, Massey University and necropsy was performed 48 h after euthanasia. Samples of all internal organs were collected and fixed in 10% buffered formalin solution. After fixation, selected tissue blocks were embedded in paraffin, cut at 4 µm and stained routinely with haematoxylin and eosin for histological examination. At necropsy the pelvic region was radiographed (dorso-ventral and lateral plain radiographs), and following this the pelvis was bisected laterally at midline. The entire left coxofemoral joint was fixed in formalin, decalcified with Osteomoll solution (Merck, KGaA, Darmstadt, Germany), and processed for histology as described above. Selected sections of bone were also stained with Gram's method, periodic acid–Schiff stain, and Young's stain. The right pelvic and femoral bones were examined grossly following boiling in water and manual defleshing.
Results
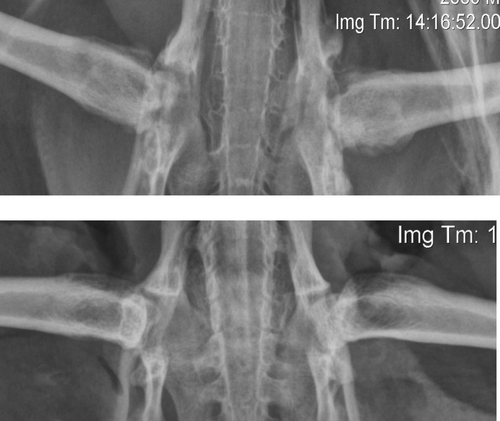
Radiographically, severe periarticular osteophyte formation associated with both coxofemoral joints was noted. There was osteolysis of the right femoral head and acetabulum, and the left acetabulum was wide and shallow. The left femoral head was entirely absent, and severe remodelling of the femoral neck was present ().
Figure 1. Post-mortem ventrodorsal radiographs of a juvenile YEP with bilateral coxofemoral degenerative joint disease (top) and unaffected age-matched control (bottom).
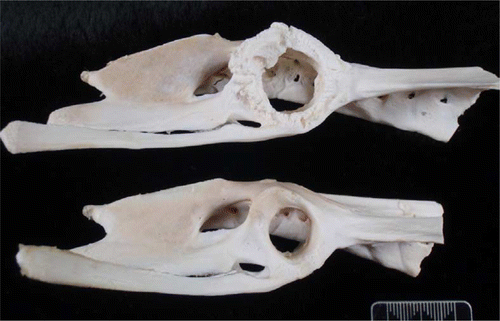
Post-mortem examination revealed that the bird was in excellent body condition (7/9, weight 4.1 kg), with good fat reserves. Both coxofemoral joints had marked laxity, and their synovial capsules were distended and thickened. The left joint cavity had been replaced by solid fibrinous and collagenous material containing pockets of haemorrhage and pale cream-coloured fibrinonecrotic debris (). The right joint contained thin, tan-coloured, opaque exudate, and the articular cartilage of the femoral head was extensively under-run so that only the ligamentum teres and a small flap of connective tissue at the lateral aspect attached it to the femur. The right femoral head was small, roughened and angular, and there was marked osteophyte formation on the femoral neck, greater trochanter, and lesser trochanter (). The acetabulum was wide (170 mm compared with 120 mm in a normal YEP of a similar age) and shallow, with marked osteolysis, roughening of articular bone, and osteophyte formation (). No other skeletal or soft tissue abnormalities were apparent grossly.
Figure 2. Gross formalin-fixed transverse section of the left coxofemoral joint of a juvenile YEP with bilateral coxofemoral degenerative joint disease. Note the spinal cord at the top (arrow), and the femoral shaft extending down and to the left. The femoral head remnant is surrounded by necrotic debris, collagen, and organizing haemorrhage. The horizontal crack in the section is artefactual. Scale bar = 1 cm.
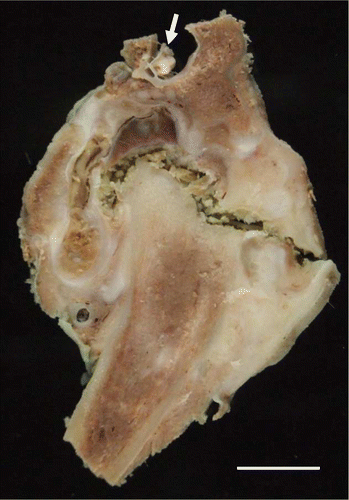
Figure 3. The defleshed right proximal femur of a juvenile YEP with bilateral coxofemoral degenerative joint disease (left) and an unaffected age-matched control (right). The affected femoral head is markedly shrunken, angular, and roughened, with osteophyte formation on the femoral neck, and greater and lesser trochanters. Scale bar = 1 cm.
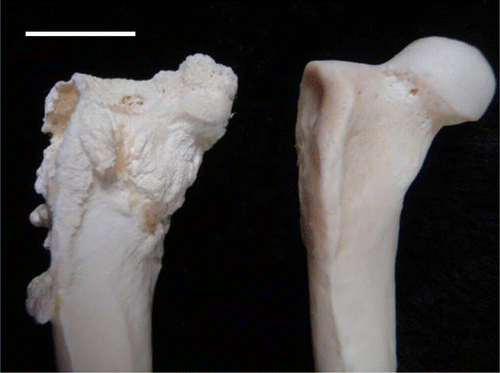
Figure 4. The defleshed right pelvis of a juvenile YEP with bilateral coxofemoral degenerative joint disease (top) with an unaffected age-matched control (bottom). The acetabulum is markedly widened and there is circumferential osteophyte formation.
Histologically, articular cartilage and subchondral bone were absent from both the left femoral head and acetabulum. Both of the articulating surfaces were composed of fibrinonecrotic debris and haemorrhage superficially, a middle layer comprising sheets of macrophages and plasma cells within a meshwork of proliferating fibroblasts, capillaries and loosely interwoven collagen fibres (granulation tissue), and a deep layer of proliferating fibroblasts that interdigitated with the underlying trabecular bone (). Occasional small fragments of mineralized bone with scalloped margins, often surrounded by osteoclasts, were embedded within all of the above layers (). In areas of decreased articulation, where synovium was intact, the synovial villi were thickened by plasma cells and macrophages, and were overlain by hyperplastic synoviocytes. There was exuberant bony proliferation along adjacent periosteum (). No microorganisms were seen on sections stained by Gram's method, periodic acid–Schiff or Young's stains. The internal organs were unremarkable on histology.
Figure 5. Histology of the left femoral head of a juvenile YEP with bilateral coxofemoral degenerative joint disease (haematoxylin and eosin), with the remains of the proximal femoral head at the top left (F). The articular cartilage is absent, and much of the normal epiphyseal bone has been resorbed. The remaining trabecular bone is covered by a thick layer of mature lamellar collagen (C). Fibrin and cellular debris occupy pockets within the remaining joint space (arrow). Note the florid periosteal reaction that forms delicate trabeculae which protrude nearly perpendicularly from the periostium (P). Scale bar = 1000 µm.
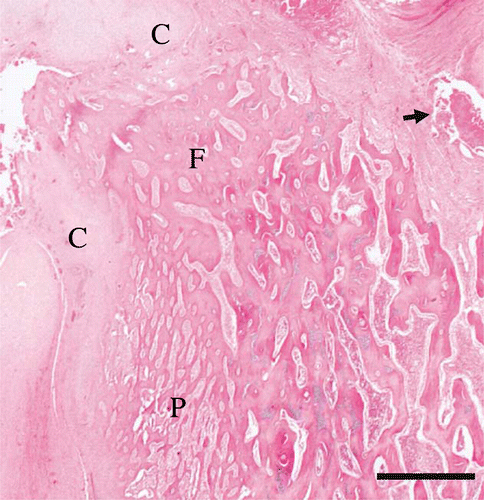
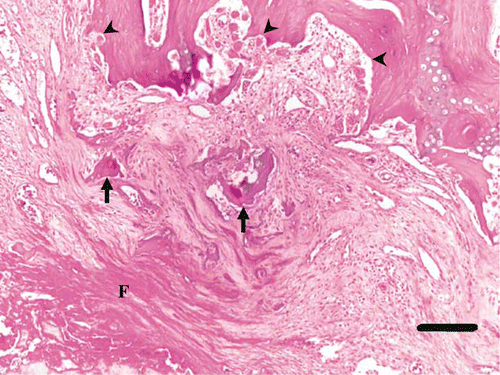
Figure 6. Histology of the articular surface of the left femoral head of a juvenile YEP with bilateral coxofemoral degenerative joint disease (haematoxylin and eosin). The cortical bone and articular cartilage are absent, and remaining superficial trabecular bone has been covered by maturing sheets of collagen interspersed with fragments of bone (arrows), with layers of superficial fibrin (F). Within intact trabecular bone there is active resorption by many osteoclasts, and Howship's lacunae are prominent (arrowheads). Scale bar = 150 µm.
Discussion
Owing to the chronic and severe nature of the degenerative joint changes and bony remodelling, the cause of this condition was not identified. The disease processes considered to be the most probable contributing factors are those that affect young animals, are commonly bilateral, and which are capable of causing severe degeneration of the femoral head. Two such processes are osteonecrosis and osteochondrosis.
Although both osteonecrosis and osteochondrosis can occur secondary to bacterial infection, we considered an infectious cause to be unlikely for several reasons. There was no gross or histological evidence of infection in any tissues examined, and the bird was clinically well having gained weight in captivity prior to antibiotic administration. Furthermore, the lesions were bilateral, a slightly unusual distribution for an infectious arthritis, and histopathology of the left coxofemoral joint failed to demonstrate microorganisms or the presence of a heterophilic exudate that would be expected with a bacterial or fungal infection.
Femoral head osteonecrosis has many causes, including ischaemic necrosis due to congenital narrowing or malpositioning of femoral head blood vessels, femoral head fracture, non-septic infarction due to trauma or blood dyscrasias; and primary infectious diseases such as septic infarction, and septic osteomyelitis or chondritis. Aseptic femoral head osteonecrosis can occur following any event that results in ischaemic necrosis of bone. A classic example is Legg–Calvé–Perthes disease, a congenital condition believed to be caused by transient occlusion of blood vessels supplying the femoral head (Boss & Misselevich, Citation2003). Many factors can predispose to femoral head ischaemia and osteonecrosis. Osteonecrosis of the femoral head occurs spontaneously in hypertensive rats (Kikkawa et al., Citation2000), and has been associated with hypercoagulability disorders in humans, but not in dogs (Mickelson et al., Citation1981; Brenig et al., Citation1999). Steroid-induced osteonecrosis is a side-effect in up to 50% of human patients treated with long-term corticosteroids (Boss & Misselevich, Citation2003), a side-effect thought to be the result of changes in lipid mobilization and storage. Osteonecrosis of the femoral head has been induced by chemical, positional, and ischaemic manipulation in experimental emu, dog, rabbit, and rat models (Launder et al., Citation1981; Conzemius et al., Citation2002; Boss & Misselevich, Citation2003).
Venous occlusion to the femoral head occurs experimentally at moderately increased intracapsular pressures in predisposed, but not in non-predisposed, breeds of dog (Kemp, Citation1981). This may indicate that the vascular arrangement of susceptible dogs predisposes them to positional femoral head ischaemia. Such a predisposition has been found in children, in which affected individuals show structural abnormalities of their lateral epiphyseal arteries, the most important femoral head arteries during development (Trueta, Citation1957; Atsumi et al., Citation2000). In susceptible dog breeds, including the miniature poodle, West Highland white terrier and Yorkshire terrier, the disease appears to be linked to an autosomal recessive gene (Robinson, Citation1992). Primary femoral head osteonecrosis has not to our knowledge been described in birds.
Femoral head osteochondrosis is thought to result from ischaemic insult to developing cartilage. Osteochondrosis is a disease of young fast-growing animals, including commercial poultry, pigs, giant dog breeds, deer, and horses (Ytrehus et al., Citation2007), and can manifest as femoral head epiphysiolysis, or detachment of epiphyseal bone. Femoral head epiphysiolysis is common in meat breeds of turkey (Julian, Citation1985, Citation2005) and fast-growing pigs (Hill, Citation1990), and has been reported in copper-deficient red deer (Thompson et al., Citation1994). In commercial poultry, osteochondrosis can occur following both sterile and bacterial infarction (Thorp et al., Citation1993; McNamee & Smyth, Citation2000), as well as trauma-associated ischaemia (Mutalib & Maslin, Citation1996). Birds lack a bony femoral head epiphysis, having instead a region of epiphyseal cartilage vascularized during development from the epiphyseal side by vessels originating from the retinaculum and teres ligament (Thorp, Citation1986). Because birds lack a true physis, the condition termed epiphysiolysis is a misnomer. However, the result in birds and mammals is similar, in that chronic epiphysiolysis can result in complete collapse of the femoral head, and in severe degenerative joint disease.
Penguin chicks are known to have high growth rates, with king penguin chicks showing the fastest known bone tissue growth rate of any wild bird (Margerie et al., Citation2004). Although comparative data for YEPs are not available, they are the fourth-largest penguin species, and chicks are capable of attaining 90% of adult weight within 55 days of hatch (vanHeezik, Citation1990). Comparison with fast-growing commercial poultry may therefore be appropriate.
Degenerative joint disease of the hips is likely to seriously impede the ability of affected individuals to forage in the wild and may be more common in YEPs than we realise. A more extensive investigation involving routine post-mortem examination of the hips is therefore justified.
Acknowledgements
The authors thank Tony Malthus for his veterinary care, and the staff and volunteers at Penguin Place, the Yellow-eyed Penguin Trust, and the Department of Conservation, Coastal Otago for their roles in submitting this case. Angela Hartman and Devon Thompson provided assistance in radiographic interpretation. Technical assistance was provided by Evelyn Lupton and Elaine Booker. Professor Keith Thompson provided invaluable advice and critique. The present work was funded by the New Zealand Department of Conservation Wildlife Pathology Contract.
References
- Atsumi , T. Yamano , K. , Muraki , M. Yoshihara , S. & Kajihara , T. 2000 . The blood supply of the lateral epiphyseal arteries in Perthes' disease The Journal of Bone and Joint Surgery , 82-B , 392 – 398 .
- Boss , J.H. and Misselevich , I. 2003 . Osteonecrosis of the femoral head of laboratory animals: the lessons learned from a comparative study of osteonecrosis in man and experimental animals . Veterinary Pathology , 40 : 345 – 354 .
- Brenig , B. , Leeb , T. , Jansen , S. and Kopp , T. 1999 . Analysis of blood clotting factor activities in canine Legg–Calvé–Perthes' disease . Journal of Veterinary Internal Medicine , 13 : 570 – 573 .
- Conzemius , M.G. Brown , T.D. Zhang , Y. & Robinson , R.A. 2002 . A new animal model of femoral head osteonecrosis: one that progresses to human-like mechanical failure Journal of Orthopaedic Research , 20 , 303 – 309 .
- Darby , J.T. and Dawson , S.M. 2000 . Bycatch of yellow-eyed penguins (Megadyptes antipodes) in gillnets in New Zealand waters 1979–1997 . Biological Conservation , 93 : 327 – 332 .
- Darby , J.T. and Seddon , P.J. 1990 . “ Breeding biology of yellow-eyed penguins (Megadyptes antipodes) ” . In Penguin Biology , Edited by: Davis , L.S. and Darby , J.T. 45 – 62 . San Diego : Academic Press .
- Helmer , P. and Reding , P. 2006 . “ Surgical resolution in orthopedic disorders ” . In Clinical Avian Medicine , Edited by: Harrison , G.J. and Lightfoot , T.L. Vol. 2 , 768 – 769 . Palm Beach, FL : Spix Publishing .
- Hill , M.A. 1990 . Causes of degenerative joint disease (osteoarthrosis) and dyschondroplasia (osteochondrosis) in pigs . Journal of the American Veterinary Medical Association , 197 : 107 – 113 .
- Hocken , A.G. 2005 . Necropsy findings in yellow-eyed penguins (Megadyptes antipodes) from Otago, New Zealand . New Zealand Journal of Zoology , 32 : 1 – 8 .
- Julian , R.T. 1985 . Osteochondrosis, dyschondroplasia, and osteomyelitis causing femoral head necrosis in turkeys . Avian Diseases , 29 : 854 – 866 .
- Julian , R.J. 2005 . Production and growth related disorders and other metabolic diseases of poultry—a review . The Veterinary Journal , 169 : 350 – 369 .
- Kemp , H.B.S. 1981 . Perthes' disease: the influence of intracapsular tamponade on the circulation in the hip joint of the dog . Clinical Orthopaedics and Related Research , 156 : 105 – 114 .
- Kikkawa , M. Imai , S. & Hukuda , S. 2000 . Altered postnatal expression of insulin-like growth factor-I (IGF-I) and type X collagen preceding the Perthes' disease-like lesion of a rat model Journal of Bone and Mineral Research , 15 , 111 – 119 .
- Launder , W.S. Hungerford , D.S. & Jones , L.H. 1981 . Hemodynamics of the femoral head The Journal of Bone and Joint Surgery , 63 , 442 – 448 .
- Lee , R. and Fry , P.D. 1969 . Some observations on the occurrence of Legg–Calvé–Perthes disease (coxaplana) in the dog, and an evaluation of excision arthroplasty as a method of treatment . Journal of Small Animal Practice , 10 : 309 – 317 .
- Ljunggren , G. 1967 . Legg–Perthes disease in the dog . Acta Orthopaedica Scandinavica , 95 : 1 – 79 .
- Margerie , E.D. Robin , J.-P. Verrier , D. Cubo , J. Groscolas , R. & Castanet , J. 2004 . Assessing a relationship between bone microstructure and growth rate: a fluorescent labelling study in the king penguin chick (Aptenodytes patagonicus) The Journal of Experimental Biology, 207 , 869 – 879 .
- McNamee , P.T. and Smyth , J.A. 2000 . Bacterial chondronecrosis with osteomyelitis (“femoral head necrosis”) of broiler chickens: a review . Avian Pathology , 29 : 253 – 270 .
- Mickelson , M.R. , McCurnin , D.M. , Awbrey , B.J. , Maynard , J.A. and Martin , B.K. 1981 . Legg–Calvé–Perthes disease in dogs: a comparison to human Legg–Calvé–Perthes disease . Clinical Orthopaedics and Related Research , 157 : 287 – 300 .
- Mutalib , A. and Maslin , W. 1996 . Proximal tibiotarsal infarcts in turkeys . Avian Diseases , 40 : 807 – 812 .
- Piek , C.J. Hazewinkel , H.A.W. Wolvekamp , W.T.C. Nap , R.C. & Mey , B.P. 1996 . Long term follow-up of avascular necrosis of the femoral head in the dog Journal of Small Animal Practice , 37 , 12 – 18 .
- Robinson , R. 1992 . Legg–Calve–Perthes disease in dogs: genetic aetiology . Journal of Small Animal Practice , 33 : 275 – 276 .
- Rothschild , B.M. and Panza , R.K. 2005 . Epidemiologic assessment of trauma-independent skeletal pathology in non-passerine birds from museum collections . Avian Pathology , 34 : 212 – 219 .
- Rothschild , B.M. and Panza , R. 2006 . Osteoarthritis is for the birds . Clinical Rheumatology , 25 : 645 – 647 .
- Stauber , E. Mulholland , J.A. , Levine , E.W. Suzuki , Y. & Hall , J. 2008 . Successful rehabilitation of a severely injured peregrine falcon Journal of Avian Medicine and Surgery , 22 , 346 – 350 .
- Thompson , K.G. Audige , L. Arthur , D.G. Julian , A.F. Orr , M.B. & McSporran , K.D. 1994 . Osteochondrosis associated with copper deficiency in young farmed red deer and wapiti x red deer hybrids New Zealand Veterinary Journal , 42 , 137 – 143 .
- Thorp , B.H. 1986 . Vascular pattern of the developing proximal femur in the domestic fowl . Research in Veterinary Science , 40 : 231 – 235 .
- Thorp , B.H. Whitehead , C.C. 1993 . Proximal femoral degeneration in growing broiler fowl . Avian Pathology , 22 : 325 – 342 .
- Trueta , J. 1957 . The normal vascular anatomy of the human femoral head during growth . The Journal of Bone and Joint Surgery , 39-B : 358 – 394 .
- van Heezik , Y. 1990 . Patterns and variability of growth in the yellow-eyed penguin . The Condor , 92 : 904 – 912 .
- Ytrehus , B. , Carlson , C.S. and Ekman , S. 2007 . Etiology and pathogenesis of osteochondrosis . Veterinary Pathology , 44 : 429 – 448 .