Abstract
Currently, the aetiology of runting and stunting syndrome (RSS) in chickens is unknown. The impact of RSS on weight gain and microscopic lesions in immunological organs and the duodenum, was investigated in 1-day-old commercial broilers at 12 days following exposure to RSS-contaminated litter. Furthermore, the presence of the viral nucleic acids of three astroviruses and one parvovirus was analysed by in situ hybridization from days 1 through 5 post exposure. A 70% decrease in weight was observed in the RSS-exposed group at the end of the experiments when compared with the unexposed controls. Lesions in the bursa of Fabricius and thymus were present in both groups but were significantly higher at the end of the study in the RSS-exposed group. In contrast, no significant difference in Harderian gland lesions was observed between the groups. Histological lesions in the duodenum were already present 24 h after exposure in the RSS-exposed group only, peaked at day 4 and declined until the end of the study. Results of the in situ hybridization studies clearly indicate replication of three astroviruses (chicken astrovirus, avian nephritis virus [ANV]-1, ANV-2) in the duodenum but not in other organs evaluated. Chicken astrovirus nucleic acids were detected on days 1 and 2 post exposure, while ANV-1 and ANV-2 nucleic acids were observed on several days during the period investigated. Surprisingly, no viral nucleic acid specific for the chicken parvovirus was observed. The results indicate that astroviruses probably play an important role during RSS due to the concurrence of viral RNA detection and lesions in the duodenum.
Introduction
Runting and stunting syndrome (RSS) in chickens is a transmissible disease of uncertain aetiology. RSS affects chickens early in life and is characterized by growth retardation, ruffled feathers, and diarrhoea, resulting in considerable economic losses especially in the commercial broiler industry. RSS was initially reported in the broiler industry during the 1970s and has since been described in various parts of the world (Olsen, Citation1977; Kouwenhoven et al., Citation1978). Due to the absence of a known aetiology, identification of the disease commonly relies on descriptive terms such as malabsorption syndrome, infectious stunting syndrome, broiler runting syndrome, and helicopter syndrome (Rebel et al., Citation2006). Currently there is no effective commercial vaccine available for control of the disease, due primarily to the absence of known aetiologic agents. One experimental vaccine, based on a recombinant baculovirus encoding for a new astrovirus capsid protein, has recently been described (Sellers et al., Citation2010). Nonetheless, clinical signs and microscopic lesions of RSS have been reproduced experimentally using oral inoculation of filtered and non-filtered intestinal homogenate from affected chickens (Montgomery et al., Citation1997; Songserm et al., Citation2000; Songserm et al., Citation2002a).
Histopathological changes in birds affected by RSS are primarily associated with lesions in the small intestine. A hallmark lesion observed in clinical and experimental cases of RSS is cystic enteropathy (Otto et al., Citation2006; Sellers et al., Citation2010). The cells lining the crypt degenerate and detach into the lumen, forming cystic crypts lined by flattened epithelial cells. The severity of clinical signs may depend on the extent of villous atrophy resulting from epithelial loss and decreased turnover. Nonetheless, the actual cause and pathogenesis of the intestinal lesions remains unclear; however, increased apoptosis along the lining of villi and crypt epithelial cells has been reported (Zekarias et al., Citation2005). Approximately 2 to 3 weeks after experimental infection, recovery of the mucosal lining has been observed (Zekarias et al., Citation2005).
Although bacteria and environmental factors may be associated as contributors to the development of disease (Otto et al., Citation2006; Rebel et al., Citation2006), small, round, non-enveloped viruses have been suggested as the most probable aetiologic agents based on electron microscopy and the ability to reproduce the disease using a chloroform-treated, bacteria-free filtrate (Sellers et al., Citation2010). Over the years, reovirus (Songserm et al., Citation2000), rotavirus (Otto et al., Citation2006), parvovirus (Kisary et al., Citation1984) and astrovirus (Baxendale & Mebatsion, Citation2004) have been detected or isolated from clinically affected chickens. Kisary et al. (Citation1984) described the presence of parvovirus in guts of RSS-affected chickens. The parvovirus, called also ABU isolate, was purified by ultracentrifugation and was able to induce growth retardation in chickens (Kisary, Citation1985). On the contrary, broiler chickens experimentally infected with either a reovirus or the parvovirus ABU isolate did not show clinical signs specific for RSS (Decaesstecker et al., Citation1986). The importance of parvovirus infection in turkeys and chickens for the occurrence of enteric diseases in both species was supported by recently published investigations in poultry flocks in Hungary (Palade et al., Citation2011) but is still not fully understood, probably due to the lack of a pure virus isolate. On the other hand, infection with an enterovirus-like virus resulted in enteric disease (Decaesstecker et al., Citation1986). The group of enterovirus-like viruses is not well defined. In fact, prior to the year 2000, astroviruses were placed taxonomically with the enterovirus-like virus group (Imada et al., Citation2000). Astroviruses are associated with gastrointestinal disease in many species of mammals and birds (reviewed in Koci & Schultz, Citation2002). In chickens with enteric disease, chicken astrovirus (Baxendale & Mebatsion, Citation2004) and avian nephritis virus (ANV) (Imada et al., Citation2000) have been identified. Recently, the genome of a parvovirus from particle-associated nucleic acid was described and suggested as a possible poultry enteric disease agent (Zsak et al., Citation2008; Day & Zsak, Citation2010). Using other techniques, different viruses have been detected in the intestines of affected birds. Reovirus antigen has been detected in the epithelial cells of the tip and middle regions of the intestinal villi in RSS-affected chickens (Songserm et al., Citation2000, Citation2002b). Similarly, in an in situ hybridization (ISH) study using astrovirus-infected turkeys, astrovirus RNA was only detected in the villous epithelial cells in the intestine (Koci et al., Citation2003). Furthermore, Pantin-Jackwood et al. (Citation2008b) observed cells positive for a turkey astrovirus in the intestinal crypts of infected turkeys using ISH and immunohistochemistry methods.
The role of these viruses in RSS-affected chickens is still poorly understood. Moreover, the viruses may cause RSS in combination with other viruses or unknown factors. The target cell predilection as well as the pathogenicity of these viruses can also differ. To further complicate identification of the disease aetiology, a steady increase in carcass condemnations for septicaemia–toxaemia was observed after the onset of the clinical RSS outbreak in late 2004 (Smith, Citation2008). While this suggests an association of RSS with immune suppression, it is not entirely clear which comes first. However, due to the extremely early onset of signs, it is possible that the agent(s) causing clinical RSS are themselves directly and profoundly immunosuppressive (Sklan, Citation2001). Due to the complexity of RSS and the possibility of multiple aetiologies, developing treatment and control strategies are hindered. In previous studies, a novel chicken astrovirus was identified in RSS-affected chickens (Sellers et al., Citation2010). The role of this astrovirus and other small round viruses may hold an important key to identification of the aetiology/aetiologies and improve our understanding of the pathogenesis of RSS. The aim of the present study was to evaluate the development of microscopic changes over time in the small intestine, bursa of Fabricius, thymus, and Harderian gland in commercial broilers challenged with RSS in a previously described challenge model (Sellers et al., Citation2010). Using riboprobes designed to hybridize to regions of ANV-1, ANV-2, chicken parvovirus and a novel chicken astrovirus, tissues were examined for the presence of replicating virus using an ISH assay.
Materials and Methods
Chickens
Three hundred 1-day-old commercial broiler chicks obtained from a commercial flock were randomly separated into two experimental groups consisting of 150 birds each. The number of chickens was necessary to obtain a chicken density comparable with commercial production conditions. The birds came from the same company and had the same genetic background as described before (Sellers et al., Citation2010). Each group of chicks was placed into a separate 10 m2 isolation house. Water and feed were provided ad libitum. One group was placed on fresh pine shavings, which served as bedding material. For the RSS-challenged group, litter material obtained from the same local commercial broiler farm with a history of clinical RSS was used as previously described (Sellers et al., Citation2010). Starting at 24 h following placement, five birds were collected randomly daily as a representative sample from each group. Birds were numbered (from 1 to 5) and weighed. After euthanizing the birds with carbon dioxide, the duodenal loop, thymus, bursa of Fabricius, and Harderian glands were collected from each bird and placed separately, by individual bird, in 10% neutral buffered formalin. The container was labelled with the bird number, so that the collected tissues could be linked with the bird weight. The same procedure was repeated until 11 days post placement. At 12 days post placement, the experiment was terminated. At this time, all chickens were euthanized and 30 out of the remaining 95 chickens were weighed and samples collected.
Treatment of the tissue samples
A cross-section of the duodenal loop, just above the tip of the pancreas including the ascending and descending sections of the loop, a section of the bursa of Fabricius, the thymus lobes and the Harderian gland, was placed in 10% buffered formalin for 24 h. The fixed tissues were embedded in paraffin blocks and labelled with the group identification number, age, and bird number. The paraffin-embedded blocks were cut consecutively into 4 µm thick sections for subsequent experiments. Sections placed on regular glass slides were stained with haematoxylin and eosin for light microscopic examination. Sections for ISH were placed on Superfrost/Plus microscopic slides (Fisher Scientific, Pittsburgh, Pennsylvania, USA).
Evaluation of the microscopic lesions
For the microscopic evaluation, the presence of cystic formation in the crypts of Lieberkühn (further designated as cystic lesions) in both parts of the duodenal loop was evaluated and the number of cystic lesions per bird was counted. For the primary immune organs (bursa of Fabricius and thymus) and the secondary immune organ (Harderian gland), the evaluation was performed by microscopic assessment of the lymphocytic population. Since it is difficult to evaluate lymphoid tissues in young birds less than 2 weeks of age due to the high dynamic of the development of the tissues, a subjective scoring system was based on comparing lymphocyte populations in the challenged group versus the control group on a daily basis. As the goal of the study was to gauge differences between the two groups, individuals with the largest lymphocyte population within the control group were considered the reference for the scoring system. The subjective microscopic evaluation of the tissues was expressed on a scale of 0 (normal lymphocyte population) to 3 (severely affected lymphocyte population). For a score of 0, there were no differences in the lymphocyte population versus the selected reference tissue. As reference tissue, a sample of a control bird was chosen that showed the highest density of the lymphocyte population, and thus was automatically scored as 0. For a score of 1, there was a subjectively mild difference (25%) in the lymphocyte population versus the selected reference tissue(s). For a score of 2, there was a subjectively moderate difference (50%) in the lymphocyte population versus the selected reference tissue(s). For a score of 3, there was a subjectively severe difference (75%) in the lymphocyte population versus the selected reference tissue. It needs to be kept in mind that even with great caution during the scoring it is highly subjective.
Data analysis
Weight measures, counts of cystic lesions in the cross-section of duodenum and microscopic scoring of immune organs were entered into an Excel spreadsheet (Microsoft® Office Excel® 2007) and averages and standard deviations were calculated. The determination of significant differences between groups was calculated using one-way analysis of variance of summary data employing a free calculation tool available online (http://danielsoper.com/).
Generation of riboprobes
Since astroviruses (Koci & Schultz-Cherry, Citation2002; Baxendale & Mebatsion, Citation2004) and a parvovirus (Kisary et al., Citation1984; Zsak et al., Citation2008; Day & Zsak, Citation2010) have been identified as potentially playing a role in the aetiology of RSS, sequences of the these viruses were amplified by reverse transcription-polymerase chain reaction (RT-PCR) for astrovirus and PCR for parvovirus. The initial material used for the preparation of plasmids for the transcription of the riboprobes was gut material obtained from chickens exposed to the RSS-contaminated litter that had been taken at day 12. The gut material was homogenized with FastPrep 24 by Bio101 (MP Biochemicals, Solon, Ohio, USA). The resulting homogenate was centrifuged at 13,000×g at 4°C for 20 min. The supernatant was taken for either RNA purification using the High-Pure-RNA-Isolation-Kit (Roche Applied Science, Indianapolis, Indiana, USA) or DNA purification using the QIAamp DNA Blood Mini Kit (QIAGEN, Hilden, Germany). The extracted nucleic acids were used in the experiments described below. Oligonucleotides (see ) were designed based on sequences available in the NCBI database for ANV-1 (Genbank accession number AB033998), ANV-2 (Genbank accession number AB046864), chicken astrovirus (Genbank accession number JF414802) and chicken parvovirus (Genbank accession number GU214704). The probes for ANV-1 and ANV-2 were located in the coding sequence of the capsid protein. The probe for the chicken parvovirus was located in the viral VP2 sequence, while the probe for the chicken astrovirus was located in the open reading frame 1a region of the virus. Appropriate restriction enzyme cleavage sites were introduced for linearization prior to the DNA-dependent RNA polymerase reaction using either T7 or T3 phage polymerase. After RT-PCR or PCR, the appropriate cDNA fragments were separated on a 1% gel and gel purified using QIAquick Gel Extraction Kit (Qiagen, Germantown, Maryland, USA). The purified PCR products were incubated with the appropriate restriction enzymes, gel purified again and ligated into the appropriately cleaved pBluescript® II Phagemid vector (Stratagene, La Jolla, California, USA). Plasmids containing the expected inserts were selected by restriction enzyme analysis and those plasmids containing an insert were sequenced as described above. A plasmid containing the target sequence was transformed into Top10 F cells (Invitrogen, Carlsbad, California, USA) and plasmid DNA was prepared using the GeneJET™ Plasmid Miniprep Kit (Fermentas, Glen Burnie, Maryland, USA). The resulting purified plasmids were cleaved with the restriction enzyme EcoRI (ANV-1, ANV-2), or XbaI (chicken astrovirus), purified and subsequently transcribed in vitro using phage polymerase T3 (Applied Biosystems/Ambion, Austin, Texas, USA) to generate an antisense RNA of approximately 500 nucleotides in length. For sense probes, cDNA constructs for ANV-1, ANV-2, and the new chicken astrovirus were linearized with SacI followed by in vitro transcription using T7 RNA-polymerase (Takara, Madison, Wisconsin, USA). For the parvovirus-specific sense probes, one recombinant plasmid for each probe was generated due to the incompatibility of the T7 polymerase for the subsequent transcription reaction. The parvovirus-specific cDNA was amplified with the appropriate primer pair (ChPVpr-FP, ChPVpr-RP, see ). The obtained PCR fragment was cleaved either with HindIII/SacII (sense probe) or EcoRI/SacI (antisense probe) and ligated into the appropriately cleaved pBluescript® II Phagemid vector (Stratagene). The plasmids obtained were cleaved with either HindIII (sense probe) or EcoRI (antisense probe), purified and used for the T3 RNA-polymerase reaction. For the polymerase reaction, the DIG RNA Labeling Mix (Roche, Basel, Switzerland) was used in accordance with the manufacturer's instructions. Following the polymerase reaction, the plasmid DNA was degraded by adding 10 u RNAse-free DNAse I (Roche) and subsequently incubating for 60 min at 37°C. The reaction was stopped by the addition of 2 µl of 0.2 M ethylenediamine tetraacetic acid (pH 8.0). The reaction products were purified using SigmaSpin™ Post-Reaction Clean-Up Columns (Sigma-Aldrich, St Louis, Missouri, USA). The presence of the synthesized RNA probe was evaluated by agarose gel electrophoresis (data not shown).
Table 1. Oligonucleotides used for amplification of the sequences for the RNA probes.
The riboprobe concentration was determined by comparison with a known amount of a DIG-labelled control RNA (Roche) in a dot-blot assay as described by the manufacturer. The dilution factor for each RNA probe was determined to include 35 ng/ml RNA into each hybridization procedure.
In situ hybridization using tissue samples of RSS-infected and control chickens. The unstained tissue slides were first heated at 70°C for 10 min and deparaffinized in Citrosolv™ (Fisher Scientific). Slides were then air-dried thoroughly and tissue sections were rehydrated in 5 mM MgCl2 in phosphate-buffered saline (PBS) for 10 min. Before enzyme digestion, slides were treated in Tris–glycine buffer (0.1 M glycine in 0.2 M Tris, pH 7.5) for 10 min at room temperature (RT) and then incubated with proteinase K (35 µg/ml) in proteinase K buffer (10 mM Tris, pH 7.5, 2 mM CaCl2) for 15 min at 37°C. The enzymatic reaction was stopped in the Tris–glycine buffer. Pre-hybridization solution (5×saline-sodium citrate buffer [SSC] containing 0.75 M NaCl, 0.075 M sodium citrate with 50% formamide, 5% blocking reagent [Roche], 0.1% N-lauroylsarcosine and 0.02% sodium dodecyl sulphate [SDS]) was added to sections for 30 min at 42°C. Seventy microlitres of the hybridization solution, which consisted of the pre-hybridization solution containing the riboprobe (35 ng/µl), was applied directly onto the section and covered with a siliconized cover slip (HybriSlip™; Grace Bio-Labs, Bend, Oregon, USA). The hybridization was performed overnight at 42°C in a humid chamber. The next day, coverslips were removed and slides were washed once at 50°C in 2×SSC (0.3 M NaCl, 0.03 M sodium citrate) with 1% SDS followed by one wash at 50°C with 1×SSC (0.15 M NaCl, 0.015 M sodium citrate) with 0.1% SDS and at RT with one wash in 1×SSC followed by one wash with 0.1×SSC (0.015 M NaCl, 0.0015 M sodium citrate) for 30 min each. After the washing steps, slides were treated in buffer I (100 mM Tris–HCl, 150 mM NaCl, pH 7.5) for 10 min then incubated with a 300-fold diluted sheep anti-digoxigenin alkaline phosphatase-conjugated Fab2′ (Roche) in Buffer I containing 1% foetal bovine serum and incubated for 2 h at 37°C. After three washes with buffer I, the binding of the conjugate was visualized by adding a chromogen mixture (200 µl NBT/BCIP stock solution [Roche] in 10 ml of 0.1 M Tris–HCl, pH 9.5, 0.1 M NaCl). The development of the signal was allowed to progress for 45 to 60 min and was stopped by rinsing the slide in distilled water. Slides were lightly counterstained with Gill's haematoxylin and coverslipped with Permount™ (Fisher Scientific). The slides were evaluated under a light microscope.
Functionality test for the parvovirus riboprobe
Cells of the chicken fibroblast cell line DF-1 (Himly et al., Citation1998) grown in Dulbecco's modified Eagles's medium with 4.5 g/l glucose (DMEM-4.5; Thermo Scientific, Waltham, Massachusetts, USA) supplemented with 10% foetal bovine serum (Mediatech, Manassas, Virginia, USA) were prepared in eight-well-chamber slides (Lab-Tek® II CC2™; Nunc, Roskilde, Denmark) 12 h before transfection. Using the TransIT-mRNA Transfection Kit (Mirus Bio LLC, Madison, Wisconsin, USA), cells were transfected with either DIG-labelled ChPV sense probe, or non-labelled ChPV sense probe, or mock transfected as negative control following the instructions of the manufacturers. Four hours after transfection of the cells, the supernatant was removed, cells were washed once with PBS followed by incubation with 10% neutral buffered formalin containing 5% acetic acid for 30 min at RT. After fixation, cells were washed with PBS and stored in 70% ethanol at 4°C until use. For ISH, cells were incubated with 5 mM MgCl2 in PBS for 10 min and then in Tris–glycine buffer (0.1 M glycine in 0.2 M Tris, pH 7.5) for 10 min. Cells were incubated with proteinase K (3.5 µg/ml) in proteinase K buffer (10 mM Tris, pH 7.5, 2 mM CaCl2) for 15 min at 37°C. The enzymatic reaction was stopped by a rinsing step using Tris–glycine buffer. The hybridization with the DIG-labelled ChPV antisense probe was performed as described above.
Results
Cystic lesions in the small intestine were present 24 h after exposure
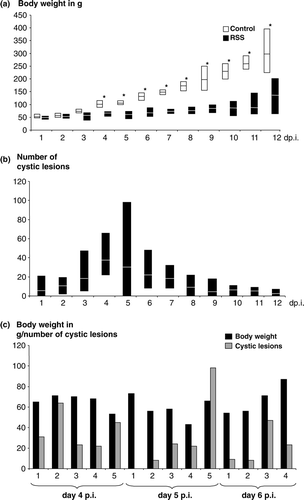
Determination of the body weights indicated a severe challenge of the birds exposed to the RSS-contaminated litter (a). The average of the body weights was significantly different (P <0.05) starting from day 3 after exposure. Five days following the start of the experiment, the body weight difference between the groups was approximately 50%. The difference in weight culminated at the end of the study where the median body weight of the chickens exposed to the RSS-contaminated litter was only 30% of the weight of the control group. The microscopic evaluation of the cross-section of the duodenal loop revealed the presence of cystic lesions only in chickens that were exposed to RSS-contaminated litter (b). No differences between control and RSS-exposed birds were observed during the evaluation of the pancreatic tissues. The average lesion numbers were determined per group and by day. One striking finding was that cystic lesions were observed in four out of five birds examined as early as 24 h after placement. Interestingly, the lesions observed at this early time point already showed the structure previously described (Songserm et al., Citation2000; Nili et al., Citation2007). The lesions observed were characterized by dilatation of the crypts of Lieberkühn in addition to atrophy of the intestinal villi ranging from mild to moderate. Furthermore, hyperplasia of the crypt region was observed. In the dilated crypt lesion, the epithelial cells were markedly flattened. The dilated crypt lumen occasionally contained cellular debris that was composed of degenerated cells and eosinophilic cellular debris. Starting with day 10 after exposure, affected crypts first became surrounded and later replaced by connective tissues. In addition, mineralization was occasionally observed within the lesions. The average number of cystic lesions increased until day 4 after placement. Four days following exposure (days 2, 3, 4 and 7), lesions were observed in all five birds examined, while on the remaining days lesions were observed in approximately 80% of the birds (see also later ). The median number of lesions peaked at day 4 after exposure and declined until the end of the study. It needs to be mentioned that the variability in the numbers of lesions was extreme, as indicated by the minimum and maximum number of lesions per bird (b). Furthermore, the data were analysed to determine whether there was a correlation between number of lesions in the duodenal loop and the weight of the same bird (c). The data of each bird for both values are shown side by side for days 4, 5, and 6 post inoculation (p.i.) since the highest lesion scores were observed at these time points (see also a). It became obvious that no direct correlation was observed between these parameters, since birds with a comparatively low body weight showed a low number of lesions (day 5 p.i., Bird 4) while in the opposite case a bird with a comparatively high body weight showed a high number of lesions (day 5 p.i., Bird 5).
Figure 1. Weight gain and presence of cystic lesions in RSS-afffected chickens. Commercial broiler chickens were exposed either to RSS-contaminated litter (RSS) or to fresh wood shavings (control). 1a: Five chickens at day 1 through day 11 and 30 chickens at day 12 after infection (day p.i.) were euthanized and the body weights were determined. The average body weights for each day is shown as a white line in the RSS-related box, and a black line in the white box representing the control chickens. In addition, the recorded minimum and maximum weights within the group were shown. *Statistically significant different values between control birds and RSS-exposed birds. 1b: The cross-section of each duodenal loop was assessed for the presence of cystic lesions for each chicken as described under (1a). The average value for the number of cystic lesions is shown for each day in the box as a white line. The minimum and maximum observed numbers of lesions were indicated. 1c: The body weight and number of cystic lesions in the duodenal loop for each chicken at days 4, 5, and 6 after exposure to the RSS-contaminated litter was shown. Body weight and cystic lesions are both plotted on the y axis.
Primary lymphoid organs were affected during infection
In order to assay whether exposure to RSS-inducing litter caused changes in two of the primary lymphoid organs (bursa of Fabricius, thymus) and one secondary lymphoid organ (Harderian gland), microscopic evaluations were performed. The results obtained for the bursa of Fabricius showed that the scores for the RSS-litter-exposed group increased over the course of the experiment (). The scores of the bursa of Fabricius were statistically significantly different between the RSS-exposed group and the control group from day 9 onwards. The changes observed in the thymus serving as the second primary lymphoid organ showed a similar trend (). Thymus sections of chickens exposed to the contaminated litter presented a significantly higher score from day 6 through day 12 after placement, in comparison with the control group which was placed on fresh shavings. One exception was observed on day 8 after placement where no significant difference in the average lesion scores between both groups was observed. In contrast, the evaluation of the Harderian gland as a representative for a secondary lymphoid organ resulted in no detectable differences between both groups in respect to microscopic tissue changes (), except for day 6 after placement where significant differences were observed.
Table 2. Average scores of microscopic changes in the bursa of Fabricius, thymus, and Harderian gland.
Sequence comparison of the probe nucleotide sequences
The length of the amplified sequence from the intestinal content of RSS-infected chickens encoding for parts of the viral capsid protein for the ANV-1 probe was 516 nucleotides (nt) and for ANV-2 was 521 nt. The homology of these nucleotide sequences to each other was 46%. The similarity to the published ANV-1 capsid encoding sequences (AB033998) was 84% while the ANV-2 sequence showed a similarity of 89% to a published ANV-2 sequence (AB046864). Nucleotide sequence similarities to other poultry astroviruses—turkey astrovirus 1 (EU143848), turkey astrovirus 2 (EU143843), duck astrovirus (NC_012437), chicken astrovirus (JF414802)—were below 30%. The sequence used for the probe for the new chicken astrovirus was also obtained from the intestinal sample. The sequence was located in the region of the new chicken astrovirus encoding for the non-structural protein. A NCBI Genbank Blastn search did locate any similar sequences, and a direct comparison with the appropriate nucleotide sequences for ANV-1 (NC_003790), turkey astrovirus 1 (EU143848), turkey astrovirus 2 (EU143843), and the duck astrovirus (NC_012437) showed a similarity of below 20%. The probe for the amplified parvovirus sequence showed an 88% identity to a recently published chicken parvovirus sequence (Day & Zsak, Citation2010)
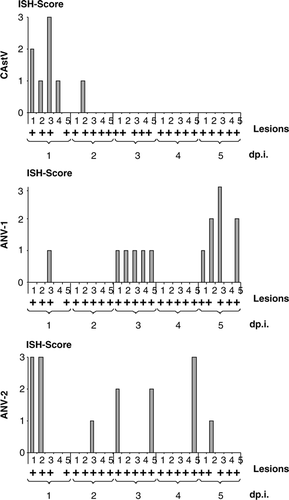
In situ hybridization revealed astroviruses as agents for RSS. The ISH was performed on all tissues, which included the bursa of Fabricius, thymus, Harderian gland, and the cross-section of the duodenum including the tip of the pancreas. Samples from day 1 through day 5 were analysed. One major problem with the investigations was that viruses of interest were not isolated in cell culture and thus the availability of a positive control for the ISH to show specificity was limited. To address this problem from the very beginning, all probes were designed so that they would have approximately the same length (ANV-1, 516 nt; ANV-2, 521 nt; chicken astrovirus, 450 nt; chicken parvovirus, 511 nt). Due to the sequence composition, the GC content was approximately 45% and very similar in all probes. For the adjustment of the ISH conditions, DF1 cells were transfected with positive-sense transcripts of each virus (ANV-1, ANV-2, chicken astrovirus, and chicken parvovirus) and cross-tested for specificity with each probe. In addition, the antisense-oriented transcripts were also transfected and probed with the sense probes. The adjustment factor for the specificity was the temperature during the washing procedure, probably due to the GC content and the similar length of probes. Interestingly, for all probes the same temperature could be used without losing specificity. Since the study was designed so that each sample could be traced to the appropriate chicken, association of ISH signals to appropriate changes observed microscopically could be evaluated. The bursa of Fabricius, thymus, tip of the pancreas, and Harderian gland showed no ISH signals, neither those samples obtained from the group that was exposed to the RSS-contaminated litter nor the tissue samples obtained from the negative control group. This indicated that neither one of the three astroviruses nor the chicken parvovirus replicated in these organs. A different result was observed when the samples representing the duodenal loop were investigated with the probes specific for chicken astrovirus, ANV-1, and ANV-2. The sense probes for the astroviruses showed no signals that could be appreciated as positive on slides of two chickens which were positive on day 1 after placement (data not shown). All antisense probes specific for each of the astroviruses showed a positive signal in a number of samples. This indicated a high specificity of the antisense ISH probes with the respective virus. Furthermore, none of the samples investigated from the negative control group showed a positive ISH signal from any of the birds that were taken on days 1 and 3 after placement regardless of whether the sense or antisense probe was used. The general signature of the astrovirus-specific antisense probes was very similar (). The majority of positive signals were observed along the villous epithelial layer although occasionally a few rare signals were seen in the lamina propria as irregular particles. The signals in the villous epithelial cells were localized in the cytoplasm, indicating the expected cytoplasmic replication of the virus. Interestingly, no matter whether tissues contained the dilated crypt lesions, the signals for astroviruses were present neither in the crypt epithelial cells nor in the adjacent tissues of the crypt. The signals were clearly distinguishable, which indicated an intense virus replication. No difference in the strength of the signal among the three different astroviruses was observed. The pattern of the staining might be important for the dynamics of the disease. Although the lesions are already shown in b, the association between lesions and presence of ISH signals is shown in . The signal for ANV-1 was observed in one chicken at day 1, all five chickens at day 3, no chickens at day 4, and four out of five chickens at day 5. The signal specific for the ANV-2 probe was scattered throughout the investigated time points. Two chickens were positive at days 1 and 3 after exposure to the litter, while at days 2, 4, and 5 after exposure one chicken out of the five investigated chickens showed a positive signal. The chronological presence of viral RNA for the new chicken astrovirus was noted. Four out of the five birds evaluated showed a positive signal 24 h after exposure to the RSS-contaminated litter and only one chicken at day 2. None of the investigated sections showed any signal from day 3 through day 5 after exposure. It needs to be mentioned that the sections were cut consecutively from the paraffin-embedded blocks and on an individual basis. The initial concern was that the probes specific for ANV-1 and ANV-2 might cross-react although the nucleotide sequence similarity was only 46% (see above). However, the data obtained show that although Birds 1 and 2 at day 1 after exposure showed ISH signals for the ANV-2 probe in a high number of cells, hybridization with the ANV-1 probe remained negative. A similar result was observed with Bird 5 on day 4. The ISH with the ANV-2 probe was positive in a high number of cells but negative for the ANV-1 ISH probe. The opposite result was present in Birds 3 and 5 on day 5 where the ISH probe for the ANV-1 was positive in a high number of cells while the ISH for ANV-2 was negative. A very similar result was observed for the ISH probe of the new chicken astrovirus, where no reaction was observed beyond day 2 following exposure while positive signals were observed with both of the other probes (ANV-1 and ANV-2). The comparison of all three ISH signals on a single-bird basis demonstrated that some birds were positive for two viruses at the same time regardless of the strength of the signal. In addition, it was also observed that the presence of cystic lesions was not necessarily related to the presence of viral RNA as was observed for Bird 4 24 h after exposure.
Figure 2. Astrovirus RNA was detected in epithelial cells of RSS-exposed chickens. 2a: Sections from the duodenal loop of commercial broiler chickens exposed either to RSS-contaminated litter (RSS) or chickens exposed to fresh wood shavings (control) were exposed to antisense DIG-labelled riboprobes specific to a chicken astrovirus (CAstV), ANV-1, ANV-2, and a chicken parvovirus (ChPV). Selected areas (asterisks) of RSS-infected chickens are shown inset. Bar in picture of lower magnification = 100 mm; bar in inset = 20 mm. 2b: DF-1 cells were either mock transfected (a), or transfected with a DIG-labelled sense cRNA riboprobe (b), or transfected with unlabelled sense riboprobe (c). The fixed cells of (a) and (c) were hybridized with DIG-labelled antisense riboprobe. The NBT/BCIP reagents were applied to all three samples for colorimetric detection of the DIG-labelled cRNA probe. Bar = 50 mm. Bar in inset with higher magnification = 20 mm. T, transfection; P, probe.
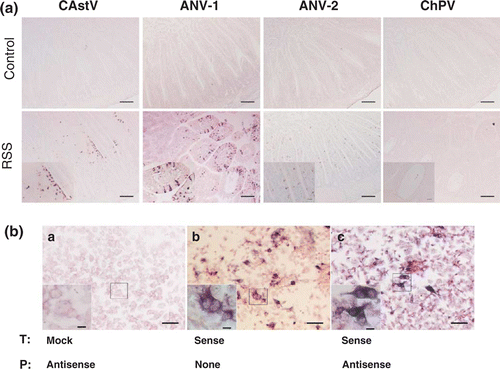
Figure 3. Presence of viral RNA specific for astroviruses during exposure to RSS-contaminated litter. Sections from the duodenal loop of commercial broiler chickens exposed either to RSS-contaminated litter (RSS) or to fresh wood shavings (control) were exposed to antisense DIG-labelled riboprobes specific to a chicken astrovirus (CAstV), ANV-1, and ANV-2 were scored for the presence of an ISH signal on an individual basis during the first 5 days after exposure (day p.i.). The presence of cystic lesions per cross-section is indicated (+). The ISH score was estimated on the basis of the following scale: 0 = no signals; 1 = < 5 signals per high-power field; 2 = five to 15 signals per high-power field; 3 = > 5 signals per high-power field.
The use of both sense and antisense ISH probes specific for parvovirus resulted in no signal in any samples at any time points investigated. Since this result was not expected, experiments were performed to evaluate whether the antisense parvovirus probe was functional. Thus, DF1 cells were left untransfected, or were transfected with either the labelled sense probe to verify the reaction conditions or were transfected with the unlabelled sense probe and probed with the labelled antisense probe as shown in b. The results showed clearly that the antisense parvovirus probe was able to bind to the sense parvovirus cRNA. This result was an additional strong indicator that no parvovirus was replicating in the duodenal loop of the investigated intestines.
Discussion
Although the aetiology for RSS remains unknown, early investigations revealed that RSS probably has a viral aetiology (Decaesstecker et al., Citation1986; Smart et al., Citation1988). These observations have been supported by experiments using either filtered intestinal content (Nili et al., Citation2007) or chloroform-treated, filtered intestinal content (Sellers et al., Citation2010). The latter supported the assumption that the viruses causing RSS are non-enveloped. Initially, reovirus was believed to be the major causative agent for RSS since these viruses have been identified in RSS-affected chickens (Rekik et al., Citation1987; Kouwenhoven et al., Citation1988; van Loon et al., Citation2001). In addition, intestinal lesions typical for RSS have been reported in specific pathogen free chickens after infection with reovirus of enteric origin (Shirai et al., Citation1990; Goodwin et al., Citation1993; Songserm et al., Citation2003). In contrast, neutralization of reovirus from infective homogenates or vaccination of breeder hens against reovirus did not reduce the severity of RSS (Eidson et al., Citation1985). Also viruses from a variety of different virus families (Adenoviridae, Parvoviridae, Togaviridae) have been associated with RSS (Kouwenhoven et al., Citation1978; McNulty et al., Citation1984; Zsak et al., Citation2008). However, the exact aetiology of the disease has not been proven to date but more than one agent has been proposed to be involved in this disease syndrome (Rebel et al., Citation2006). Despite viral multiplication in experimentally inoculated birds, no clinical signs or growth retardation were observed in specific pathogen free and broiler chickens infected with a reovirus or parvovirus, but abnormal faeces and reduction in weight gains were observed after infection with the field materials and entero-like viruses (Decaesstecker et al., Citation1986). Otto et al. (Citation2006) described a correlation between the presence of cystic lesions in the intestine and the presence of rotavirus. Furthermore, it has been described that infection of specific pathogen free Leghorn chickens with a chicken astrovirus resulted in mild diarrhoea and some distension of the small intestine (Baxendale & Mebatsion, Citation2004).
In order to investigate the aetiology of RSS, the investigations described here focused on three members of the Astroviridae family and a chicken parvovirus. In addition, the possibility of an immuno-compromising component associated with the disease was investigated. The data clearly show that the RSS challenge was severe, as indicated by the dramatic difference in weight gain and presence of cystic lesions in the small intestine. The difference in weight at day 12 p.i. in about 70% of the RSS-litter exposed group was greater than described before (50%) in a very similar challenge model (Sellers et al., Citation2010). In both cases, the number of birds with cystic lesions at day 12 p.i. was very similar. In this study, 22 out of 30 birds showed cystic lesions compared with 20 out of 30 birds in the study previously described (Sellers et al., Citation2010). This indicated that the presence of either cystic lesions in the duodenum or decreased weight gain cannot be used as a standalone indicator for RSS, but the presence of lesions and a decreased weight gain can be used as a strong indicator for RSS when a certain number of chickens are included in the study. The latter holds true since chickens with a comparable higher weight showed a high number of lesions while birds with a low weight showed a low number of lesions (see also c). Another reason might be that the load of the infectious agent(s) was much higher. But since the exact cause for RSS is unknown it cannot be investigated. Interestingly, the average of the cystic lesions were detected in the small intestine in 80% of the RSS group as early as 24 h following exposure, and peaked at day 4 after exposure but declined until the end of the study. The dynamics for the presence of the lesions was described previously by Smart et al. (Citation1988), where the first cystic intestinal lesions were observed as early as 3 days after inoculation due to the sampling schedule used in that study. It is possible that lesions in this study were present at an earlier time point. The immediate presence of cystic lesions in the intestine followed by lymphocytic depletion in the immune organs 5 to 6 days later is probably not a direct result of any of the four viruses targeted in this study. The absence of ISH signals in the immune organ supports this notion. These observations indicate rather that RSS-contaminated litter from poultry houses harbours other pathogens that cause lesions in the bursa of Fabricius and the thymus of the chicken. This was not surprising and has been shown before (Reece et al., Citation1984; Montgomery et al., Citation1997; Nili et al., Citation2007). Another possibility is that an organ is involved which regulates the growth of chickens, such as the pituitary gland that synthesizes the growth hormone. But to test this hypothesis, another set of experiments needs to be performed. Also other organs could be included, such as the kidney and liver, since these organs are important for growth and therefore have the potential for involvement in the disease complex.
The absence of ISH signals using both parvovirus probes provided critical evidence that parvovirus, although described to be present in RSS cases (Zsak et al., Citation2008), may not play an important role in the aetiology of the disease. Although Kisary et al. (Citation1984) described the presence of parvovirus in guts of chickens that suffered from RSS and showed later that the obtained parvovirus ABU isolate was able to induce growth retardation in chickens (Kisary, Citation1985), other experiments in broiler chickens using the same ABU isolate could not confirm the results (Decaesstecker et al., Citation1986) since neither weight depression nor lesions in the intestines were observed. Additional evidence has been reported that implies chicken parvoviruses might play an important role in enteric diseases in poultry since parvoviruses were detected in most cases with an enteric disease in chickens and turkeys (Palade et al., Citation2011). Due to the lack of a virus isolate and induction of the disease complex with such an isolate, however, the evidence presented to date is only circumstantial (Kisary, Citation1985; Zsak et al., Citation2008; Palade et al., Citation2011). As described here, the presence of signals for three members of the family Astroviridae (a new chicken astrovirus, ANV-1, ANV-2) may provide additional in vivo support that members of this virus family play an important role for the induction of the disease. Some known avian astroviruses have previously been detected by RT-PCR in materials obtained from RSS-affected chickens (Pantin-Jackwood et al., Citation2008a, Pantin-Jackwood et al., Citation2011; Smyth et al., Citation2009; Spackman et al., Citation2010).
We showed, for the first time, a physical presence of astroviruses at locations in the intestine where RSS-associated lesions were also observed. Initially, we were concerned that ISH probes for ANV-1 and ANV-2 might cross-react with the viral RNA of the other ANV, but the results showed that while ISH signals in the consecutive intestinal sections were present for ANV-1, the next section was negative for both chicken astrovirus and ANV-2, respectively. Although cystic lesions were present soon after exposure to the RSS-contaminated chicken litter, the ISH signals observed were limited to the epithelial cells in the intestine. The results of this study implied that the lesions in the intestine were caused by another viral agent or that cystic enteropathy is coincidentally present but not associated with the outcome of the disease. This hypothesis is supported by the early observation of cystic enteropathy at 24 h after exposure but a lack of ISH staining in the crypts. On the other hand, experimental evidence using an recombinant astrovirus capsid-based vaccine indicated that the new chicken astrovirus might play a role in this disease (Sellers et al., Citation2010). Thus, further experiments are necessary and already underway to clarify these remaining questions.
References
- Baxendale , W. and Mebatsion , T. 2004 . The isolation and characterisation of astroviruses from chickens . Avian Pathology , 33 : 364 – 370 .
- Day , J.M. and Zsak , L. 2010 . Determination and analysis of the full-length chicken parvovirus genome . Virology , 399 : 59 – 64 .
- Decaesstecker , M. , Charlier , G. and Meulemans , G. 1986 . Significance of parvoviruses, entero-like viruses and reoviruses in the aetiology of the chicken malabsorption syndrome . Avian Pathology , 15 : 769 – 782 .
- Eidson , C.S. , Kleven , S.H. and Fletcher , O.J. 1985 . Performance of broiler progeny of breeder flocks vaccinated with inactivated oil emulsion malabsorption syndrome virus vaccine . Poultry Science , 64 : 2081 – 2086 .
- Goodwin , M.A. , Davis , J.F. and Player , E.C. 1993 . Reovirus-associated enteritis in Georgia broiler chicks . Avian Diseases , 37 : 229 – 233 .
- Himly , M. , Foster , D.N. , Bottoli , I. , Iacovoni , J.S. and Vogt , P.K. 1998 . The DF-1 chicken fibroblast cell line: transformation induced by diverse oncogenes and cell death resulting from infection by avian leukosis viruses . Virology , 248 : 295 – 304 .
- Imada , T. , Yamaguchi , S. , Mase , M. , Tsukamoto , K. , Kubo , M. and Morooka , A. 2000 . Avian nephritis virus (ANV) as a new member of the family Astroviridae and construction of infectious ANV cDNA . Journal of Virology , 74 : 8487 – 8493 .
- Kisary , J. 1985 . Experimental infection of chicken embryos and day-old chickens with parvovirus of chicken origin . Avian Pathology , 14 : 1 – 7 .
- Kisary , J. , Nagy , B. and Bitay , Z. 1984 . Presence of parvoviruses in the intestine of chickens showing stunting syndrome . Avian Pathology , 13 : 339 – 343 .
- Koci , M.D. and Schultz-Cherry , S. 2002 . Avian astroviruses . Avian Pathology , 31 : 213 – 227 .
- Koci , M.D. , Moser , L.A. , Kelley , L.A. , Larsen , D. , Brown , C.C. and Schultz-Cherry , S. 2003 . Astrovirus induces diarrhea in the absence of inflammation and cell death . Journal of Virology , 77 : 11798 – 11808 .
- Kouwenhoven , B. , Davelaar , F.G. and Van Walsum , J. 1978 . Infectious proventriculitis causing runting in broilers . Avian Pathology , 7 : 183 – 187 .
- Kouwenhoven , B. , Vertommen , M. and Goren , E. 1988 . Investigations into the role of reovirus in the malabsorption syndrome . Avian Pathology , 17 : 879 – 892 .
- McNulty , M.S. , Allan , G.M. , Connor , T.J. , McFerran , J.B. and McCracken , R.M. 1984 . An entero-like virus associated with the runting syndrome in broiler chickens . Avian Pathology , 13 : 429 – 439 .
- Montgomery , R.D. , Boyle , C.R. , Maslin , W.R. and Magee , D.L. 1997 . Attempts to reproduce a runting/stunting-type syndrome using infectious agents isolated from affected Mississippi broilers . Avian Diseases , 41 : 80 – 92 .
- Nili , H. , Jahantigh , M. and Nazifi , S. 2007 . Clinical observation, pathology, and serum biochemical changes in infectious stunting syndrome of broiler chickens . Comparative Clinical Pathology , 16 : 161 – 166 .
- Olsen , D.E. 1977 . Isolation of a reovirus-like agent from broiler chicks with diarrhea and stunting . Proceedings of the 26th Western Poultry Diseases Conference pp. 131 – 139 . Davis , California .
- Otto , P. , Liebler-Tenorio , E.M. , Elschner , M. , Reetz , J. , Lohren , U. and Diller , R. 2006 . Detection of rotaviruses and intestinal lesions in broiler chicks from flocks with runting and stunting syndrome (RSS) . Avian Diseases , 50 : 411 – 418 .
- Palade , E.A , Kisary , J. , Benyeda , Z. , Mándoki , M. , Balka , G. , Jakab , C. , Végh , B. , Demeter , Z. and Rusvai , M. 2011 . Naturally occurring parvoviral infection in Hungarian broiler flocks . Avian Pathology , 40 : 191 – 197 .
- Pantin-Jackwood , M.J. , Day , J.M. , Jackwood , M.W. and Spackman , E. 2008a . Enteric viruses detected by molecular methods in commercial chicken and turkey flocks in the United States between 2005 and 2006 . Avian Diseases , 52 : 235 – 244 .
- Pantin-Jackwood , M.J. , Spackman , E. and Day , J.M. 2008b . Pathogenesis of type 2 turkey astroviruses with variant capsid genes in 2-day-old specific pathogen free poults . Avian Pathology , 37 : 193 – 201 .
- Pantin-Jackwood , M.J. , Strother , K.O. , Mundt , E. , Zsak , L. , Day , J.M. and Spackman , E. 2011 . Molecular characterization of avian astroviruses . Archives of Virology , 156 : 235 – 244 .
- Rebel , J. M. J. , Balk , F. R. M. , Post , J. , Van Hemert , S. , Zekarias , B. and Stockhofe , N. 2006 . Malabsorption syndrome in broilers . World's Poultry Science Journal , 62 : 17 – 30 .
- Reece , R.L. , Hooper , P.T. , Tate , S.H. , Beddome , V.D. , Forsyth , W.M. , Scott , P.C. and Barr , D.A. 1984 . Field, clinical and pathological observations of a runting and stunting syndrome in broilers . Veterinary Record , 115 : 483 – 485 .
- Rekik , M.R. , Silim , A. , & Bernier , G. 1987 . Experimental infection of broiler chickens using different serotypes of reovirus from malabsorption syndrome cases . Proceedings of the 36th Western Poultry Diseases Conference pp. 146 – 148 . Davis , California .
- Sellers , H. , Linneman , E. , Icard , A.H. and Mundt , E. 2010 . A purified recombinant baculovirus expressed capsid protein of a new astrovirus provides partial protection to runting-stunting syndrome in chickens . Vaccine , 28 : 1253 – 1263 .
- Shirai , J. , Obata , H. , Nakamura , K. , Furuta , K. , Hihara , H. and Kawamura , H. 1990 . Experimental infection in specific-pathogen-free chicks with avian reovirus and avian nephritis virus isolated from broiler chicks showing runting syndrome . Avian Diseases , 34 : 295 – 303 .
- Sklan , D. 2001 . Development of the digestive tract of poultry . World's Poultry Science Journal , 57 : 415 – 428 .
- Smart , I.J. , Barr , D.A. , Reece , R.L. , Forsyth , W.M. and Ewing , I. 1988 . Experimental reproduction of the runting-stunting syndrome of broiler chickens . Avian Pathology , 17 : 617 – 627 .
- Smith , J.A. 2008 . Runting/Stunting/Cystic Enteritis Syndrome . Available online at http://www.thepoultrysite.com/articles/1114/runting-stunting-cystic-enteritis-syndrome (accessed Mar 24 2011) .
- Smyth , V.J. , Jewhurst , H.L. , Adair , B.M. and Todd , D. 2009 . Detection of chicken astrovirus by reverse transcriptase-polymerase chain reaction . Avian Pathology , 38 : 293 – 299 .
- Songserm , T. , Pol , J.M. , van Roozelaar , D. , Kok , G.L. , Wagenaar , F. and ter Huurne , A.A. 2000 . A comparative study of the pathogenesis of malabsorption syndrome in broilers . Avian Diseases , 44 : 556 – 567 .
- Songserm , T. , Engel , B. , van Roozelaar , D.J. , Kok , G.L. , Pijpers , A. , Pol , J.M. and ter Huurne , A.A. 2002a . Cellular immune response in the small intestine of two broiler chicken lines orally inoculated with malabsorption syndrome homogenates . Veterinary Immunology and Immunopathology , 85 : 51 – 62 .
- Songserm , T. , Zekarias , B. , van Roozelaar , D.J. , Kok , R.S. , Pol , J.M. , Pijpers , A.A. and ter Huurne , A.A. 2002b . Experimental reproduction of malabsorption syndrome with different combinations of reovirus, Escherichia coli, and treated homogenates obtained from broilers . Avian Diseases , 46 : 87 – 94 .
- Songserm , T. , van Roozelaar , D. , Kant , A. , Pol , J. , Pijpers , A. and ter Huurne , A. 2003 . Enteropathogenicity of Dutch and German avian reoviruses in SPF white leghorn chickens and broilers . Veterinary Record , 34 : 285 – 295 .
- Spackman , E. , Day , J.M. and Pantin-Jackwood , M.J. 2010 . Astrovirus, reovirus, and rotavirus concomitant infection causes decreased weight gain in broad-breasted white poults . Avian Diseases , 54 : 16 – 21 .
- van Loon , A.A. , Koopman , H.C. , Kosman , W. , Mumczur , J. , Szeleszczuk , O. , Karpinska , E. , Kosowska , G. and Lutticken , D. 2001 . Isolation of a new serotype of avian reovirus associated with malabsorption syndrome in chickens . Veterinary Quarterly , 23 : 129 – 133 .
- Zekarias , B. , Stockhofe-Zurwieden , N. , Post , J. , Balk , F. , van Reenen , C. , Gruys , E. and Rebel , J.M. 2005 . The pathogenesis of and susceptibility to malabsorption syndrome in broilers is associated with heterophil influx into the intestinal mucosa and epithelial apoptosis . Avian Pathology , 34 : 402 – 407 .
- Zsak , L. , Strother , K.O. and Kisary , J. 2008 . Partial genome sequence analysis of parvoviruses associated with enteric disease in poultry . Avian Pathology , 37 : 435 – 441 .