Abstract
Four meat turkey farms with a history of lameness were investigated for the presence of Mycoplasma synoviae by testing one flock per farm for antibodies with the rapid plate agglutination (RPA) test and/or for M. synoviae DNA by polymerase chain reaction (PCR). Flocks were sampled every 2 weeks from 6 weeks of age until slaughter. If PCR results were positive, tracheal swabs were taken for mycoplasma isolation and swollen joints were sampled for general bacteriology, mycoplasma culture and virology. In one flock, all M. synoviae diagnostic tests were negative but reovirus was isolated. In the other flocks, M. synoviae was the only pathogen associated with lameness due to joint disease. M. synoviae RPA tests became positive 6 to 8 weeks later than PCR. An experimental infection was then conducted in male meat turkeys in which a negative control group was sham inoculated at 8 weeks of age, while three other groups were inoculated intravenously with M. synoviae. Turkeys in group LCh were given 105 colony-forming units (CFU) of an arthropathic M. synoviae chicken strain at 8 weeks; group LHCh was given a low (105 CFU) dose at 8 weeks, followed by a high (108 CFU) dose at 12 weeks, of the same chicken strain; and group HTu was inoculated with 108 CFU of a M. synoviae turkey joint isolate from the field study. Post-mortem examination, histopathology, serology, bacteriology and mycoplasma culture were performed at 19 weeks of age. A dose effect was found after comparing the LCh and the LHCh groups. No significant difference was observed between the HTu (108 CFU/bird) and the LCh (105 CFU/bird) group regarding the number of turkeys with arthritis, the number of M. synoviae reisolations and the mean microscopic lesion scores of joints, indicating that the M. synoviae chicken strain has greater arthropathogenic potential and that infection of turkeys in the field with such a strain may possibly have a greater clinical and economic impact.
Introduction
Lameness is an important cause of economic loss in the poultry industry and particularly in turkey production. Both, infectious and non-infectious conditions may be responsible for lameness due to joint problems in the young and the adult turkey (Olson et al., Citation1956; Clark et al., Citation1981; Huff et al., Citation2000; Kleven & Ferguson-Noel, Citation2008). Escherichia coli, Staphylococcus aureus and Ornithobacterium rhinotracheale are commonly associated with joint disease in turkeys (Nairn, Citation1973; Huff et al., Citation2000; Szalay et al., Citation2002). Mycoplasma meleagridis and Mycoplasma iowae have also been reported as a cause of leg disorders resulting in lameness in turkeys; however, field reports of this are scarce (Bradbury & Kleven, Citation2008; Chin et al., Citation2008). In contrast, Mycoplasma synoviae is a fairly common cause of lameness in commercial turkeys (Kleven & Ferguson-Noel, Citation2008).
E. coli and S. aureus arthritis and osteomyelitis of septic origin have been described as the cause of mild to severe lameness and retarded growth in turkeys. There is a high correlation between these lesions and green liver discolouration as well as higher liver weights, which has been described as the turkey osteomyelitis complex. The osteomyelitis is generally found in the tibiotarsus, femur, humerus and vertebrae, while the arthritis is localized in the hock, hip and wing joints (Nairn, Citation1973; Huff et al., Citation2000). Histologically, the inflammatory response is characterized by an increase in heterophils, leucocytes and monocytes in affected joints.
O. rhinotracheale has been reported to induce osteomyelitis of the cranial bones in turkeys causing nervous signs, movement disturbances and recumbence (Szalay et al., Citation2002). In the cranial bones a severe heterophilic osteomyelitis and in the muscles surrounding the bones a moderate inflammation with mainly mononuclear cells have been observed (Szalay et al., Citation2002).
M. iowae has been associated with the occurrence of valgus and skeletal deformities in turkeys in the field, where deformities of the vertebrae, shortening of the tarsometatarsal bones and curling of toes were observed (Trampel & Goll, Citation1994; Ley et al., Citation2010). Histological examination revealed irregular joint surfaces due to degeneration and necrosis. Degeneration of the cartilage was characterized by accumulation of eosinophilic material with loss and necrosis of chondrocytes (Ley et al., Citation2010).
M. meleagridis has also been associated with skeletal abnormalities in young poults causing the so-called turkey syndrome ‘65 (Wise et al., Citation1974), which is characterized by bowing and shortening of the tarsometatarsal bones and deformation of the vertebrae. Hock swelling may also be observed. The articular cartilage surfaces may show lower cell density and abnormal chrondrocytes at microscopy (Wise et al., Citation1973).
M. synoviae infections with arthropathic strains in turkeys induce synovitis and subsequent lameness. Usually, the synovial membranes of the sternal bursa, foot and hock joints are affected. The diseased joints, tendons and bursa are warm, swollen and often filled with fibrinopurulent exudate (Landman & Feberwee, Citation2001; Kleven & Ferguson-Noel, Citation2008). Microscopically, infiltrates of inflammatory cells are observed (heterophils, lymphocytes and macrophages) and also hyperplasia of the affected synovial membranes (Landman & Feberwee, Citation2001; Kleven & Ferguson-Noel, Citation2008).
Non-infectious causes of lameness, induced by rapid growth and heavy body weight in particular, have been associated with musculoskeletal disorders in turkeys. The articular cartilage of rapidly growing birds can show focal avascular or ischaemic necrosis in the intratarsal and femorotibial joint, frequently resulting in joint lesions that are often missed during necropsy (Julian, 1998; 2005).
In parallel with outbreaks of arthropathic M. synoviae infections in chickens in the Netherlands (Landman & Feberwee, 2001; 2004), poultry veterinarians reported an increasing incidence of lameness, growth retardation, increased number of treatments with antibiotics and increased condemnation rates at the slaughter house in meat turkeys. The results of field and experimental studies in chickens showed an association between lameness and M. synoviae infection (Landman & Feberwee, Citation2001), suggesting that infections with arthropathic M. synoviae strains could also be responsible for the joint pathology affecting the turkey industry. To address this question, a longitudinal field study was performed during 2001 and 2002 in order to verify whether M. synoviae was indeed associated with the emerging joint pathology in turkeys. Subsequently, during 2003 and 2004, an experimental infection was conducted with M. synoviae isolates obtained from field cases to assess the causal relationship between them and with the turkey lameness problems.
Materials and Methods
Longitudinal field study
A longitudinal field study was performed in close collaboration with five veterinary practices, the turkey industry and the Animal Health Service (GD). Flocks that were serologically positive for Mycoplasma gallisepticum were excluded from the study to avoid possible misinterpretation of results in cases of mixed infection with M. synoviae. Moreover, all flocks originated from M. gallisepticum-free and M. meleagridis-free parent stock, complying with European legislation (Commission decision: 2009/158/EU).
A total of eight flocks (one house per farm) was set as the primary study target, with a maximum of two flocks per veterinary practice. The selection of flocks was based on the history of two previous flocks; that is, a slaughterhouse report showing increased condemnation rates due to joint pathology and clinical problems with lameness on the farm, characterized by swollen joints as noted by the farmer, and/or by post-mortem analysis performed by the practitioner. Also the previous flock was required to be free of M. gallisepticum.
Sampling and monitoring for the longitudinal field study
Sampling of participating flocks started at 6 weeks of age and continued until slaughter at 20 weeks. Twenty-four blood samples per flock were collected every 14 days and tested by the rapid plate agglutination (RPA) test for M. gallisepticum and M. synoviae as described under the section ‘Serology’. At the same time 24 tracheal swabs were taken for M. synoviae polymerase chain reaction (PCR) as described below. If the M. synoviae PCR gave a positive result, 60 tracheal swabs were collected for mycoplasma isolation. If M. synoviae was isolated, no further tracheal swabs were taken. As soon as birds with swollen joints were observed, a maximum of five birds were selected for post-mortem examination, general bacteriology, mycoplasma culture and virus isolation of affected joints.
Experimental infection
The arthropathic potential of one M. synoviae isolate originating from an affected joint of a meat turkey of Farm 1 from the field study (turkey/NL/Dev/SP1240/01) and of another M. synoviae isolate with proven arthropathogenic potential from chickens (chicken/NL/Dev/801979Rob/00) (Landman & Feberwee, Citation2001) was assessed in male meat turkeys. Four experimental groups were used, and were inoculated intravenously at 8 weeks of age. The first group consisted of 24 birds, which were sham inoculated with 1 ml Mycoplasma Experience (ME) broth (Avian Mycoplasma Liquid Medium; Mycoplasma Experience, Reigate, UK) and acted as a negative control (NC) group. A second group (LCh) consisted of 18 birds, each being inoculated with 1 ml ME broth containing 105 colony-forming units (CFU) of the M. synoviae chicken isolate. The third group (LHCh) consisted of 18 birds, which were each inoculated with 1 ml ME broth containing 105 CFU of the M. synoviae chicken isolate at 8 weeks of age and then with 1 ml ME broth containing 108 CFU of the isolate at 12 weeks of age. The fourth group (HTu) consisted of 36 birds, and each received 1 ml ME broth containing 108 CFU of the M. synoviae turkey isolate at 8 weeks of age. Preparation and control of the inocula were performed as described under general bacteriology, mycoplasma culture and virus isolation. The experiment lasted 11 weeks.
Experimental birds and housing
Male meat turkeys were obtained at 6 weeks of age from a commercial farm. The turkey parent flock repeatedly tested serologically negative for M. gallisepticum, M. meleagridis and M. synoviae. The birds were weighed, divided into weight classes and allocated at random into 32 identical floor pen units of 1.5×1.7 m2 (0.6 m2/bird) with wood shavings as bedding, a hanging bell drinker (40 cm diameter) and a feed trough of 525 cm (29 cm/bird). Birds were provided with 16 hours of light daily, and feed and drinking water were given ad libitum.
Sampling and monitoring of experimental birds
All birds were weighed at the beginning and at the end of the experiment. Blood samples were collected for serology before inoculation of M. synoviae and at the end of the experiment (19 weeks of age). Also, before inoculation of M. synoviae, a tracheal swab was collected from each bird for M. synoviae PCR. During the whole experiment the occurrence of lameness was recorded daily.
All experimental birds were subjected to post-mortem examination at 19 weeks of age and joint lesions were assessed macroscopically. Routine bacteriological analysis and mycoplasma culture of affected joints was performed. Additionally, one affected joint per bird was fixed in formaldehyde solution and processed for histopathology (haematoxylin and eosin stain). Joint specimens for bacteriology and histology were collected from approximately one-half of the negative control birds.
Serology
M. synoviae serology was performed using the RPA test as described earlier (Feberwee et al., Citation2005b). Sera were analysed immediately after collection of the blood samples. In short, the undiluted sera were tested with the RPA antigen (Nobilis MS antigen; Intervet International, Boxmeer, the Netherlands). If there was a positive result (agglutination), sera were serially diluted from 1:4 to 1:32 in 0.5 M phosphate-buffered saline (pH 7.2) and re-tested in the RPA test. If a serum dilution of 1:8 or higher showed agglutination, then it was considered to be specifically positive for M. synoviae. M. gallisepticum serology was carried out using the RPA (Nobilis MG antigen; Intervet International) and the haemagglutination inhibition test (Feberwee et al., Citation2005b).
M. synoviae PCR test
Tracheal samples were collected using sterile cotton swabs (Tubed Sterile Dryswab™ Rayon MW102; Medical Wire & Equipment, Corsham, UK). For the longitudinal study, pools of six samples were tested while they were individually investigated in the experimental infection. The swabs were washed in 1 ml phosphate-buffered saline, which was then used for DNA extraction and PCR testing. The M. synoviae PCR test was performed using a commercial M. synoviae DNA Test Kit (IDEXX Laboratories, Westbrook, Maine, USA). DNA extraction and interpretation of results were performed according to the manufacturer's instructions.
General bacteriology, mycoplasma culture and virus isolation
In the longitudinal field study, tracheal swabs for mycoplasma culture were dipped in ME broth (Avian Mycoplasma Liquid Medium; Mycoplasma Experience) before sampling, transported at 4°C and individually plated out on ME agar within 3 h of sampling.
For preparation of the inocula, a frozen vial containing 1 ml ME broth with M. synoviae (107 CFU/ml) was thawed, added to fresh 50 ml ME broth and incubated at 37°C until a change of colour was observed. Viable counts were established following the International Organization for Standardization (Citation1985) guidelines. Briefly, decimal dilutions of culture were made in ME broth (10−1, 10−2, 10−3, 10−4, 10−5, 10−6 and 10−7) (International Organization for Standardization, Citation1983), and 20 µl of the undiluted broth and of each dilution were plated on ME agar (Avian Mycoplasma Solid Medium; Mycoplasma Experience). Incubation was performed at 37°C until mycoplasma colonies were visible, and then the initial bacterial concentration was calculated.
Bacteriology and mycoplasma culture of the affected joints from birds of both the longitudinal and experimental studies was performed after sterilizing the outer surface of the joint with a hot scalpel blade. Subsequently, an incision was made with a sterile scalpel and two dry sterile cotton swabs (Tubed Sterile Dryswab™ Rayon MW102; Medical Wire & Equipment) were used to sample the affected joint. One swab was plated out on a 5% sheep blood agar plate (K004; BioTrading Benelux B.V., Mijdrecht, the Netherlands) for general bacteriology and the other on a ME agar plate for mycoplasma culture. The sheep blood agar was incubated at 37°C and 10% CO2 and examined after 24 and 48 h of incubation. The ME agar plates were incubated at 37°C in a humid environment and examined for colonies every 2 to 3 days up to 28 days. If mycoplasma growth occurred, one separate colony was plated out on a fresh ME agar and incubated at 37°C. Thereafter, approximately 2×0.5 cm2 agar with mycoplasma colonies was transferred to ME broth and incubated at 37°C. Identification of M. synoviae was performed with the previously described PCR using broths showing a colour change (Feberwee et al., Citation2005a).
In addition, joint fluid samples—which were first treated with benzyl penicillin sodium (10,000 IE/ml), streptomycin (10 mg/ml) and amphotericin B (25 µg/ml)—were examined for viruses by inoculation of the allantoic sac, the yolk sac and chorioallantoic membrane of 9-day-old, 5-day-old and 10-day-old specific pathogen free chicken embryos, respectively. The eggs were candled daily in order to identify dead and/or deformed embryos, which were then removed from the incubator and tested for the most important avian viruses: avian encephalomyelitis virus, infectious bronchitis virus, infectious bursal disease virus, infectious laryngotracheitis virus, adenovirus and reovirus (Cottral, 1978). In cases where no virus was detected, up to three additional blind passages were performed at 6-day intervals.
Histopathology
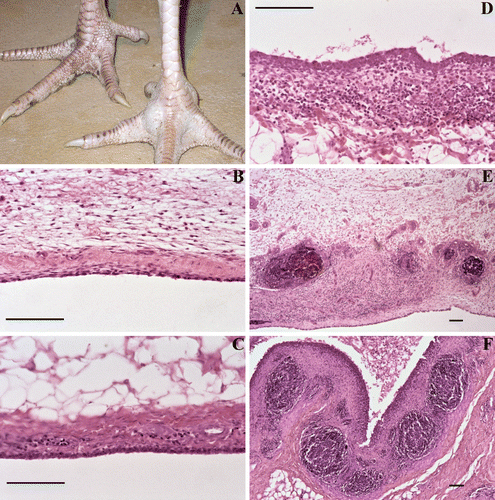
One affected joint per bird was fixed in 4% formaldehyde solution, paraffin embedded and processed for histology. (Para)sagittal sections were stained with haematoxylin and eosin and viewed at 100× to 400× magnification by light microscopy. Detailed examination of the synovial membrane was used to grade lesions semi-quantitatively as follows: score 0=90 to 95% of the tissue samples did not show abnormalities, in the remaining area no free lying plasma cells and/or granulocytes were found in some cases, hypertrophy of the synovial membrane was noted and/or a few haemorrhages that, based on morphology, were attributed to mechanical stress/damage; score 1=in a larger area than score 0, there were diffuse infiltrates of plasma cells and granulocytes; score 2=in a large part of the tissue sample, confluent areas with plasma cells and granulocytes were found the synovial membrane was altered; score 3=as score 2 but including lymphoid follicles and/or (fibrino-)purulent exudate; and score 4=lesions as described for score 3 were found in the whole tissue section, while (fibrino-)purulent exudate was noted in the articular pouches (). As microscopic lesion score 1 was observed in some birds of the negative control group and regarded as mild aseptic arthritis, only lesion scores ≥2 were used to calculate the mean lesion score per group and for statistical analysis.
Figure 1. A swollen turkey foot joint with experimentally induced M. synoviae arthritis is shown in panel A (right view). Detailed examination of the synovial membrane was used to grade arthritic lesions semi-quantitatively as shown in the remaining panels and the table below. Lesion score ≥2 was considered relevant for calculation of mean lesion scores per group and statistical analysis. Bar = 100 µm.
Statistical analysis
Growth of the birds was analysed by means of Kruskal–Wallis one-way analysis of variance. The Kruskal–Wallis all-pairwise comparisons test was performed as post-hoc analysis in order to compare all possible pairwise differences between the means of the different treatment groups. The effect of different treatments on the occurrence of arthritis was analysed with the chi-square test. Homogeneity of groups was assessed after applying Bonferroni correction. Bonferroni's correction was developed by Keppel to compensate for the alpha inflation. If one or more cells had a frequency <5, the Yates correction was applied on the P value. In all other cases the Pearson chi-square test was applied. Significant differences in the inflammation scores of joints were analysed by Kruskal–Wallis test including a Dunn's test as the post-hoc test. SPSS 11.0 was used for all statistical analyses (SPSS, Citation1999).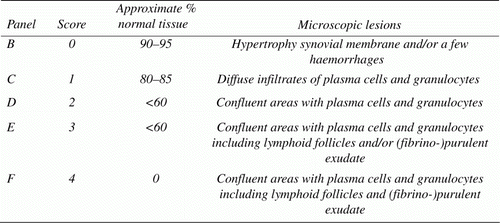
Ethical statement
Experiments were approved by the Institutional Animal Experimental Committee, DEC-Consult Foundation, according to Dutch law on experimental animals (Wet op de dierproeven).
Results
Longitudinal field study
Eight farms, with one house per farm, participated in the study. Flock sizes ranged from 1900 to 7440 birds, which belonged to one commercial breed. Four flocks did not develop arthritis or problems with lameness and yielded negative test results. In contrast, the other four flocks (for convenience identified as Flocks 1 to 4) had problems with lameness, which started at an age varying from 14 to 19 weeks. In Flocks 1 and 2 the onset of lameness was at 14 weeks of age and tracheal swabs were positive by M. synoviae PCR (15 of 60) and by culture (51 of 60) at 8 and 10 weeks of age, respectively. The first positive M. synoviae serological results in these two flocks occurred at 14 and 18 weeks of age, respectively. However, the RPA test was not done at 12 weeks in Flock 1 and at 16 weeks in Flock 2. Therefore, both flocks may have seroconverted two weeks earlier. At post-mortem examination, M. synoviae was also isolated from affected joints (one of four and one of five birds with affected joints of Flocks 1 and 2, respectively). The macroscopic lesions (arthritis) in Flocks 1 and 2 were confined to the hock joints and the breast (breast blister), both being filled with seropurulent material. In Flock 3, the lameness started at 19 weeks of age and the M. synoviae PCR was positive at 18 weeks of age, but no M. synoviae-positive serological result was detected until slaughter (19 weeks of age). No tracheal swabs were available for culture as the flock was slaughtered before sampling. At post-mortem examination, M. synoviae was isolated from affected joints (one of two birds with affected joints). In Flock 4, lameness was detected at 18 weeks of age while M. synoviae PCR and serology results remained negative up to slaughter. However, reovirus was isolated from one affected joint of this flock, and no M. synoviae could be cultured. All flocks were serologically negative for M. gallisepticum (data not shown). Results of general bacteriology of affected joints were negative in all four flocks (). The distribution of the macroscopic lesions found at post-mortem in the birds submitted from the four flocks with lameness is shown in .
Figure 2. Field study: ventral view of the distribution of joint and sternal bursa lesions in birds with arthritis at post-mortem of Flock 1 (n=5), Flock 2 (n=5), Flock 3 (n=2) and Flock 4 (n=1). M. synoviae was isolated from the affected joints of birds of Flock 1 (n=1), Flock 2 (n=1) and Flock 3 (n=1). In Flock 4, only reovirus was isolated from the affected joint of one bird. A dot represents an affected hock joint and a square an affected sternal bursa.
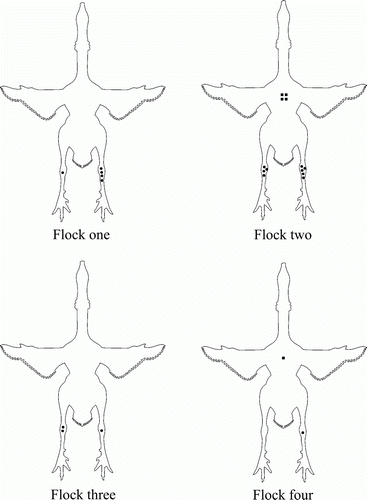
Table 1. Field study: age at onset of arthritis, first positive M. synoviae PCR of tracheal swabs, first M. synoviae positive RPA test and results of mycoplasma culture from trachea and joint specimens.
Experimental infection
Both the M. synoviae chicken strain and the turkey isolate induced severe joint disease in turkey poults after intravenous inoculation. Respiratory signs were not observed during the experiment and, in agreement with this, airsacculitis was not found post-mortem (). In the LCh group, lameness occurred and post-mortem examination revealed increased synovial fluid in 16 of 17 birds. This fluid was purulent in three turkeys, haemorrhagic in five birds and clear in eight birds. Only the birds with purulent or haemorrhagic synovial fluid showed lesion scores ≥2 at microscopy (8/15), while two birds with clear synovial fluid had lesion score 1. The mean histological lesion score of this group was 1.3. M. synoviae was cultured in one of 18 joints (5.5%) and eight birds were serologically positive for M. synoviae (44%) ().
Arthritis was more severe with more joints involved in the LHCh group compared with the LCh and HTu group. At post-mortem examination, moderate to severe joint swelling was observed in all turkeys. Purulent (whitish), haemorrhagic or clear content was found in altered joints. However, histologically arthritis/synovitis (lesion score ≥3) was observed in all birds with a mean histological lesion score of 3.7. M. synoviae was isolated from 10 of 18 affected joints (55.5%) and all tested birds (n=17) were serologically positive for M. synoviae antibodies (). At post-mortem examination, four of 36 birds in the HTu group had severe joint swelling with purulent (whitish) content. Eleven of 36 birds showed haemorrhagic content in the joints, while these were somewhat swollen. Another 10 birds showed increased clear synovial content. In 23 of 34 birds, arthritis was confirmed at histopathology; however, only 15 birds (44%) showed a lesion score ≥2. The mean histological lesion score of this group was 1.5. M. synoviae was cultured in two of 36 joints (5.5%), while 24 of 34 birds (70.5%) were serologically positive for M. synoviae. The lesion score as well as the rate of mycoplasma positive cultures were significantly higher (P <0.05) in the group inoculated twice with the chicken strain compared with all other groups ().
Table 2. Experimental infection: macroscopic and microscopic joint lesions, mycoplasma culture of joint swabs, serology and growth of male meat turkey poults inoculated intravenously with M. synoviae.
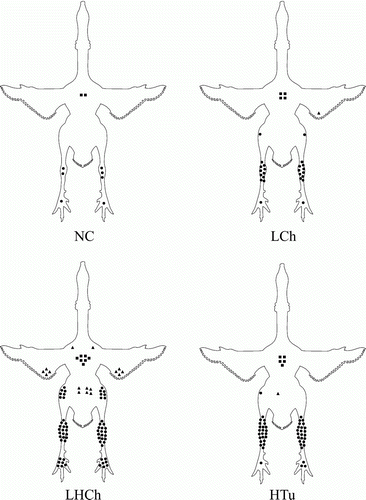
In the NC group, no lameness or airsacculitis were observed during the experiment. However, at post-mortem examination large amounts of synovial fluid were observed frequently. The joint fluid appeared as clear (10 of 24) or had a haemorrhagic aspect (six of 24). At histological examination, three of 12 joints were diagnosed as having aseptic arthritis (lesion score 1). Bacteriological examination and mycoplasma culture results from these joints were negative. Moreover, all birds were also serologically negative for M. synoviae (). The macroscopic distribution of joint lesions confirmed at microscopy (lesion scores 1–4) of all experimental groups is shown in .
Figure 3. Experimental infection: ventral view of the distribution of joint and sternal bursa lesions in birds with arthritis at post-mortem confirmed by histopathology (lesion score 1 to 4) in: the NC group, 3/12 affected with aseptic arthritis (lesion score 1); the LCh group, intravenous inoculation with a low dose (105 CFU/bird) of an arthropathic M. synoviae chicken strain (10/15 affected); the LHCh group, intravenous inoculation with a low dose (105 CFU/bird) and a high dose (108 CFU/bird) of an arthropathic M. synoviae chicken strain (18/18 affected); and the HTu group, intravenous inoculation with a high dose (108 CFU/bird) of an arthropathic M. synoviae turkey strain (23/34 affected). Each symbol represents an affected joint or sternal bursa. A triangle depicts a shoulder, elbow or hip joint; a dot a knee, hock or foot joint; a square represents a sternal bursa.
Discussion
Lameness is an important cause of economic losses in turkey production. Besides bacterial and viral causes, non-infectious aetiologies have also been described (Nairn, Citation1973; Julian, Citation1998; Huff et al., Citation2000; Landman & Feberwee, Citation2001; Szalay et al., Citation2002; Julian, Citation2005; Kleven et al., 2008). In order to address the question of whether M. synoviae was playing a key role in problems with lameness in the turkey industry in the Netherlands, a field study and subsequent experimental study were conducted.
The flocks included in the field study were tested for freedom from M. gallisepticum antibodies; moreover, they originated from M. gallisepticum-negative and M. meleagridis-negative parent stock, while musculoskeletal disorders suggesting a M. meleagridis or M. iowae infection were not observed and have never been reported in the Netherlands. Similarly, the experimental birds originated from Dutch parent stock turkeys that repeatedly tested negative for M. gallisepticum, M. meleagridis and also M. synoviae. Clinical signs of the musculoskeletal system suggesting infection with M. meleagridis or M. iowae were not seen in any of the experimental groups. Additionally, all mycoplasma-positive cultures from joint specimens were confirmed as M. synoviae by PCR, while mycoplasmas were never cultured from joints of the negative control group that remained free of disease, indicating that infection of these birds with M. meleagridis and/or M. iowae before and during the experiment was unlikely to have occurred.
Similar to studies with layer hens (Landman & Feberwee, Citation2001), the results of the field study showed an association between M. synoviae and joint pathology in turkeys, while the experimental infection confirmed that M. synoviae was the cause of the severe problems with lameness in turkeys in our country. However, reovirus was isolated from birds with swollen joints of one flock. Reovirus has been described as the cause of tenosynovitis and arthritis in broilers (Wood & Thornton, Citation1981; Jones et al., Citation1984). Although its isolation from turkeys with tenosynovitis has been reported previously, a causal relationship with the disease has not yet been demonstrated (Al-Alfaleq & Jones, Citation1989).
The field study also showed that the M. synoviae PCR test is more sensitive than the M. synoviae RPA test for the early diagnosis of a M. synoviae infection in turkeys. This finding is in agreement with the results of other studies in turkeys and chickens reporting that antibody response to spontaneous infection with M. synoviae can be delayed, or in some cases even absent, and indicating that additional tests such as PCR are needed for the timely detection of infection (Ortiz & Kleven, Citation1992; Ewing et al., Citation1998; Kleven et al., Citation2001). Moreover, the M. synoviae infection was detected by PCR before development of arthritis, suggesting that the use of M. synoviae PCR test for monitoring purposes may contribute to the reduction of the economic losses caused by arthropathogenic M. synoviae strains because intervention strategies may be applied in the early phases of a disease outbreak.
The experimental infection showed that the lameness and joint pathology could be reproduced in turkeys with both a chicken isolate and a turkey isolate. Signs of lameness were not observed in the NC group; however, microscopically hypertrophy of the synovial membrane and haemorrhages were present, which were explained by mechanical damage due to the large body weights of the turkeys at the end of the fattening period. Particularly in rapidly growing male turkeys, heavy body weights have been associated with musculoskeletal disorders. The articular cartilage of rapidly growing turkeys can show focal avascular or ischaemic necrosis in the intertarsal and femorotibial joints frequently resulting in joint lesions, which are often missed during a necropsy examination of turkeys (Julian, 1998; 2005). No significant difference in growth between the negative control and M. synoviae inoculated groups was observed. This might be explained by the fact that the floor space per bird was much larger than that in the field (0.6 m2/bird in the experiment compared with 0.2 m2/bird in the field), which probably allowed the turkeys easy access to the feed and drinking water despite the occurrence of lameness. It has been shown previously in birds with M. synoviae-associated lameness that weight gain can still be satisfactory if lame birds are housed with more space (Kleven & Ferguson-Noel, Citation2008). Moreover, another study also showed no significant difference in weight gain between the negative control group and a group challenged with an arthropathic M. synoviae strain (Kang et al., Citation2002).
The percentage of turkeys with arthritis, the number of M. synoviae-positive cultures from joints and the microscopic lesion score were higher in the LHCh group (reinoculated 4 weeks later with a higher dose of the arthropathic chicken strain) compared with the LCh group that was challenged once with a lower dose, indicating, not unexpectedly, the occurrence of a dose effect. A similar dose effect was described earlier by Landman & Feberwee (Citation2004) in the experimental induction of arthritis with M. synoviae in brown layer hens.
The fact that the number of turkeys with arthritis, the number of M. synoviae reisolates of affected joints and the mean lesion score were not significantly different in the group inoculated with the lower dose of the chicken isolate compared with the group inoculated with the high dose of the turkey isolate indicates that these M. synoviae strains differ in their arthropathogenic potential. These results agree with the work of Kang et al. (Citation2002), who showed that recent M. synoviae turkey field isolates were less virulent for turkeys than a previously known virulent isolate from turkeys (M. synoviae K1968). The latter isolate also induced synovitis in broilers (Lockaby et al., Citation1998).
A low number of positive mycoplasma cultures was obtained from joints of the LCh and HTu groups (1/18 and 2/36, respectively [=5.5% positive]). This was attributed to the length of the time between M. synoviae inoculation and the culture of joint specimens. The groups inoculated with M. synoviae at 8 weeks of age (LCh and HTu groups) had significantly lower positive cultures compared with the LHCh group, which was reinoculated with M. synoviae at 12 weeks of age. This is in accordance with other studies performed in chickens, where the isolation of M. synoviae was shown to decrease with the chronicity of the infection, with few or no mycoplasmas being detected in blood and/or joint samples examined 6 to 8 weeks after inoculation (Olson & Kerr, Citation1967; Kawakubo et al., Citation1980; Landman & Feberwee, Citation2004). Another factor contributing to the poor yields may have been the low sensitivity of mycoplasma culture as it is currently performed, which could be circumvented by using a more sensitive PCR for the detection of M. synoviae in joints (Dijkman et al., Citation2011). However, at the time the present studies were conducted, there was no validated PCR available for joint specimens.
Although Flocks 1 and 2 of the longitudinal field study showed a relatively low number of positive mycoplasma cultures (1/5=20% positive), this was still in range with what Kawakubo et al. (Citation1980) found in some experimental groups of chickens 2 weeks post-inoculation with M. synoviae.
Airsacculitis was not observed in our study, which is a notable difference from the findings of Kang et al. (Citation2002). A possible explanation is that in the latter study the birds were not only challenged intravenously, but also intratracheally and into the footpad. It is probable that the intravenous administration of M. synoviae prevented the colonization of air sacs (in large numbers).
In conclusion, our findings show that the introduction of a M. synoviae PCR for monitoring may contribute to the early detection of arthropathic M. synoviae isolates, while results of the experimental infection underline the importance of biosecurity in order to avoid cross-infection with M. synoviae between different poultry types, as arthropathic M. synoviae isolates from chickens appear to be more detrimental to turkey poults than those of turkey origin.
Acknowledgements
The present research was funded by the Dutch Commodity Board for Poultry and Eggs. The authors thank Dr R.M. Dwars for performing the histological examinations and DVM P.N.G.M. van Beek for his assistance in the longitudinal field study.
References
- Al-Alfaleq , A.I. and Jones , R.C. 1989 . Pathogenicity of three turkey and three chicken reoviruses for poults and chickens with particular reference to arthritis/tenosynovitis . Avian Pathology , 18 : 433 – 440 .
- Bradbury , J.M. and Kleven , S.H. 2008 . “ Mycoplasma iowae infection ” . In Diseases of Poultry , 12th edn , Edited by: Saif , Y.M. , Barnes , H.J. , Glisson , J.R. , Fadly , A.M. , McDougald , L.R. and Swayne , D.E. 856 – 862 . London , , UK : Blackwell Publishing .
- Chin , R.P. , Yan Ghazikhanian , G. and Kempf , I. 2008 . “ Mycoplasma meleagridis infection ” . In Diseases of Poultry , 12th edn , Edited by: Saif , Y.M. , Barnes , H.J. , Glisson , J.R. , Fadly , A.M. , McDougald , L.R. and Swayne , D.E. 834 – 845 . London , , UK : Blackwell Publishing .
- Clark , S.R. , Barnes , H.J. , Bickford , A.A. , Chin , R.P. and Droual , R. 1981 . Relationship of osteomyelitis and associated soft-tissue lesions with green liver discoloration in tom turkeys . Avian Diseases , 35 : 139 – 147 .
- Cottral , G.E. 1978 . Manual of Standardized Methods for Veterinary Microbiology , 47 – 52 . Ithaca , N.Y : Cornell University Press .
- Ewing , M.L. , Cookson , K.C. , Phillips , R.A. , Turner , K.R. and Kleven , S.H. 1998 . Experimental infection and transmissibility of Mycoplasma synoviae with delayed serologic response in chickens . Avian Diseases , 42 : 230 – 238 .
- Dijkman , R. , Feberwee , A. & Landman , W.J.M. 2011 . Mycoplasma synoviae concentrations in synovial fluid with time. Proceedings of the XVIIth World Veterinary Poultry Association Congress 647 – 652 . Cancún Mexico .
- Feberwee , A. , Dijkstra , J.R. , von Banniseht-Wysmuller , Th.E. , Gielkens , A.L.J. and Wagenaar , J.A. 2005a . Genotyping of Mycoplasma gallisepticum and M. synoviae by amplified fragment length polymorphism (AFLP) analysis and digitalized random amplified polymorphic DNA (RAPD) analysis . Veterinary Microbiology , 111 : 125 – 131 .
- Feberwee , A. , Mekkes , D.R. , de Wit , J.J. , Hartman , E.G. and Pijpers , A. 2005b . Comparison of culture PCR and different serological tests for the detection of Mycoplasma gallisepticum and Mycoplasma synoviae infections . Avian Diseases , 49 : 260 – 268 .
- Huff , G.R. , Huff , W.E. , Rath , N.C. and Balog , J.M. 2000 . Turkey osteomyelitis complex . Poultry Science , 79 : 1050 – 1056 .
- International Organization for Standardization 1983 . Microbiology – General Guidance for the Preparation of Dilutions for Microbiological Examination, ISO 6887 , 1st . Geneva : International Standard Organization .
- International Organization for Standardization 1985 . Microbiology – General Guidance for the Enumeration of Enterobacteriaceae without Resuscitation – MPN Technique and Colony Count Technique, ISO 7402 , 1st . Geneva : International Standard Organization .
- Jones , R.C. , Al-Afaleq , A. , Savage , C.E. and Islam , M.R. 1984 . Reovirus-induced tenosynovitis in chickens: the influence of age at infection . Avian Pathology , 13 : 441 – 457 .
- Julian , R.J. 1998 . Rapid growth problems: ascites and skeletal deformities in broilers . Poultry Science , 77 : 1773 – 1780 .
- Julian , R.J. 2005 . Production and growth related disorders and other metabolic diseases of poultry . Veterinary Journal , 169 : 530 – 569 .
- Kang , M.S. , Gazdzinski , P. and Kleven , S.H. 2002 . Virulence of recent isolates of Mycoplasma synoviae in turkeys . Avian Diseases , 46 : 102 – 110 .
- Kawakubo , Y. , Kume , K. , Yoshioka , M. and Nishiyama , Y. 1980 . Histo- and immuno-pathological studies on experimental Mycoplasma synoviae infection of the chicken . Journal of Comparative Pathology , 90 : 457 – 467 .
- Kleven , S.H. and Ferguson-Noel , N. 2008 . “ Mycoplasma synoviae infection ” . In Diseases of Poultry , 12th edn , Edited by: Saif , Y.M. , Barnes , H.J. , Glisson , J.R. , Fadly , A.M. , McDougald , L.R. and Swayne , D.E. 845 – 856 . London , , UK : Blackwell Publishing .
- Kleven , S.H. , Rowland , G.N. and Kumar , M.C. 2001 . Poor serological response to upper respiratory infection with Mycoplasma synoviae in turkeys . Avian Diseases , 45 : 719 – 723 .
- Landman , W.J.M. and Feberwee , A. 2001 . Field studies on the association between amyloid arthropathy and Mycoplasma synoviae infection, and experimental reproduction of the condition in brown layers . Avian Pathology , 30 : 629 – 639 .
- Landman , W.J.M. and Feberwee , A. 2004 . Aerosol-induced Mycoplasma synoviae arthritis: the synergistic effect of infectious bronchitis virus infection . Avian Pathology , 33 : 591 – 598 .
- Ley , D.H. , Marusak , R.A. , Vivas , E.J. , Barnes , H.J. and Fletcher , O.J. 2010 . Mycoplasma iowae associated with chondrodystrophy in commercial turkeys . Avian Pathology , 39 : 87 – 93 .
- Lockaby , S.B. , Hoerr , F.J. , Lauerman , L.H. and Kleven , S.H. 1998 . Pathogenicity of Mycoplasma synoviae in broiler chickens . Veterinary Pathology , 35 : 178 – 190 .
- Nairn , M.E. 1973 . Bacterial osteomyelitis and synovitis of the turkey . Avian Diseases , 17 : 504 – 517 .
- Olson , N.O. and Kerr , K.M. 1967 . The duration and distribution of synovitis-producing agents in chickens . Avian Diseases , 11 : 578 – 585 .
- Olson , N.O. , Shelton , D.C. , Bletner , J.K. , Munro , D.A. and Anderson , G.C. 1956 . Studies of infectious synovitis in chickens . The American Journal of Veterinary Research , 17 : 747 – 754 .
- Ortiz , A. and Kleven , S.H. 1992 . Serological detection of Mycoplasma synoviae infection in turkeys . Avian Diseases , 36 : 749 – 752 .
- SPSS Inc . 1999 . SPSS Base 11.0 for Windows User's Guide . Chicago , IL : SPSS Inc .
- Szalay , D. , Glavits , R. , Nemes , C. , Kosa , A. and Fodor , L. 2002 . Clinical signs and mortality caused by Ornithobacterium rhinotracheale in turkey flocks . Acta Veterinaria Hungarica , 50 : 297 – 305 .
- Trampel , D.W. and Goll , F. 1994 . Outbreak of Mycoplasma iowae infection in commercial turkey poults . Avian Diseases , 38 : 905 – 909 .
- Wise , D.R. , Boldero , M.K. and Thornton , G.A. 1973 . The pathology and aetiology of turkey syndrome '65 (T.S. 65) . Research in Veterinary Science , 14 : 194 – 200 .
- Wise , D.R. , Fuller , M.K. and Thornton , G.A. 1974 . Experimental reproduction of turkey syndrome '65 with Mycoplasma meleagridis . Research in Veterinary Science , 17 : 236 – 241 .
- Wood , G.W. and Thornton , D.H. 1981 . Experimental infection of broiler chicks with an avian reovirus . Journal of Comparative Pathology , 90 : 29 – 38 .