Abstract
The aim of the present study was to determine whether avian metapneumovirus (aMPV)-related viruses were present in wild and synanthropic birds in Brazil. Therefore, we analysed samples from wild birds, feral pigeons and domestic chickens in order to perform a phylogenetic comparison. To detect the presence of aMPV, a nested reverse transcriptase-polymerase chain reaction was performed with the aim of amplifying a fragment of 270 bases for subtype A and 330 bases for subtype B, comprising the gene coding the G glycoprotein. Positive samples for aMPV subtypes A and B were found in seven (13.2%) different asymptomatic wild birds and pigeons (50%) that had been received at the Bosque dos Jequitibás Zoo Triage Center, Brazil. Also analysed were positive samples from 15 (12.9%) domestic chickens with swollen head syndrome from several regions of Brazil. The positive samples from wild birds, pigeons and domestic chickens clustered in two major phylogenetic groups: some with aMPV subtype A and others with subtype B. The similarity of the G fragment nucleotide sequence of aMPV isolated from chickens and synanthropic and wild avian species ranged from 100 to 97.5% (from 100 to 92.5% for the amino acids). Some positive aMPV samples, which were obtained from wild birds classified in the Orders Psittaciformes, Anseriformes and Craciformes, clustered with subtype A, and others from the Anas and Dendrocygma genera (Anseriformes Order) with subtype B. The understanding of the epizootiology of aMPV is very important, especially if this involves the participation of non-domestic bird species, which would add complexity to their control on farms and to implementation of vaccination programmes for aMPV.
Introduction
Avian metapneumovirus (aMPV) belongs to the subfamily Pneumovirinae of the Paramyxoviridae family, in which four types (subtypes A, B, C and D) have been recognized based on genetic and serological properties (Juhasz & Easton, 1994; Bayon-Auboyer et al., Citation2000; Seal, Citation2000). Since the first detection in South Africa in 1978 (Buys & De Preez, Citation1980), aMPV has induced infections in turkeys worldwide resulting in an acute rhinotracheitis, characterized by coughing, nasal discharge and conjunctivitis (turkey rhinotracheitis). Moreover, aMPV has also been involved in the aetiology of multifactorial diseases such as swollen head syndrome (SHS) in chickens (McDougall & Cook, Citation1986; Pringle, Citation1999; van den Hoogen et al., Citation2001).
Isolates belonging to different subtypes of aMPV have been reported worldwide, the great majority being of subtypes A and B, and were reported in Israel (Banet-Noach et al., Citation2005), Mexico (Decanini et al., Citation1991), Jordan (Roussan et al., Citation2008), Brazil (Dani et al., Citation1999; D'Arce et al., Citation2005), Japan (Tanaka et al., Citation1995; Mase et al., Citation2003) and many European countries (Giraud & Bennejean, Citation1986; Lister & Alexander, Citation1986; McDougall & Cook, 1986; Wilding et al., Citation1986; Naylor & Jones, Citation1993; Naylor et al., Citation1997; Hafez et al., Citation2000). Subtype C (aMPV-C) was first detected in the USA in 1996 (Cook et al., Citation1999; Panigrahy et al., Citation2000; Seal, Citation1998, 2000) and is more closely related to the human metapneumovirus than to any other aMPV (Toquin et al., Citation2003; Yunus et al., Citation2003; Govidanrajan & Samal, Citation2005; Graaf et al., Citation2008). Finally, subtype D was reported once in France (Bayon-Auboyer et al., Citation1999; Toquin et al., Citation2000).
Live vaccines are widely used for the control of aMPV in turkey and chicken flocks and have been shown to provide good protection under experimental conditions. However, disease in vaccinated birds is still encountered and some of this remains unexplained (Banet-Noach et al., Citation2009). There is evidence that in some instances disease might be due to poor application of the vaccine, involvement of a virus subtype different to the vaccine (Naylor et al., 1997; Van de Zande et al., Citation1998), derivatives of the vaccine itself (Catelli et al., Citation2006) and, in other cases, to evolution of aMPV in the field, thus avoiding vaccine-induced immunity (Catelli et al., Citation2010).
A seasonal pattern in the occurrence of aMPV-C has been observed in the USA with a high incidence in spring and autumn (Shin et al., 2000). One of the reasons for the seasonality of aMPV-C was speculated to be the migratory pattern of wild birds that are involved in the transmission of the virus (Shin et al., 2000). In support of this, aMPV-C antibodies or viral RNA were detected in many wild avian species, including American coots, American crows, Canada geese, cattle egrets, rock pigeons, blue-winged teal, wild geese, wild ducks and mallard ducks (Shin et al., 2000, Citation2002; Bennett et al., 2002, Citation2004, Citation2005; Turpin et al., Citation2008; Velayudhan et al., Citation2008; Padhi & Poss, Citation2009). The ability of aMPV-C viruses to infect and replicate in ducks has also been reported, suggesting that isolates from poultry can infect this avian species (Shin et al., Citation2001). In Brazil, where only subtypes A and B have been detected, the role of wild and synanthropic birds in the epidemiology of SHS is not yet clear, nor is it known whether the aMPV A and B subtypes can be isolated from these avian species. This information could be of great importance for the control and prevention of disease in this and other countries.
The aim of the present study was to determine whether aMPV was present in wild and synanthropic birds in Brazil and to compare any virus detected with the aMPV subtypes A and B already detected in Brazilian chickens.
Materials and Methods
Samples from wild birds, feral pigeons and domestic chickens
Tracheal and cloacal swabs from 53 different wild, free-living species of bird and a total of 14 different pigeons were analysed. The wild avian species and feral pigeons were received at the Bosque dos Jequitibás Zoo Triage Center, Campinas City, São Paulo State, Brazil (22°90′S; 47°05′W). The samples were collected immediately after the arrival of the birds (in the quarantine period). Neither the wild birds nor the pigeons had signs of respiratory disease or SHS at the moment of collection, between 2005 and 2008. In addition, samples from commercial poultry were also analysed in order to perform a comparison between different groups of birds. Samples of tracheal swabs from 116 domestic chickens (Gallus gallus domesticus) with respiratory signs or SHS were obtained from the main poultry-producing regions of Brazil between 2008 and 2009. All these samples were tested in parallel for infectious bronchitis virus with a nested reverse transcriptase-polymerase chain reaction (RT-PCR) (Felippe et al., Citation2010).
Virus samples
Two aMPV vaccines used commercially in Brazil, one of subtype A (vaccine A) and one of subtype B (vaccine B), were obtained and submitted to the same RT-PCR and sequencing protocol as the samples from commercial chickens, wild birds and pigeons. The strains were propagated in the chicken embryo-related cell line (Coswig et al., Citation2010).
RNA extraction and viral nucleic acid amplification
Swabs from wild birds, pigeons and chickens were suspended in 1 ml of minimum essential medium (Sigma-Aldrich, St. Louis, Missouri, USA) and centrifuged for 5 min at 3,500 x g to sediment the cellular debris. RNA was extracted from the supernatant using the High Pure Viral Nucleic extraction kit™ (Roche Diagnostics, Manheim, Germany). cDNA was synthesized using the High Capacity cDNA kit (Applied Biosystems, Foster City, California, USA). Both procedures were performed according to the manufacturer's instructions for use with random hexamers. All samples were investigated for aMPV using amplification of specific genome fragments. The amplification of the G protein gene was the target for the PCR. The first step of the nested RT-PCR was carried out with the forward primer G1 (5′-gggacaagtatct/cc/at/g-3′) and the reverse primer G6 (5′ctgacaaattggtcctgatt-3′) (Juhasz & Easton, 1994; Cavanagh et al., 1999) corresponding to amplified products of 440 bp and was carried out with 50 µl mixture, containing 5 µl of 10x PCR buffer (10 mM Tris–HCl, pH 8.0, 50 mM KCl), 1.5 1, 5 µl MgCl2 (0.2 mM), 1.5 µl of a 10 mM dNTP mixture, 2.5 µl each primer (10 mM), 5 µl cDNA, and 0.2 µl (5 units/µl) Platinum™ DNA Polymerase (Invitrogen Ltd, California, Carlsbad, USA). In the amplification of the first PCR reaction, the cDNA was pre-heated for 2 min at 94°C, then 30 cycles of 30 s at 94°C (denaturation), 45 s at 58°C (annealing) and 1 min at 72°C (extension). After completion of the 30 cycles, a final extension of 7 min at 72°C was performed. The nested PCR for aMPV subtypes A and B was the same, the only difference being the annealing temperature (57°C for subtype A and 59°C for subtype B). A second nested PCR step was carried out from the amplified products of the template of the first round, using G8-A (5′-cactcactgttagcgtcata-3′) and G5 (5′-caaagaa/gccaataagccca-3′) primers for subtype A, and G8-B (5′-tagtcctcaagcaagtcctc-3′) and G5 for subtype B (Juhasz & Easton, Citation1994; Cavanagh et al., Citation1999). Amplifications were carried out in a thermal cycler PCR System 9700 (Gene Amp; Applied Biosystems). The PCR products were run on 1% agarose gel and visualized under UV light after staining with ethidium bromide.
Sequencing and phylogenetic analysis
RT-PCR products were sequenced three times each, in both the forward and reverse directions using the ABI PRISM Big Dye Terminator Cycle Sequencing Ready Reaction kit (Applied Biosystems). The BioEdit software, version 7.0.5.2 (Hall, 1999), was used to manipulate the nucleotide and amino acid retrieved sequences. The sequence alignments were performed with the Clustal W software, version 1.83 (Thompson et al., Citation1994) using full alignment and a total of 2000 replications on the bootstrap in order to ensure a higher level of confidence in our analysis (Thompson et al., 1994). Phylogenetic analyses were performed using neighbour-joining as implemented in the MEGA version 4 software package (Tamura et al., Citation2007), based on the Kimura two-parameter distance estimation method. Bootstrap resampling was performed for each analysis (1000 replications) (Kimura, Citation1980). Reference aMPV nucleotide sequences were retrieved from the GenBank database. The sample name and GenBank accession number of the sequences used in the phylogenetic analyses are given in .
Table 1. Sample names and GenBank accession number of sequences used in the phylogenetic analyses.
Results
Positive aMPV from wild birds, feral pigeons and domestic chickens
A total of seven (13.2%) positive aMPV samples were detected from different free-living wild species of birds in Brazil. Among the positive samples, five from wild birds (four different species) belonged to subtype A (Anas bahamensis, Penelope superciliaris, Aratinga leucophtalmus, Neochen jubata) and two belonged to subtype B (A. bahamensis, Dendrocygma viduata). From the feral pigeons, a total of seven (50%) positive aMPVs were detected from samples of different specimens of Columba livia. Among the positive samples, two belonged to subtype A and five belonged to subtype B. A total of 15 (12.9%) aMPV-positive samples (one belonging to subtype A and 14 belonging to subtype B) were detected from commercial chickens from different regions in Brazil between 2008 and 2009 (). These samples were negative by nested RT-PCR for infectious bronchitis virus.
Table 2. Name, abbreviation and GenBank accession number of avian metapneumovirus samples studied here, including the subtype and avian species in which it was detected.
Similarity of G fragment studied from different samples
In the present study, the similarity of the G fragment nucleotide sequence from aMPV subtype A detected in G. gallus domesticus to that from feral pigeons was 100%; from wild avian species it ranged from 100 to 97.5% (from 100 to 95% for the amino acid sequence). The similarity of the nucleotide sequence from chickens and non-domestic avian species isolates to vaccine subtype A ranged from 99.1 to 96.6% (from 97.5 to 92.5% for the amino acid sequence) (). With subtype B, the similarity between chicken and feral pigeon isolates ranged from 100 to 98.1% (from 100 to 97.2% for the amino acid sequence) and from wild avian species from 100 to 99% (from 100 to 97.2% for the amino acid sequence). The similarity to vaccine subtype B from chickens and non-domestic avian species ranged from 100 to 99% (from 100 to 97.2% for the amino acid sequence) ().
Table 3. Identity matrix of G gene partial nucleotide and amino acid (italic) sequences of avian metapneumovirus subtype A from Brazilian chicken field samples, feral pigeons and wild avian species.
Phylogenetic analysis
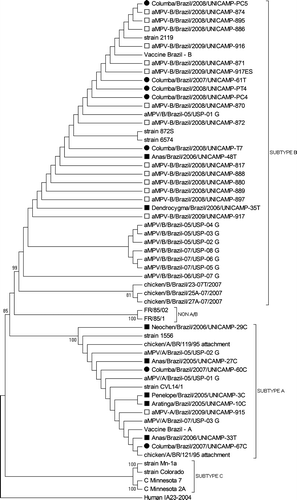
The majority of chicken samples clustered with subtype B (aMPV-B/Brazil/UNICAMP-874, 895, 886, 916, 871, 917ES, 870, 872, 817, 888, 880, 889, 897 and 917) and only one with subtype A (aMPV-A/Brazil/UNICAMP-915). Two positive samples from wild avian species clustered together with subtype B (Dendrocygma/Brazil/2006/UNICAMP-35T and Anas/Brazil/2006/UNICAMP-48T), and five with subtype A (Anas/Brazil/2005/UNICAMP-27C, Anas/Brazil/2006/UNICAMP-33T, Penelope/Brazil/2005/UNICAMP-3C, Aratinga/Brazil/2005/UNICAMP-10C and Neochen/Brazil/2006/UNICAMP-29C). Of the samples originating from feral pigeons, two clustered with subtype A (Columba/Brazil/2007/UNICAMP-60C and 67C) and five with subtype B (Columba/Brazil/2007/UNICAMP-61T; PT4; PC4; PC5 and PT7) ().
Discussion
The co-circulation of aMPV subtypes A and B in commercial poultry flocks in Brazil has previously been described (D′Arce et al., 2005; Chacón et al., Citation2007). In the present study we detected a higher incidence of subtype B (n = 14) than of subtype A (n = 1), suggesting that subtype B is either more prevalent in Brazilian commercial chicken breeding flocks or is easier to retrieve. The aMPV subtype B found in this study belonged to a different group to that of other Brazilian samples. Our samples could only be grouped with the aMPV/B/Brazil-05/USP-01G strain. This corroborates the findings of Villarreal et al. (Citation2009) who suggested that there could be at least two subtype B subpopulations in Brazil.
Table 4. Identity matrix of G gene partial nucleotide and amino acid (italic) sequences of metapneumovirus subtype B, from Brazilian chicken field samples, feral pigeon and wild avian species.
The present study is the first to report detection of aMPV subtypes A and B in wild and synanthropic avian species. Interestingly, Gough et al. (Citation1988) failed to isolate the virus or even detect antibodies in experimental infections with the aMPV subtype A (CVL 14/1) in ducks, geese, guinea fowls, pheasants and pigeons. Nonetheless, differences in susceptibility to viral infection between different species of wild birds from different countries are to be expected. In this study we observed differences in both nucleotide and amino acid sequence between subtype A of chickens from different origins and subtype A from wild avian species, which suggests that this subtype has probably adapted to these birds.
Regarding samples from wild avian species that clustered with subtype A, the virus was found in free-living birds of the Brazilian fauna from different Orders (Psittaciformes, Anseriformes and Craciformes), representing four different zoological Genera (Anas, Aratinga, Neochen and Penelope). For subtype B, aMPV was detected in two species from the Anatidae family (genera Anas and Dendrocygma). Hence, we can speculate about the importance of waterfowl for the survival and evolution of metapneumoviruses in the environment. Although none of the wild birds detected with aMPV in this study were on the list of Brazilian migratory birds, certain migratory waterfowl species share the same lakes and ponds for breeding. This suggests a potentially important means of dissemination of aMPV to other Brazilian states and to other countries. Wild avian species carrying the virus, which did not belong to the Anatidae family, have different ecological niches, making it very difficult to understand their role in the epidemiology of the aMPV. Further studies with specific wild bird species are therefore necessary.
Current methods used to detect aMPV subtype C infections, such as virus isolation, positive RT-PCR and detection of aMPV-C-specific antibodies, have demonstrated infection in several wild bird species, including rock pigeons, Canadian geese, blue-winged teals, swallows, house sparrows, ring-billed gulls, Muscovy, Pekin ducks, mallards and ostriches (Toquin et al., Citation1999; Shin et al., Citation2000, 2001; Bennett et al., Citation2002, 2004; Turpin et al., Citation2003, 2008). Thus, studies involving other aMPV subtypes, especially subtype C, in the Brazilian wild bird population are still needed.
Similarities found between the RNA detected in pigeons and wild birds and in the vaccine strains (99.1 to 96.6% for A subtype and 100 to 99.5% for subtype B) suggest an environmental contamination from this attenuated virus. Previous studies have demonstrated the survival and loss of attenuation of vaccine strains in the environment after vaccination, which could lead to the contamination of wild birds (Catelli et al., 2006). The infection of birds other than the target species could pose different selective challenges to the vaccine virus, which, in turn, could re-infect commercial poultry with virus with a new pathogenic profile. Interestingly, a 20-year retrospective study conducted with subtype B in Italy and other European countries revealed changes to the G protein in field strains, following the introduction of the vaccine for this subtype (Cecchinato et al., Citation2010).
It is imperative that future studies target the complete sequence of the viral RNA found in these types of birds, as well as the isolation and study of the pathogenicity of these viruses for chickens and turkeys. Nonetheless, the detection of aMPV in wild birds is important for the understanding of the epizootiology of rhinotracheitis in turkeys and, therefore, for the planning of biosecurity measures and vaccination protocols for poultry farms.
References
- Banet-Noach , C. , Simanov , L. , Laham-Karam , N. , Perk , S. and Bacharach , E. 2009 . Longitudinal survey of avian metapneumovirus in poultry in Israel: infiltration of field strains into vaccinated flocks . Avian Diseases , 53 : 184 – 189 .
- Banet-Noach , C. , Simanov , L. and Perk , S. 2005 . Characterization of Israeli avian metapneumovirus strains in turkeys and chickens . Avian Pathology , 34 : 220 – 226 .
- Bayon-Auboyer , M.H. , Arnaud , C. , Toquin , D. and Eterradossi , N. 2000 . Nucleotide sequence of the F, L and G protein genes of two non-A/non-B avian metapneumovirus (aMPV) reveal a novel aMPV subtype . Journal of General Virology , 81 : 2723 – 2733 .
- Bayon-Auboyer , M.H. , Jestin , V. , Toquin , D. , Cherbonnel , M. and Eterradossi , N. 1999 . Comparison of F-, G- and N-based RT-PCR protocols with conventional virological procedures for the detection and typing of turkey rhinotracheitis virus . Archives of Virology , 144 : 1091 – 1109 .
- Bennett , R.S. , LaRue , R. , Shaw , D. , Yu , Q. , Nagaraja , K.V. , Halvorson , D.A. and Njenga , M.K. 2005 . A wild goose metapneumovirus containing a large attachment glycoprotein is avirulent but immune-protective in domestic turkeys . Journal of Virology , 79 : 14834 – 14842 .
- Bennett , R.S. , McComb , B. , Shin , H.J. , Njenga , M.K. , Nagaraja , K.V. and Halvorson , D.A. 2002 . Detection of avian pneumovirus in wild Canada geese (Branta canadensis) and blue-winged teal (Anas discors) . Avian Diseases , 46 : 1025 – 1029 .
- Bennett , R.S. , Nezworski , J. , Velayudhan , B.T. , Nagaraja , K.V. , Zeman , D.H. , Dyer , N. , Grahan , T. , Lauer , D.C. , Nenga , M.K. and Halvorson , D.A. 2004 . Evidence of avian pneumovirus spread beyond Minnesota among wild and domestic birds in central North America . Avian Diseases , 48 : 902 – 908 .
- Buys , S.B. and De Preez , J.H. 1980 . A preliminary report on the isolation of a virus causing sinusitis in turkeys in South Africa and attempt to attenuate the virus . Turkeys , 28 : 36
- Catelli , E. , Cecchinato , M. , Savage , C.E. , Jones , R.C. and Naylor , C.J. 2006 . Demonstration of loss of attenuation and extended field persistence of a live avian metapneumovirus vaccine . Vaccine , 24 : 6476 – 6482 .
- Catelli , E. , Lupini , C. , Cecchiato , M. , Ricchizzi , E. , Brown , P. and Naylor , C.J. 2010 . Field avian metapneumovirus evolution avoiding induced immunity . Vaccine , 28 : 916 – 921 .
- Cavanagh , D. , Mawditt , K. , Britton , P. and Naylor , C.J. 1999 . Longitudinal field studies of infectious bronchitis virus and avian pneumovirus in broilers using type-specific polymerase chain reactions . Avian Pathology , 28 : 593 – 605 .
- Cecchinato , M. , Catelli , E. , Lupini , C. , Ricchizzi , E. , Clubbe , J. , Battilani , M. and Naylor , C.J. 2010 . Avian metapneumovirus (AMPV) attachment protein involvement in probable virus evolution concurrent with mass live vaccine introduction . Veterinary Microbiology , 146 : 24 – 34 .
- Chacón , J.L. , Brandão , P.E. , Buim , M. , Villarreal , L. and Ferreira , A.J. 2007 . Detection by reverse transcriptase-polymerase chain reaction and molecular characterization of subtype B avian metapneumovirus isolated in Brazil . Avian Pathology , 36 : 383 – 387 .
- Cook , J.K.A. , Huggins , M.B. , Orbell , S.J. and Senne , D.A. 1999 . Preliminary antigenic characterization of an avian pneumovirus isolated from commercial turkeys in Colorado, USA . Avian Pathology , 28 : 607 – 617 .
- Coswig , L.T. , Santos , M.B. , Hafez , H.M. , Ferreira , H.L. and Arns , C.W. 2010 . Propagation of avian metapneumovirus subtypes A and B using chicken embryo-related and other cell systems . Journal of Virological Methods , 41 : 368 – 375 .
- Dani , M.A. , Durigon , E.L. and Arns , C.W. 1999 . Molecular characterization of Brazilian avian pneumovirus isolates: comparison between immunochemiluminescent Southern blot and nested PCR . Journal of Virological Methods , 79 : 237 – 241 .
- D'Arce , R.C.F. , Coswig , L.T. , Almeida , R.S. , Trevisol , I.M. , Monteiro , M.C.B. , Rossini , L.I. , Di Fabio , J. , Hafez , M.H. and Arns , C.W. 2005 . Subtyping of new Brazilian avian metapneumovirus isolates from chickens and turkeys by reverse transcriptase-nested-polymerase chain reaction . Avian Pathology , 34 : 133 – 136 .
- Decanini E.L. Miranda E.C. LeGros F.X. 1991 Swollen head syndrome in heavy breeders in Mexico . In . Proceedings of the 40th Western Poultry Disease Conference 158 – 161 Acapulco, , Mexico
- Felippe , P.A. , da Silva , L.H.A. , Santos , M.M.A.B. , Spilki , F.R. and Arns , C.W. 2010 . Genetic diversity of avian infectious bronchitis virus isolated from domestic chicken flocks and coronaviruses from feral pigeons in Brazil between 2003 and 2009 . Avian Diseases , 54 : 1191 – 1196 .
- Giraud , P. and Bennejean , G. 1986 . Turkey rhinotracheitis in France: preliminary investigations on ciliostatic virus . The Veterinary Record , 119 : 606 – 607 .
- Gough , R.E. , Collins , M.S. , Cox , W.J. and Chettle , N.J. 1988 . Experimental infection of turkeys, chickens, ducks, geese, guinea fowl, pheasants and pigeons with turkey rhinotracheitis virus . The Veterinary Record , 123 : 58 – 59 .
- Govidanrajan , D. and Samal , S.K. 2005 . Analysis of the complete genome sequence of avian metapneumovirus subtype C indicates that it possesses the longest genome among metapneumoviruses . Virus Genes , 30 : 331 – 333 .
- Graaf , M. , Osterhaus , A.D.M.E. , Fouchier , R.A.M. and Holmes , E.C. 2008 . Evolutionary dynamics of human and avian metapneumoviruses . Journal of General Virology , 89 : 2933 – 2942 .
- Hafez , M.H. , Hess , M. , Prusas , C. , Naylor , C.J. and Cavanagh , D. 2000 . Presence of avian pneumovirus type A in continental Europe during the 1980s . Journal of Veterinary Medicine B , 47 : 629 – 633 .
- Hall T.A. Bioedit a user-friendly biological sequence alignment editor and analysis 1999 Available online at: http://www.mbio.ncsu.edu/BioEdit/bioedit.html
- Juhasz , K. and Easton , A.J. 1994 . Extensive sequence variation in the attachment (G) protein gene of avian metapneumovirus: evidence of two distinct subtypes . Journal of General Virology , 75 : 2873 – 2880 .
- Kimura , M. 1980 . A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences . Journal of Molecular Evolution , 16 : 111 – 120 .
- Lister , S.A. and Alexander , D.J. 1986 . Turkey rhinotracheitis: a review . Veterinary Bulletin , 56 : 637 – 663 .
- Mase , M. , Yamaguchi , S. , Tsukamoto , K. , Imada , T. , Imai , K. and Nakamura , K. 2003 . Presence of avian pneumovirus subtypes A and B in Japan . Avian Diseases , 47 : 481 – 484 .
- McDougall , J.S. and Cook , J.K.A. 1986 . Turkey rhinotracheitis: preliminary investigations . The Veterinary Record , 118 : 206 – 207 .
- Naylor , C.J. and Jones , R.C. 1993 . Turkey rhinotracheitis: a review . Veterinary Bulletin , 63 : 339 – 349 .
- Naylor , C.J. , Shaw , K. , Britton , P. and Cavanagh , D. 1997 . Appearance of type B avian pneumovirus in Great Britain . Avian Pathology , 26 : 327 – 338 .
- Padhi , A. and Poss , M. 2009 . Population dynamics and rates of molecular evolution of a recently emerged paramyxovirus, avian metapneumovirus subtype C . Journal of Virology , 83 : 2015 – 2019 .
- Panigrahy , B. , Senne , D.A. , Pedersen , J.C. , Gidlewski , T. and Edson , R.K. 2000 . Experimental and serologic observations on avian pneumovirus (APV/turkey/Colorado/97) infection in turkeys . Avian Diseases , 44 : 17 – 22 .
- Pringle , C.R. 1999 . Virus taxonomy—1999. The universal system of virus taxonomy, updated to include the new proposals ratified by the International Committee on Taxonomy of Viruses during 1988 . Archives of Virology , 144 : 421 – 429 .
- Roussan , D.A. , Haddad , R. and Khawaldeh , G. 2008 . Molecular survey of avian respiratory pathogens in commercial broiler chicken flocks with respiratory diseases in Jordan . Poultry Science , 87 : 444 – 448 .
- Seal , B.S. 1998 . Matrix protein gene nucleotide and predicted amino acid sequence demonstrate that the first US avian pneumovirus isolate is distinct from European strains . Virus Research , 58 : 45 – 52 .
- Seal , B.S. 2000 . Avian pneumovirus and emergence of a new type in the United State of America . Animal Health Research Reviews , 1 : 67 – 72 .
- Shin , H.J. , Nagaraja , K.V. , McComb , B. , Halvorson , D.A. , Jirjis , F.F. , Shaw , D.P. , Seal , B.S. and Njenga , M.K. 2002 . Isolation of avian pneumovirus from mallard ducks that is genetically similar to viruses isolated from neighboring commercial turkeys . Virus Research , 83 : 207 – 212 .
- Shin , H.J. , Njenga , M.K. , Halvorson , D.A. , Shaw , D.P. and Nagaraja , K.V. 2001 . Susceptibility of ducks to avian pneumovirus of turkey origin . The American Journal of Veterinary Research , 62 : 991 – 994 .
- Shin , H.J. , Njenga , M.K. , McComb , B. , Halvorson , D.A. and Nagaraja , K.V. 2000 . Avian pneumovirus (APV) RNA from wild and sentinel birds in the United States has genetic homology with RNA from APV isolates from domestic turkeys . Journal of Clinical Microbiology , 38 : 4282 – 4284 .
- Tamura , K. , Dudley , J. , Nei , M. and Kumar , S. 2007 . MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0 . Molecular Biology and Evolution , 24 : 1596 – 1599 .
- Tanaka , M. , Takuma , H. , Kokumai , N. , Oishi , E. , Obi , T. , Hiramatsu , K. and Shimizu , Y. 1995 . Turkey rhinotracheitis virus isolated from broiler chicken with swollen head syndrome in Japan . Journal of Veterinary Medical Science , 57 : 939 – 941 .
- Thompson , J.D. , Higgins , D.G. and Gibson , T.J. 1994 . Clustal W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice . Nucleic Acids Research , 11 : 4673 – 4680 .
- Toquin , D. , Bäyon-Auboyer , M.H. and Jestin , V. 1999 . Isolation of a pneumovirus from a Muscovy duck . The Veterinary Record , 145 : 680
- Toquin , D. , Bayon-Auboyer , M.H. , Senne , D.A. and Eterradossi , N. 2000 . Lack of antigenic relationship between French and recent North American non-A/non-B turkey rhinotracheitis viruses . Avian Diseases , 44 : 977 – 982 .
- Toquin , D. , de Boisseson , C. , Beven , V. , Senne , D.A. and Eterradossi , N. 2003 . Subgroup C avian metapnumovirus (MPV) and the recently isolated human MPV exhibit a common organization but have extensive sequence divergence in their putative SH and G genes . Journal of General Virology , 84 : 2169 – 2178 .
- Turpin , E.A. , Lauer , D.C. and Swayne , D.E. 2003 . Development and evaluation of a blocking enzyme-linked immunosorbent assay for detection of avian metapneumovirus type C-specific antibodies in multiple domestic avian species . Journal of Clinical Microbiology , 41 : 3579 – 3583 .
- Turpin , E.A. , Stallknecht , D.E. , Slemons , R.D. , Zsak , L. and Swayne , D.E. 2008 . Evidence of avian metapneumovirus subtype C infection of wild birds in Georgia, South Carolina, Arkansas and Ohio, USA . Avian Pathology , 37 : 343 – 351 .
- van den Hoogen , B.G. , Jong , J.C. , Kuiker , G.T. , Groot , R. , Fouchier , R.A. and Osterhaus , A.D. 2001 . A newly discovered human pneumovirus isolated from young children with respiratory tract disease . Nature Medicine , 7 : 719 – 724 .
- Van de Zande , S. , Nauwynck , H. , Cavanagh , D. and Pensaert , M. 1998 . Infection and reinfection with avian pneumovirus subtype A and B on Belgian turkey farms and relation to respiratory problems . Journal of Veterinary Medicine B , 45 : 621 – 626 .
- Velayudhan , B.T. , Yu , Q. , Estevez , C.N. , Nagaraja , K.V. and Halvorson , D.A. 2008 . Glycoprotein gene truncation in avian metapneumovirus subtype C isolates from United States . Virus Genes , 37 : 266 – 272 .
- Villarreal , L.Y.B. , Sandri , T.L. , Assayag , M.S. , Richtzenhain , L.J. , Malo , A. and Brandão , P.E. 2009 . “ Field observations after natural infection of Brazilian layer chickens with a phylogenetically divergent lineage of subtype B aMPV ” . In Proceedings of the VI International Symposium on Avian Corona- and Pneumoviruses and Complicating Pathogens, June , Edited by: Kaleta , E.F. and Heffels-Redmann , U. 255 – 259 . Germany : Rauischholzhausen .
- Wilding , G.P. , Baxter-Jones , C. and Grant , M. 1986 . Ciliostatic agent found in rhinotracheitis . The Veterinary Record , 118 : 735
- Yunus , A.S. , Govindarajan , D. , Huang , Z. and Samal , S.K. 2003 . Deduced amino acid sequence of the small hydrophobic protein of US avian pneumovirus has greater identity with that of human metapneumovirus than those of non-US avian pneumovirus . Virus Research , 93 : 91 – 97 .