Abstract
The chicken infectious anaemia virus (CIAV) infection may induce immunosuppression and persistent infection. The use of vaccination in young chicks is still controversial due to its low immune efficiency. In order to verify the viral persistency of a vaccinal strain of CIAV and its associated-lymphoid cell disorders, 54 1-day-old specific pathogen free chicks were vaccinated (CIAV-VAC®; Intervet, Millsboro, Delaware, USA) and haematologic examination, expression of viral VP3 gene, humoral response and phenotyping of lymphoid cells were studied in lymphoid organs at various times post vaccination (p.v.). No clinical signs were observed but light heteropaenia was detected in CIAV-vaccinated chicks. The VP3 gene of CIAV was detected by polymerase chain reaction in the thymus and spleen from day 7 until 28 days p.v. Thymic larger CD4+CD8+ cells increased only at 7 days p.v. while smaller CD4+CD8+ cells decreased after 14 and 28 days in CIAV-vaccinated birds. The CD4 expression, in contrast to that seen for CD8, decreased in thymocytes from the CIAV-vaccinated group. In the spleen and bursa, the percentage of CD8+ cells increased at 7 and 28 days p.v. only, while CD4+ cells decreased simultaneously. The vaccinated chicks also exhibited a higher number of splenic CD3–CD8+ cells (natural killer cells). The anti-CIAV antibody responses, however, remained low in most vaccinated chicks and did not persist up to 18 days p.v. These results suggest that the vaccinal virus strain is clinically attenuated but persists in the thymus and spleen in some birds, inducing a low humoral immune response and altering thymopoiesis.
Introduction
Chicken infectious anaemia virus (CIAV) was first isolated by Yuasa et al. (Citation1983). The virus is a Gyrovirus belonging to the family Circoviridae with a circular single-stranded DNA genome (Gelderblom et al., Citation1989). The genome encodes for three viral proteins designated as VP1, VP2 and VP3. These viral proteins are expressed in the infected cells, whereas only VP1—which is the capsid polyprotein—is present in the purified virus particles (Todd et al., Citation1990). The disease is transmitted vertically and horizontally (Chettle et al., Citation1989; Hoop, Citation1992). It is characterized by aplastic anaemia, heterophil decrease, generalized lymphoid atrophy, skin lesions, haemorrhages, immunosuppression, enhancement of the pathogenicity of secondary infectious agents, suboptimal antibody responses and mortality in chicks younger than 3 weeks old (Taniguchi et al., Citation1982; Goryo et al., Citation1985; McNulty et al., Citation1988; Vielitz & Landgraf, Citation1988; Jeurissen et al., Citation1992; Otaki et al., Citation1992). In younger chicks, extensive lesions occurred in thymus and bone marrow between 10 and 17 days post infection (p.i.) (Kuscu & Gurel, Citation2008).
The clinical or subclinical features result from virus replication and apoptosis of haemacytoblasts in bone marrow and T-cell precursors in the thymus of infected chicks leading to the anaemia, intramuscular haemorrhages and granulocytopaenia, the reduction in size of the thymic cortex and the immunosuppression (Noteborn et al., Citation1994; Noteborn, Citation2004; Kuscu & Gurel, Citation2008). In the thymus, it has been shown that the virus replication and cell destruction occur in immature cortical lymphocytes (Jeurissen et al., Citation1989; McNeilly et al., Citation1991).
It has been reported that CIAV infection either destroys cells expressing CD4, CD8, and CT1 molecules on their surface or interferes with the expression of these molecules on thymic cells (Hu et al., Citation1993a, Citationb). In addition to the infection of precursor T cells in the thymus, Adair et al. (Citation1993) demonstrated that mature T lymphocytes in the spleen are also affected by CIAV. In many experimental studies, a greater destruction of CD8+ cells than CD4+ cells was observed (Cloud et al., Citation1992; Adair et al., Citation1993), while in some other studies no selective decrease in cytotoxic T lymphocytes (CTL) was detected by flow cytometric analysis of CD4+ and CD8+ subpopulations (Hu et al., Citation1993b). However, impairment of CTL activity has also been reported (Cloud et al., Citation1992; Bounos et al., Citation1995). VP3 gene presence in CIAV-infected cells is shown to induce apoptosis in chicken lymphoblastoid T cells and myeloid cells, which are susceptible to the infection, but not in chicken embryo fibroblasts, which are not susceptible to CIAV (Noteborn et al., Citation1994).). The B cells are not susceptible to the infection (Markowski-Grimsrud & Schat, Citation2001). After infection with CIAV, antibodies are produced in immunologically mature chickens that prevent lesion development. Therefore, the age-related resistance to CIAV is antibody mediated since older birds can develop lesions or persistent viraemia when the antibody system is compromised by embryonal bursectomy (Hu et al., Citation1993a). In addition, it was proposed that CIAV can persist as a latent virus in spite of occurrence of neutralizing antibodies (McNulty, Citation1991; Miller & Schat, Citation2004).
Considering the ubiquitous and contagious nature of CIAV, some attenuated viral strains have been studied or used as vaccine strains. It was demonstrated that attenuated viral strains may induce lower T-cell depletion in the thymus and reduced severity of lesions in 1-day-old chicks than those induced by a pathogenic viral strain (McKenna et al., Citation2003). However, the current vaccines cannot be used for younger birds because of the age-dependent susceptibility to CIAV (Miller & Schat, Citation2004). No information is available on viral persistency of vaccinal strains in younger birds.
In the present study, the viral persistency of CIAV-VAC® vaccine virus (Intervet, Millsboro, Delaware, USA) and T-cell disorders were investigated in 1-day-old specific pathogen free (SPF) chicks.
Materials and Methods
Chicken and experimental design. Embryonated SPF eggs were obtained from the Veterinary Laboratories Agency (Nepean, Ontario, Canada) and incubated, hatched and reared in the Faculty of Veterinary Medicine facilities (St-Hyacinthe, Québec, Canada). All procedures were approved by the Université de Montréal animal care committee. Thirty-six 1-day-old SPF chicks were divided in two groups and housed separately in isolators for chickens under sterile condition in room under negative pressure. Eighteen chicks of the vaccinated group received intraperitoneally (i.p.) 5 µl CIA vaccine (CIAV-VAC®) while 18 chicks in the control group were inoculated with phosphate-buffered saline. At 7, 14 and 28 days post vaccination (p.v.), six chicks from each group were weighed, blood-sampled by cardiac puncture and euthanized by CO2 chamber. Eighteen more chicks were vaccinated by i.p. injection, as indicated above, kept under the same conditions and euthanized at 18, 21 and 28 days p.v. for additional antibody assay and virus genome detection by polymerase chain reaction (PCR).
Sampling and cell extraction
The blood samples were collected directly into heparinized microhaematocrit tubes for packed cell volume (PCV) determinations and also for white blood cell counts (WBC) and differential analysis. The thymus, spleen, bone marrow and bursa were collected under sterile conditions and subjected to the lymphocyte extraction procedure. Samples of sera and caecal tonsils were also collected and kept frozen until tested. Isolation of lymphocytes from the spleen, thymus and bursa was conducted by mincing each tissue into fragments in RPMI 1640 media (GIBCO Laboratories, Grand Island, New York, USA) supplemented with 20% foetal bovine serum (FBS) and antibiotic-antimycotic solution (GIBCO Laboratories), and then pushing it through a 70 µm cell strainer (Falcon Scientific Co., Montreal, Québec, Canada). Lymphocytes from the spleen and thymus cell suspensions were further enriched by centrifugation at 1000×g for 20 min on a Lymphoprep gradient (Cedarlane, Hornby, Ontario, Canada). The recovered lymphocyte layer from the spleen and thymus and the original cell suspension from bursa samples were washed in fresh media by centrifugation at 500×g for 10 min. Bone marrow cells were collected from the femur in RPMI 1640 with 5% FBS after cutting the two epiphyses and pushing the media through the medular cavity using a 1-ml insulin syringe. The suspensions were placed on an FBS cushion and incubated on ice for 10 min to remove the debris. The top layer from each bone marrow cell suspension was recovered in a fresh tube. The cell suspensions of the thymus, spleen, bursa and bone marrow suspensions have been enumerated in a haemacytometer with trypan blue (Fischer Scientific, Montréal, Québec, Canada), adjusted at 106 viable cells per 1 ml and used for different assays.
Haematology
Peripheral leukocyte analyses such as PCV, WBC and differential percentages of heterophils, monocytes, lymphocytes, eosinophils, and basophils were performed by May–Grunwald staining and light microscopic examination.
Immunolabelling of lymphocyte subsets
The phenotype of lymphocyte subpopulations such as CD4+CD8−, CD4−CD8+, CD4+CD8+, CD3−CD8+, CD3−IgM+, CD3+TCRγδ+ cells was determined by double-immunolabelling (CD4 and CD8, CD3 and CD8, CD3 and IgM, or CD3 and TCRγδ markers) using fluorescein isothiocyanate (FITC)-conjugated anti-CD4, anti-IgM, anti-CD3 or anti-TCRγδ, and phycoerythrine (PE)-conjugated anti-CD8a or anti-CD3 monoclonal antibodies (Southern Biotech, Anaheim, California, USA). For double-staining, 1×106 cells from thymic, splenic or bursal lymphocyte suspensions were incubated with 1 µg anti-chicken monoclonal antibodies labelled with FITC or PE for 30 min at 4°C. Cells were then washed gently three times in RPMI 1640 and cells were fixed overnight at 4°C in phosphate-buffered saline, pH 7.2, containing 1% formaldehyde (Fischer Scientific). Cytofluorometric analysis of positive FITC-stained and PE-stained cells was performed on a FACScan cytofluorometer (Becton Dickinson, Mountain View, California, USA) using CellQuest software (Becton Dickinson, San Jose, California, USA). Analysis was done on 10,000 events and discrete viable lymphoid cell populations were gated according to forward scatter versus 90° angle scatter parameters. Percentages of different lymphoid cell subpopulations in the thymus, spleen and bursa were determined by multiparametric analysis.
Viral VP3 DNA of CIAV detection by nested PCR
Total DNA was extracted from the thymus, spleen and bursa, caecal tonsils, bone marrow and liver using Trizol_LS Reagent (Life Technologies, Grand Island, New York, USA) according to the manufacturer's procedure. Bursa tissues were homogenized by beads previous to Trizol extraction. To detect the viral genome in the samples, fragments of 374 base pairs (bp) situated between nucleotides 472 and 846, and of 203 bp situated between nucleotides 588 and 791, were targeted to be amplified in conventional and nested PCR, respectively. A set of primers were designed and used as follow: forward (5′-CTCTCCAAGAAGATACTCCAC-3′), reverse (5′-GCTCGTCTTGCCATCTTA-3′), forward nested (5′-ATCACTCTATCGCTGTGTGG-3′) and reverse nested (5′-GGAGTAGTGGTAATCAAGC-3′). The PCR program consisted of an initial denaturation at 94°C for 5 min, and 35 cycles of 94°C for 35 sec, 58°C for 55 sec, and 72°C for 1 min followed by a final extension at 72°C for 5 min. PCR products were analysed by electrophoresis on 1.4% agarose gel in TAE buffer (40 mM Tris and 2 mM ethylenediamine tetraacetic acid, pH 8.0) containing 0.5 mg/ml ethidium bromide for 60 min at 100 V and visualized under an ultraviolet light transilluminator.
Specific anti-CIAV antibodies
Specific anti-CIAV antibodies were quantified in serum by enzyme-linked immunosorbent assay (ELISA) using the IDEXX FlockChek* CIAV test Kit (IDEXX Laboratories, Inc., Westbrook, Massachusetts, USA) according to the manufacturer's procedure.
Statistical analysis
The percentages of blood cells and lymphocytes subsets in lymphoid organs from CIAV-vaccinated young birds were compared with those from sham birds using Student's t test. Values of P≤0.05 were considered significant.
Results
Haematologic evaluation of CIAV-vaccinated SPF chicks
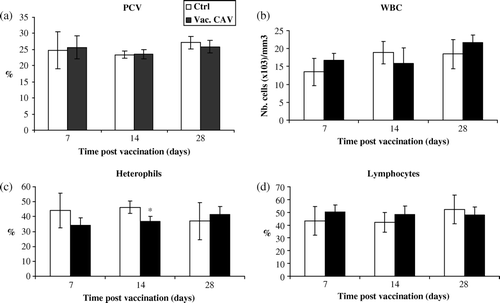
Thirty-six 1-day-old CIAV-vaccinated SPF birds or controls were euthanized at 7, 14 and 28 days p.v. Birds in both the CIAV-vaccinated and control groups did not show any clinical signs or anaemia following vaccination. No weight losses were observed in the vaccinated and control groups. No thymic atrophy, as revealed by gross examination, was observed in vaccinated chicks at various times. The PCV, WBC and percentages of lymphocytes in blood of CIAV-vaccinated birds did not show any significant variations up to 28 days p.v. when compared with chicks in the control group (a,b,d). However, the percentage of blood heterophils slightly decreased at 14 days p.v. only in vaccinated chicks (P<0.05) (c). Bone marrow cell numbers were not altered in these chicks (results not shown).
Figure 1. Haematological examination of blood from CIAV-vaccinated SPF chicks at hatch. (1a) Haematocrit (PCV), (1b) WBC, (1c) percentages of heterophils and (1d) lymphocytes in the blood of CIAV-vaccinated birds (▪) and control birds (□) were determined at 7, 14 and 28 days p.v. The mean of each value for vaccinated and control birds (n = 6) was calculated and compared. *P≤0.05.
Persistency of CIAV vaccinal strain in lymphoid organs
The presence of the viral VP3 gene of the CIAV vaccinal strain was detected by PCR in the thymus, spleen, bursa, and caecal tonsils. As shown in , viral VP3 gene was expressed in the thymus of five (out of six) vaccinated birds at day 7 p.v. There were no viral VP3-positive cases in the thymus samples at 14, 18 and 21 days p.v., whereas the presence of viral genome in the thymus was revealed at 28 days p.v. in two of the 12 tested vaccinated chicks. In the spleen, the vaccine virus was detected in one chick at 7 days p.v. and persisted in some vaccinated birds until 28 days p.v. All other specimens collected from the bursa and caecal tonsils remained negative for viral DNA. In addition, the bone marrow, sera and liver were also negative in the nested PCR (results not shown).
Table 1. Detection of CIAV vaccinal virus genome in the thymus, spleen, bursa of Fabricius and caecal tonsils in CIAV-vaccinated and control groups of chicks at 7 to 28 days p.v.
Analysis of thymic cell subpopulations
To verify whether the viral persistency of the CIAV genome of the vaccinal strain in the thymus induces disorders in lymphoid cell populations, the percentages of the different lymphoid subsets in the thymus from CIAV-vaccinated chicks were compared with those from control group birds at various times p.v. The lymphoid cells were isolated from the thymus and double-immunolabelled with fluorescent antibodies against CD4, CD8, CD3, TCRγδ and IgM, and were analysed using cytofluorometry by comparing the co-expression of markers among the gated cells.
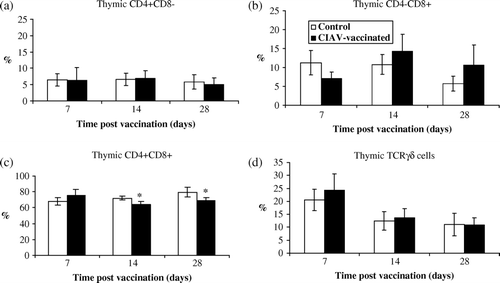
As shown in , percentages of thymic CD4+CD8−, CD4–CD8+ or TCRγδ+ cells were not altered in vaccinated chicks and the values were similar to those from control birds (a,b,d). The percentages of CD4+CD8+ thymic cells, however, slightly decreased after 14 and 28 days in the CIAV-vaccinated group (P<0.05)(c). No significant modification in the percentages of thymic CD3–CD8+, corresponding to natural killer (NK) cells, was detected in vaccinated chicks (results not shown).
Figure 2. Percentages of lymphocyte subpopulations in thymus of SPF chicks at 7, 14 and 28 days following CIAV vaccination at hatch. Thymocytes from CIAV-vaccinated (▪) and control birds (□) were double-labelled with anti-CD4, anti-CD8, anti-TCRγδ conjugated to FITC or PE and analysed by cytofluorometry. The mean percentage of thymic (2a) CD4+CD8–, (2b) CD4–CD8+, (2c) CD4+CD8+ and (2d) TCRγ▵+ subpopulations for CIAV-vaccinated and control groups (n=6) were calculated and compared. *P≤0.05.
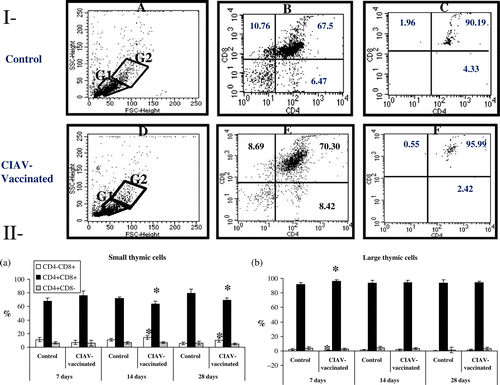
To verify whether the thymopoiesis was altered by CIAV vaccination, thymocytes were analysed according to forward scatter/90° angle scatter parameters. Thymocytes were separated into smaller (G1) and larger (G2) cells, as shown in . The G1/G2 ratio of thymic cells did not significantly differ between CIAV-vaccinated or control birds (results not shown). The multiparametric analysis of cells within the G1 region revealed the presence of CD4+CD8−, CD4+CD8+ and CD4−CD8+ cell subsets (Ib,e), whereas the cells located in the G2 area were mostly double-positive (CD4+CD8+) (Ic,f). Percentages of small CD4+CD8+ cells (in the G1 area) decreased at 14 and 28 days p.v. in the thymus of CIAV-vaccinated birds (P<0.05) (IIa), whereas the large CD4+CD8+ cells increased only at 7 days p.v. (P<0.05) (IIb). Percentages of small CD4−CD8+ cells increased at 14 and 28 days p.v. in CIAV-vaccinated chicks (P<0.05) (IIa). Large CD4+CD8− cells transiently decreased at 7 days p.v. (P<0.05) (IIb).
Figure 3. Analysis of small and large lymphocyte subpopulations in thymus of SPF chicks at 7, 14 and 28 days following CIAV vaccination at hatch. Thymocytes from CIAV-vaccinated (▪) and control birds (□) were double-labelled with anti-CD4, anti-CD8, anti-TCRγδ conjugated to FITC or PE and analysed by cytofluorometry. (3I) Thymocytes were separated into small (G1 area) and large (G2 area) cells according to forward scatter/90° angle scatter parameters: (3Ia) control and (3Id) CIAV-vaccinated groups of birds; multiparametric analysis of CD4/CD8 (3Ib, 3Ie) small and (3Ic, 3If) larger thymocytes. (3II) Percentages of (3IIa) small and (3IIb) large cell subpopulations in the thymus from control and CIAV-vaccinated groups at various days p.v. *P≤0.05.
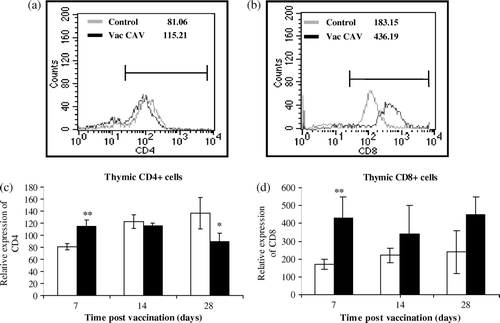
The intensity of CD4 or CD8 expression in thymocytes may reflect their maturation level. Cytofluorometric analysis of CD4 and CD8 expression on thymocytes is presented in . It revealed that the CD4 expression on thymocytes increased at 7 days p.v. (P<0.001) while it decreased at 28 days p.v. (P < 0.05) (a,c). The CD8 expression on the thymocyte surface, however, strongly increased in the CIAV-vaccinated group at 7 days p.v. (P<0.01) and remained variable until 28 days p.v. in the group of CIAV-vaccinated chicks (b,d).
Figure 4. Analysis of relative expression of CD4 and CD8 molecules on thymocytes from CIAV-vaccinated and control groups at various times p.v. Lines illustrate the intensity of (4a) CD4 or (4b) CD8 molecules on thymocytes of one control bird and one CIAV-vaccinated bird. Thymocytes from CIAV-vaccinated (▪) and control birds (□) were double-labelled with anti-CD4 and anti-CD8, conjugated to FITC and PE, and analysed by cytofluorometry. The relative expression levels of (4c) CD4 and (4d) CD8 were compared on thymocytes from each group (n=6) of birds at 7, 14 and 28 days p.v. *P≤0.05,**P≤0.01.
Analysis of splenic and bursal lymphocyte subpopulations
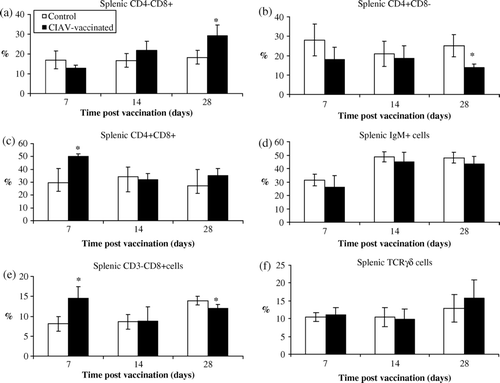
In the spleen, the percentage of CD4–CD8+ cells increased at 28 days p.v. only (P<0.05) (a) while CD4+CD8– cells decreased simultaneously (P<0.05) (b) in CIAV-vaccinated chicks. The subpopulation of CD4+CD8+ spleen cells exhibited a paramount elevation only 7 days after vaccination (P<0.05) (c). The splenic IgM+ B cells as well as TCRγδ cells were not significantly altered following CIAV vaccination (d,f). The vaccinated chicks, however, exhibited a higher number of CD3–CD8+ cells (corresponding to NK cells) in the spleen at 7 days p.v. (P<0.05) (e). Nevertheless, at 28 days p.v., the number of NK cells increased less in CIAV-vaccinated birds than that of the control group (P<0.05) (e) in spite of the fact that NK cells increased in older control birds.
Figure 5. Percentages of lymphocyte subpopulations in the spleen of SPF chicks at 7, 14 and 28 days following CIAV vaccination at hatch. Splenic cells from CIAV-vaccinated (▪) and control birds (□) were double-labelled with anti-CD4, anti-CD8, anti-TCRγδ, anti-IgM, and anti-CD3 conjugated to FITC or PE and analysed by cytofluorometry. The mean percentage of splenic (5a) CD4–CD8+, (5b) CD4+CD8–, (5c) CD4+CD8+, (5d) IgM+, (5e) CD3–CD8+, and (5f) TCRγ▵+ subpopulations for CIAV-vaccinated and control groups (n=6) were calculated and compared. *P≤0.05.
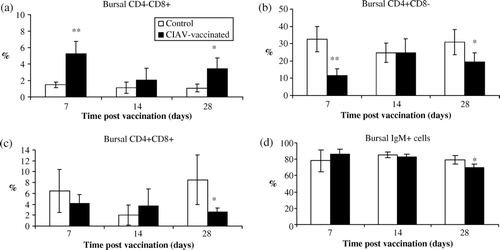
In the bursa of Fabricius, percentages of CD4–CD8+ cells increased at 7 and 28 days p.v. (P<0.05) (a) whereas percentages of CD4+CD8– cells simultaneously decreased (P<0.05) (b). The number of double-positive (CD4+CD8+) bursal cells were slightly lowered in the CIAV-vaccinated group only at 28 days p.v. (P<0.05) (c). Percentages of IgM+ bursal lymphocytes were relatively steady until day 28, when the percentage of these cells was lower in CIAV-vaccinated chicks (P<0.05) (d).
Figure 6. Percentages of lymphocyte subpopulations in the bursa of Fabricius of SPF chicks at 7, 14 and 28 days following CIAV vaccination at hatch. Lymphoid cells from CIAV-vaccinated (▪) and control birds (□) were double-labelled with anti-CD4, anti-CD8, anti-IgM conjugated to FITC or PE and analysed by cytofluorometry. The mean percentage of bursal (6a) CD4–CD8+, (6b) CD4+CD8–, (6c) CD4+CD8+ and (6d) IgM+ subpopulations for CIAV-vaccinated and control groups (n = 6) were calculated and compared. *P≤0.05, **P≤0.01.
Humoral immune response
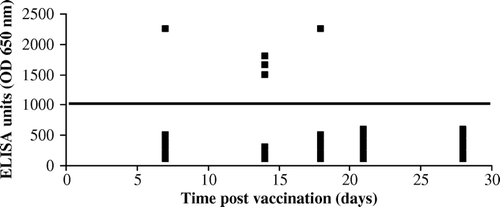
The efficiency of the vaccine virus to induce anti-CIAV antibodies in 1-day-old SPF chicks was monitored using ELISA. As shown in , vaccination with CIAV-VAC® did not produce a notable humoral response in the majority of the vaccinated chicks when administered at hatch. Among the 30 vaccinated birds sampled at five different times p.i., the anti-CIAV titres were detected in one chick, three chicks and one chick at 7, 14 and 18 days p.i., respectively. The anti-CIAV antibodies did not persist up to 18 days p.v. in seropositive chicks.
Figure 7. Anti-CIAV antibody titres after vaccination of 1-day-old SPF chicks by CIAV-VAC®. Serum samples from the CIAV-vaccinated and control groups were tested for anti-CIAV antibodies at 7, 14, 18, 21 and 28 days p.v. by ELISA test. Each point represents the ELISA titre of one chick tested. The line corresponds to the negative threshold.
Discussion
In the present work, we report that a commercial CIAV vaccinal strain induces a subclinical infection in 1-day-old chicks associated with viral persistence in the spleen and thymus, transient humoral response and alterations in thymopoiesis.
Clinical signs of CIAV infection in very young chicken are characterized by anorexia, depression, pallor, and decrease of weight gain, haematocrit and WBC, especially blood lymphocytes and heterophils. Pale bone marrow and atrophy of the thymus, spleen and bursal reflect depletion of T cells and B cells, predisposing the birds to secondary infections (reviewed in Todd, Citation2004). CIAV infection in 1-day-old SPF chicks rendered them anaemic with depleted bone marrow and thymus at 14 days p.i. (Yuasa & Imai, Citation1986).
The attenuated CIAV strains are commercially used as vaccines but some technical and practical problems affect the widespread use of these vaccines (reviewed in Schat, Citation2009). In the present work, we addressed whether a commercial vaccinal strain may retain the ability to induce disorders in percentages of thymus and spleen cells and on thymopoiesis as the pathogenic CIAV strain. We have shown that a commercial attenuated vaccine (CIAV-VAC®) did not induce clinical signs in 1-day-old chicks, did not significantly decrease total WBC in spite of low significant decrease of heterophil percentage at 14 days only, and induced no significant cell depletion in the thymus, spleen, bursa and bone marrow. The transient decrease in the percentage of heterophils may reflect either cell recruitment in infected organs or a low decrease in myelopoiesis. However, the absence of anaemia and no bone marrow cell depletion do not support the second hypothesis. These observations are in agreement with those reported by McKenna et al. (Citation2003), who have shown that some attenuated CIAV strains may induce subclinical infection with no anaemia and no or low mild lesions.
Replication of the vaccinal viral strain tested in our work was detected in the thymus in most of vaccinated chicks, at 7 days p.v. only, indicating that viral replication was rapidly aborted in the thymus, reflecting the attenuation of this viral strain. Kaffashi et al. (Citation2006) have demonstrated that pathogenic CIAV can replicate in many organs, both in 1-day-old and 6-week-old infected chicks, up to day 18 p.i., and reach a peak in the thymus, spleen and liver at 18 or 20 days p.i. At 28 days p.v., however, the VP3 gene was still observed in the thymus of some birds, indicating viral persistence. Hu et al. (Citation1993a) showed that persistent viraemia occurs in CIAV-infected birds in the absence of antibody production. The viral persistence of pathogenic CIAV was confirmed by Imai et al. (Citation1999), who suggested that CIAV can induce persistent infection in infected birds. The detection of the VP3 gene in total DNA cannot permit one to clearly distinguish between viral replication or integrated viral DNA in cellular genome. However, porcine circoviruses can persist in infected cells but they are not endogenous as retroviruses (Victoria et al., Citation2010).
The replication of vaccinal strain in the thymus and its persistence in some birds suggest the occurrence of thymopoiesis disorders, as previously reported in the thymus of pathogenic CIAV-infected birds (Jeurissen et al., Citation1989; Kuscu & Gurel, Citation2008). The cytofluorometric analysis of thymocyte subsets from CIAV-vaccinated birds revealed that larger CD4+CD8+ thymic cells transiently increased but that was followed by a relative decrease of CD4+CD8+ cells in spite of no significant decrease in total thymic cells. This observation suggests that replication of vaccinal strain slightly alters the thymocyte maturation process or that mature antiviral CD4+CD8– and CD4–CD8+ cells are recruited into the thymus from the blood and spleen. The absence of significant changes in percentages of CD4+CD8– or TCRγδ cells and the slight increase of CD4–CD8+ cells do not support the second hypothesis. On the other hand, the discrimination between dividing cells and resting cells by the forward scatter/90° angle scatter parameters revealed that smaller thymic CD4+CD8+ cells were depleted in vaccinated chicks, suggesting that viral infection can block cell mitosis. Infection of the T-cell precursor in the thymus and inhibition of anaphase-promoting complex/cyclosome by the viral apoptin, which leads to G2/M arrest and apoptosis, has already been reported in pathogenic CIAV infection (Teodoro et al., Citation2004).
The analysis of the expression intensity of CD4 and CD8 markers revealed that CD4 expression, but not CD8 expression, was diminished in thymocytes—suggesting that a vaccinal strain of CIAV may specifically interfere with CD4+ expression, leading to a decrease in percentage of small CD4+CD8+ cells. The specific decrease of CD4 expression on thymocytes by CIAV viral infection has never been reported. However, the T-helper subset is known to be a target cell in the spleen for CIAV (Adair et al., Citation1993). Hu et al. (Citation1993b) demonstrated that percentages of both CD4+ and CD8+ thymic cells similarly decreased in the thymus of 1-day-old chicks infected with the pathogenic CIAV (CIA-1 strain) due to cell destruction by viral infection or interference with CD4+ and CD8+ expression. The CD4+CD8+ cell subset, however, represents the most important population in the thymus, and a decrease of CD4+ cells would involve also a decrease in CD8+ cells. The decrease of CD4 expression only in thymic cells induced by the attenuated vaccinal CIAV strain rather revealed a discrimination between the thymocyte subsets expressing CD4 and/or CD8. It is well known that the thymic CD4–CD8+ cell subset occurs earlier than the CD4+CD8– or CD4+CD8+ cell subsets during the avian thymopoiesis (Davidson & Boyd, Citation1992). As a result, the lower expression of CD4+ in presence of higher expression of CD8 may reflect the accumulation of CD4–CD8+ cells rather than a differential decrease in CD4+-expressing thymocytes. The decreases in CD4+CD8+ cells observed at 14 and 28 days p.v. indicate that thymocyte precursors can support the replication of the CIAV vaccinal strain, as confirmed by VP3 gene presence in the thymus of some birds at 28 days p.v., such as seen with pathogenic CIAV (reviewed in Schat, Citation2009).
Contrary to that seen in the thymus, the VP3 gene was gradually expressed in the spleen of vaccinated 1-day-old chicks with time. The percentage of splenic CD4–CD8+ cells increased while CD4+CD8– cells decreased at 28 days p.v. only, such as seen in the thymus, suggesting the occurrence of a delayed CTL immune response. However, the CTL responses may not lead to viral elimination. Markowski-Grimsrud & Schat (Citation2003) have demonstrated that CIAV-infected chicks without anti-CIAV maternal antibodies have lost the ability to mount a CTL response at 7 days p.i. against secondary viral infections, suggesting that CIAV can impair the CTL functions. Adair et al. (Citation1991) have shown that interferon-γ production decreased after up to 43 days p.i. in CIAV infection, indicating suppression of CTL responses. We have not observed any increase in the interferon-γ production in CIAV-vaccinated chicks (results not shown), suggesting that CD8+-dependent cytotoxic function is not strongly stimulated by viral vaccination. In addition, no significant stimulation of lymphocytes to Concanavalin A (ConA) has been detected in thymic or splenic cells from CIAV-vaccinated chicks (results not shown), supporting the hypothesis that vaccination did not strongly stimulate lymphocyte activities. Depressed responsiveness of lymphocytes to mitogens following CIAV infection has been well documented (Otaki et al., Citation1988; Adair et al., Citation1991; McConnell et al., Citation1993; Bounous et al., Citation1995).
Transient increase of CD3–CD8+ (NK cells) suggests the occurrence of an innate antiviral immune response. It was previously demonstrated that pathogenic CIAV did not alter the NK cell activity (Markowsi-Grimsrud & Schat, 2001).
Finally, the percentage increases of CD4–CD8+ cells in the bursa of Fabricius correlated with concurrent decreases of CD4+CD8– cells, suggesting that these modifications reflect the thymus disorders rather than a specific viral replication. No VP3 gene was detected in the bursa or caecal tonsils. However, the late decrease of IgM+ cells and low and transient humoral immune responses following the CIAV-VAC® administration suggest disorders in B-cell-dependent immunity. It is well known that the infection with pathogenic CIAV strain triggers antibody production in immunologically mature chickens, which mediates the age-related resistance to the virus. It was previously reported that pathogenic CIAV was recovered from blood cells or lymphoid organs of infected birds at different days p.i. even in the presence of low or high viral-neutralizing antibodies (Yuasa et al., Citation1983; Imai et al., Citation1999). We can hypothesize that the incompetent immune system of the very young chicks favours a decrease of the CD4+ cells in the thymus and spleen, a decrease in the percentage of IgM+ B cells, low antibody response and persistence of vaccinal strain in the thymus and spleen.
The virus persistence and the lymphoid disorders induced by the CIAV vaccine virus in very young birds lead to practical consequences and may potentially play an important role in the subclinical infections and decreased responsiveness to other avian pathogens in the poultry industry.
Work is in progress to determine the effects of infection with vaccinal CIAV strain on the efficiency of bursal viral disease vaccination in young chicks.
References
- Adair , B.M. , McNeilly , F. , McConnell , C.D. and McNulty , M.S. 1993 . Characterization of surface markers present on cells infected by chicken anemia virus in experimentally infected chickens . Avian Diseases , 37 : 943 – 950 .
- Adair , B.M. , McNeilly , F. , McConnell , C.D.G. , Todd , D. , Nelson , R.T. and McNulty , M.S. 1991 . Effects of chicken anemia agent on lymphokine production and lymphocyte transformation in experimentally infected chickens . Avian Diseases , 35 : 783 – 792 .
- Bounous , D.I. , Goodwin , M.A. , Brooks , R.L. , Lamichhane , C.M. , Campagnoli , R.P. , Brown , J. and Snyder , D.B. 1995 . Immunosuppression and intracellular calcium signaling in splenocytes from chicks infected with chicken anemia virus, CL-1 isolate . Avian Diseases , 39 : 135 – 140 .
- Chettle , N.J. , Eddy , R.K. , Wyeth , P.J. and Lister , S.A. 1989 . An outbreak of disease due to chicken anaemia agent in broiler chickens in England . The Veterinary Record , 124 : 211 – 215 .
- Cloud , S.S. , Lillehoj , H.S. and Rosenberger , J.K. 1992 . Immune dysfunction following infection with chicken anemia agent and infectious bursal disease virus. I. Kinetic alterations of avian lymphocyte subpopulations . Veterinary Immunology & Immunopathology , 34 : 337 – 352 .
- Davidson , N.J. and Boyd , R.L. 1992 . Delineation of chicken thymocytes by CD3–TCR complex, CD4 and CD8 antigen expression reveals phylogenically conserved and novel thymocyte subsets . International Immunology , 4 : 1175 – 1182 .
- Gelderblom , H. , Kling , S. , Lurz , R. , Tiseher , I. and Von Bulow , V. 1989 . Morphological characterization of chicken anaemia agent (CAA) . Archives of Virology , 109 : 115 – 120 .
- Goryo , M. , Sugimura , H. , Matsumoto , S. , Umemura , T. and Itakura , C. 1985 . Isolation of an agent inducing chicken anaemia . Avian Pathology , 14 : 483 – 496 .
- Hoop , R.K. 1992 . Persistence and vertical transmission of chicken anaemia agent in experimentally infected laying hens . Avian Pathology , 21 : 493 – 501 .
- Hu , L.B. , Lucio , B. and Schat , K.A. 1993a . Abrogation of age-related resistance to chicken infectious anemia by embryonal bursectomy . Avian Diseases , 37 : 157 – 169 .
- Hu , L.B. , Lucio , B. and Schat , K.A. 1993b . Depletion of CD4+ and CD8+ T lymphocyte subpopulations by CIA-1, a chicken infectious anemia virus . Avian Diseases , 37 : 492 – 500 .
- Imai , K. , Mase , M. , Tsukamoto , K. , Hihara , H. and Yuasa , N. 1999 . Persistent infection with chicken anaemia virus and some effects of highly virulent infectious bursal disease virus infection on its persistency . Research in Veterinary Science , 67 : 233 – 238 .
- Jeurissen , S.H. , Janse , M.E. , Van Roozelaar , D.J. , Koch , G. & De Boer , G.F. 1992 . Susceptibility of thymocytes for infection by chicken anemia virus is related to pre- and posthatching development . Developmental Immunology , 2 , 123 – 129 .
- Jeurissen , S.H. , Pol , J.M. and De Boer , G.F. 1989 . Transient depletion of cortical thymocytes induced by chicken anaemia agent . Thymus , 14 : 115 – 123 .
- Kaffashi , A. , Noormohammadi , A.J. , Allott , M.L. and Browning , G.F. 2006 . Virus load in 1-day-old and 6-week-old chickens infected with chicken anaemia virus by the intraocular route . Avian Pathology , 35 : 471 – 474 .
- Kuscu , B. and Gurel , A. 2008 . Lesions in the thymus and bone marrow in chicks with experimentally induced chicken infectious anemia disease . Journal of Veterinary Science , 9 : 15 – 23 .
- Markowski-Grimsrud , C.J. & Schat , K.A. 2001 . Impairment of cell-mediated immune responses during chicken infectious anemia virus infection . In Proceedings of the 2nd International Symposium on Infectious Bursal Disease and Chicken Infectious Anaemia (pp. 395 – 402 ). Rauischholzhausen , Germany .
- Markowski-Grimsrud , C.J. and Schat , K.A. 2003 . Infection with chicken anemia virus impairs the generation of antigen-specific cytotoxic T lymphocytes . Immunology , 109 : 283 – 294 .
- McConnell , C.D. , Adair , B.M. & McNulty , M.S. 1993 . Effects of chicken anemia virus on cell-mediated immune function in chickens exposed to the virus by a natural route . Avian Diseases , 37 , 366 – 374 .
- McKenna , G.F. , Todd , D. , Borghmans , B.J. , Welsh , M.D. and Adair , B.M. 2003 . Immunopathologic investigations with an attenuated chicken anemia virus in day-old chickens . Avian Diseases , 47 : 1339 – 1345 .
- McNeilly , F. , Allan , G.M. , Moffat , D.A. and McNulty , M.S. 1991 . Detection of chicken anaemia agent in chickens by immunofluorescence and immunoperoxidase staining . Avian Pathology , 20 : 125 – 132 .
- McNulty , M.S. 1991 . Chicken anemia agent: a review . Avian Pathology , 20 : 187 – 203 .
- McNulty , M.S. , Connor , T.J. , Mcneilly , F. , Kirkpatrick , K.S. and Mcferran , J.B. 1988 . A serological survey of domestic poultry in the United Kingdom for antibody to chicken anaemia agent . Avian Pathology , 17 : 315 – 324 .
- Miller , M.M. and Schat , K.A. 2004 . Chicken infectious anemia virus: an example of the ultimate host-parasite relationship . Avian Diseases , 48 : 734 – 745 .
- Noteborn , M.H. 2004 . Chicken anemia virus induced apoptosis: underlying molecular mechanisms . Veterinary Microbiology , 98 : 89 – 94 .
- Noteborn , M.H. , Todd , D. , Verschueren , C.A. , de Gauw , H.W. , Curran , W.L. Veldkamp , S. 1994 . A single chicken anemia virus protein induces apoptosis . Journal of Virology , 68 : 346 – 351 .
- Otaki , Y. , Nunoya , T. , Tajima , M. , Kato , A. and Nomura , Y. 1988 . Depression of vaccinal immunity to Marek's disease by infection with chicken anaemia agent . Avian Pathology , 17 : 333 – 347 .
- Otaki , Y. , Saito , K. , Tajima , M. and Nomura , Y. 1992 . Persistence of maternal antibody to chicken anaemia agent and its effect on the susceptibility of young chickens . Avian Pathology , 21 : 147 – 151 .
- Schat , K.A. 2009 . Chicken anemia virus . Current Topics of Microbiology and Immunology , 331 : 151 – 183 .
- Taniguchi , T , Yuasa , N. , Maeda , M. and Horiuchi , T. 1982 . Hematopathological changes in dead and moribund chicks induced by chicken anemia agent . National Institute of Animal Health Quarterly (Tokyo) , 22 : 61 – 69 .
- Teodoro , J.G. , Heilman , D.W. , Parker , A.E. and Green , M.R. 2004 . The viral protein apoptin associates with the anaphase-promoting complex to induce G2/M arrest and apoptosis in the absence of p53 . Genes Development , 18 : 1952 – 1957 .
- Todd , D. 2004 . Avian circovirus diseases: lessons for the study of PMWS . Veterinary Microbiology , 98 : 169 – 174 .
- Todd , D. , Creelan , J.L. , Mackie , D.P. , Rixon , F. and McNulty , M.S. 1990 . Purification and biochemical characterisation of chicken anaemia agent . Journal of General Virology , 71 : 819 – 823 .
- Victoria , J.G. , Wang , C. , Jones , A.S. , Jaing , C. , McLoughlin , K. , Gardner , S. and Delwart , E.L. 2010 . Viral nucleic acids in live-attenuated vaccines: detection of minority variants and an adventitious virus . Journal of Virology , 84 : 6033 – 6040 .
- Vielitz , E. and Landgraf , H. 1988 . Anaemia-dermatitis of broilers: field observations on its occurence, transmission and prevention . Avian Pathology , 17 : 113 – 120 .
- Yuasa , N. and Imai , K. 1986 . Pathogenicity and antigenicity of eleven isolates of chicken anaemia agent (CAA) . Avian Pathology , 15 : 639 – 645 .
- Yuasa , N. , Taniguchi , T. , Imada , T. and Hihara , H. 1983 . Distribution of chicken anemia agent (CAV) and detection of neutralizing antibody in chicks experimentally inoculated with CAA . National Institute of Animal Health Quarterly (Tokyo) , 23 : 78 – 81 .