Abstract
The present study describes the pathological and bacteriological findings and diagnosis by immunoperoxidase and immunofluorescence methods in budgerigars (Melopsittacus undulatus) naturally infected with Salmonella gallinarum obtained from three commercial budgerigar rearing farms. The course of the disease in young budgerigars was peracute or acute, whereas in adult budgerigars the disease was acute or chronic. Clinically, yellow–white diarrhoea was observed in the young budgerigars with the acute form. In the adult budgerigars with the acute and chronic forms, a decrease in feed and water consumption with loss in body condition together with greenish-yellow diarrhoea was generally noted. Peritonitis and pericarditis were the most common findings in young budgerigars at necropsy, while in adult budgerigars scattered grey–white necrotic foci were found in the livers. Histopathologically, the lesions in young budgerigars were characterized with fibrinonecrotic peritonitis and/or pericarditis and necrotic hepatitis. In adult budgerigars with acute infection, hepatic necrosis with focal heterophil infiltration was present; whilst lesions in the chronic cases were granulomatous in nature with the infiltration of macrophages, lymphocytes and histiocytes. For the detection of S. Gallinarum in formalin-fixed, paraffin-embedded tissues, the avidin–biotin peroxidase complex and immunofluorescence methods were used. Both methods showed bacteria to be localized in the liver, kidney, peritoneum, heart, spleen and intestines of both young and adult budgerigars. The results of the present study indicate that the avidin–biotin peroxidase complex method was more sensitive than the immunofluorescence method in the detection of the bacteria.
Introduction
Salmonella enterica serovar Gallinarum (Salmonella Gallinarum) is a non-motile host-adapted salmonella that causes fowl typhoid (FT), a severe systemic disease responsible for heavy economic losses to the commercial poultry industry through morbidity, mortality and reduced egg production (Berchieri et al., Citation2001; Song et al., Citation2002; Parmer & Davies, Citation2007). S. Gallinarum infection primarily causes disease in chickens and turkeys of all ages, whilst the disease has also been described in ducks, quail, ring doves, pheasants, peacocks, peafowl and canaries (Berchieri et al., Citation2001; Shivaprasad & Barrow, Citation2008). The morbidity and mortality of FT are highly variable in poultry and are influenced by host age, host susceptibility, nutrition, flock management, and virulence of S. Gallinarum (the latter being a major factor affecting the severity of the disease; Sato et al., Citation1997). Even though the disease has been well documented (Song et al., Citation2002; Shivaprasad & Barrow, Citation2008), there is no detailed report about the pathological and immunohistochemical findings of the disease in budgerigars (Melopsittacus undulatus).
To date, the causative agent of FT has been reported to cause acute and chronic infections with high morbidity and mortality rates in various young and adult avian species (Sato et al., Citation1997; Lee et al., Citation2001; Audisio & Terzolo, Citation2002). The disease usually follows the ingestion of food or water contaminated by the excreta of clinically infected birds or carriers but can also be transmitted by attendants through contaminated hands, feet and clothes (Lowry et al., Citation1999; Berchieri et al., Citation2001). In the subacute and chronic stages of the disease, the liver and spleen are mostly affected, with multiple white foci, severe swelling and discolouration being observed (Hossain et al., 2006; Shivaprasad & Barrow, Citation2008).
The aim of this study was to describe the pathomorphological and bacteriological findings in young and adult budgerigars naturally infected with S. Gallinarum and to compare the avidin–biotin peroxidase complex (ABC) and direct immunofluorescence (IF) methods for the immunohistochemical localization and distribution of the organism.
Materials and Methods
Three outbreaks of avian salmonellosis were observed in three budgerigar flocks at different localities in Aydın province of the Aegean region, Turkey. Each flock was composed of 600, 450 and 245 budgerigars, respectively. The number of the budgerigars and the main clinical and necropsy findings are presented in . A total of 41 budgerigars (17 young [10 female and seven male] and 24 adult [15 female and nine male]) were submitted to the laboratory for necropsy. Tissue samples were taken at necropsy, fixed in 10% neutral buffered formalin, dehydrated in ethanol and embedded in paraffin wax. Sections were cut at 5 µm and stained routinely with haematoxylin and eosin. Replicate sections were used for immunohistochemistry. At the same time, tissue samples (heart, liver and spleen from young and adult infected budgerigars, and ovaries, oviduct, testes and fertilized eggs from adults) were collected for bacteriology.
Table 1. Clinical and necropsy findings in budgerigars naturally infected with S. Gallinarum.
Immunohistochemistry
Immunoperoxidase method
The ABC method was used according to the manufacturer's instructions. Briefly, sections (5 µm) were mounted on poly-l-lysine-coated glass slides. After incubating for 2 h at 40°C, sections were dewaxed in xylene and hydrated through graded alcohols. Endogenous peroxidase was then blocked with 3% H2O2 in 70% methanol. The tissues were pretreated with 10 mmol citrate buffer at pH 6.0 then microwaved for 10 min at 500 W for antigen retrieval, and then the slides were washed for 10 min in phosphate-buffered-saline (PBS; pH 7.3). Non-specific staining was blocked by treating with 2% normal goat serum for 10 min. The blocking serum was then replaced by rabbit anti-S. Gallinarum 9 serum diluted one in 64, followed by overnight incubation at 4°C. After washing for 10 min, sections were flooded with biotinylated goat anti-rabbit immunoglobulin for 10 min. After another wash, the sections were covered with streptavidin peroxidase and incubated for 10 min. Finally, they were treated for 7 min with diaminobenzidine containing 3% H2O2. The sections were then counterstained with Mayer's haematoxylin, washed in tap water, dehydrated in graded alcohols, and mounted. For control slides, replicate sections of selected infected tissues were processed. Unless stated otherwise, all incubating was performed at room temperature in a humidified chamber.
Immunofluorescence method
For the IF method, the sections were deparaffinized in xylene and washed briefly in PBS. Tissues were digested with 0.1% protease K for 10 min at 37°C. Slides were then washed for 15 min in PBS. Sections were incubated with rabbit anti-S. Gallinarum 9 serum and then washed again for 15 min in PBS. After the addition of rabbit anti-chicken gamma globulin serum conjugated with fluorescein isothiocyanate (Sigma, Rehovot, Israel), sections were incubated for 30 min at 37°C, washed in PBS for 15 min and mounted in phosphate-buffered glycerol (pH 9.0). The results of the fluorescent antibody reaction were determined using a fluorescence microscope (Leica DMLB, Wetzlar, Franfurkt, Germany).
Isolation and identification of S. Gallinarum
Tissue samples obtained at necropsy (heart, liver and spleen from all budgerigars; ovaries, oviduct, fertilized eggs and testes from adult budgerigars) were applied to pre-enrichment, selective enrichment and selective-differential enrichment culture media for Salmonella isolation. Buffered peptone water was used for pre-enrichment, selenite cystine broth and tetrathionate broth were used for selective enrichment, and Brillant Green phenol red agar and bismuth sulphite agar were used for selective-differential enrichment. Selenite cystine broths were cultivated at 37°C and tetrathionate broths were cultivated at 42°C for 24 h. A colony was then taken from selenite cystine broth and tetrathionate broth and harvested to Brillant Green phenol red agar and bismuth sulphite agar. Cultures were incubated at 37°C for 24 to 48 h. After incubation, lactose-negative, pink–reddish colonies in Brillant Green phenol red agar and H2S-positive black-coloured colonies in bismuth sulphite agar were tentatively identified as Salmonella (Holt et al., Citation1994; Koneman et al., Citation1997).
These colonies were then Gram stained and examined microscopically. Motility was also determined (Koneman et al., Citation1997). The colonies were also harvested to solid-media triple sugar iron agar, lysine decarboxylase–sulfhydrase medium and urea agar. For the indol test, colonies were harvested to tripticase soy broth. Biochemical tests were then applied to these isolates (Holt et al., Citation1994; Koneman et al., Citation1997) and they were considered positive S. Gallinarum when the motility, gas production, H2S production, methyl red, lysine and ornitine tests were positive and the Voges–Proskauer, indole, citrate, phenylalanine deaminase, urease and arginine tests were negative. The Salmonella Polyvalent “O” Antiserum agglutination test was applied to the colonies identified as S. Gallinarum by the biochemical tests. Agglutination occurring in 1 to 2 min was considered a positive reaction. Agglutination tests with Salmonella group-specific antiserum were then applied to the colonies that gave a positive reaction with the Piolyvalent “O” Antiserum.
Results
Clinical findings
The disease had a peracute or acute presentation in young budgerigars while in adults a peracute to chronic presentation was observed. Clinical findings are summarized in .
The peracute presentation in young budgerigars was that of death with no clinical signs. In the acute form, lethargy and yellow–white diarrhoea were observed. Budgerigars with these clinical findings generally died in 2 to 3 days.
In adult budgerigars with the acute form, the clinical signs were decreased feed and water consumption and greenish-yellow diarrhoea. Budgerigars with clinical signs mostly died in 5 to 8 days. In chronic cases, loss of condition, unwillingness to fly, inability to perch, and gathering in the bottom of the cage were noted. Drops in egg production and quality as well as a 50% increase in embryonic mortality rate at the end of the incubation period were noted.
Macroscopic findings
Gross findings are summarized in . In young budgerigars, the parietal and visceral peritoneum as well as the pericardium was thickened with a fibrinous exudate. The livers were congested and friable with scattered petechial haemorrhages on the capsular surface. In a few cases, hardly discernible white foci were also observed in the liver. In severe cases, fibrin on the pericardium was seen as a white, thick layer (A). The spleen was enlarged, dark coloured and variable. Petechial haemorrhages were present on the outer surface of the pericardium and myocardium and on the serosal surfaces of the ventriculus (gizzard), duodenum and iliocaecum.
Figure 1. 1A: The pericardium (arrow), peritoneum (arrowhead) and liver covered with white exudate (young budgerigar). 1B: The liver showed scattered gray to white necrotic foci (arrows, adult budgerigar).
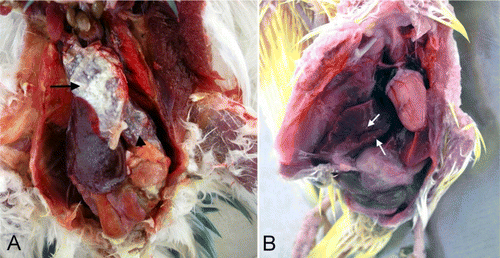
In adult budgerigars, the livers were friable, green–yellow or dark red in colour. In most cases, multifocal gray to white necrotic foci were present throughout the liver (B). In some cases, the liver surface was also covered with a layer of fibrin. Petechial haemorrhages were observed on the serosal surface of the small intestines and caecum.
Microscopic findings
Histopathological findings are summarized in . In most cases (both young and adult budgerigars) the blood vessel walls in many of the organs were hyalinized in appearance (A), with many containing various-sized microthrombi. In peracute cases, the only microscopic findings were hyperaemia, oedema and haemorrhage.
Figure 2. 2A: Hyalinization in the blood vessel wall (arrows, young budgerigar). Haematoxylin and eosin (HE). Bar = 50 µm. 2B: Focal coagulative necrosis with fibrin in the liver (arrows, young budgerigar). HE. Bar = 50 µm. 2C: Fibrinonecrotic epicarditis in the heart (arrows, young budgerigar). HE. Bar = 100 µm. 2D: Hyperaemia, haemorrhage and coagulative necrosis in the proximal tubules (arrows, young budgerigar). HE. Bar = 50 µm. 2E: A granulomatous lesion consisting of a macrophage, lymphocyte and histiocyte infiltrate in the liver (arrows, adult budgerigar). HE. Bar = 50 µm. 2F: Mononuclear cell infiltrate in the mucosa of the intestine (arrows, adult budgerigar). HE. Bar = 50 µm.
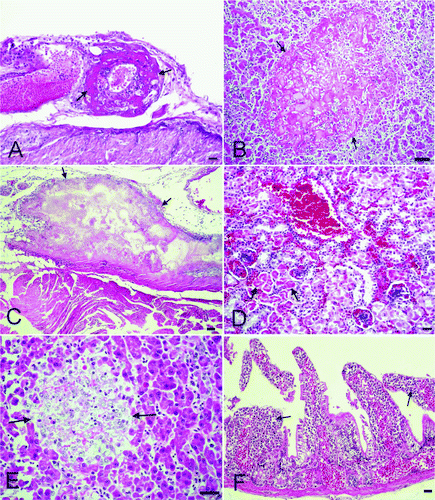
Table 2. Histopathological and immunohistochemical findings in budgerigars naturally infected with S. Gallinarum.
In young budgerigars, the liver capsule was thickened by oedema, serofibrinous to fibrinous exudate and scattered heterophils. The sinosoids were dilated and in some areas haemorrhages were noted. The hepatocytes were enlarged with granular cytoplasm and indistinct cell borders. In many cases, a focal to multifocal coagulative necrosis with fibrin exudation and a mild heterophil and lymphocyte infiltration were observed (B).
The pericardium was thickened by oedema and a fibrinonecrotic exudate containing heterophils and macrophages. In a few cases, the epicardial lesions were limited to a cellular infiltrate (C). In a few cases, multifocal coagulative necrosis was present in the spleen.
Hydropic degeneration and coagulative necrosis was present in the proximal and distal tubules of the kidney (D) with haemorrhage and a mononuclear cell infiltrate in the renal interstitium.
In the intestinal tract there was oedema, hyperaemia and submucosal haemorrhages in the proventriculus and ventriculus, while in the serosa of the small and large intestines oedema, haemorrhage and fibrin with a scant heterophil infiltrate were observed. In cases with no inflammatory changes, the only microscopic finding was haemorrhage.
In adult budgerigars with the acute form, focal necrosis with heterophil infiltration was observed; while in the chronic cases, the lesion was more granulomatous with a macrophage, lymphocyte and histiocyte infiltrate (E). The pericardium was covered with fibrin admixed with heterophils, macrophages and few mononuclear cells. The myocardial fibrils had either lost their staining characteristics or were darkly eosinophilic. An interstitial mononuclear cell infiltrate was observed in the chronic cases. In the spleen, vascular changes and mononuclear phagocytic cell hyperplasia was observed in severe cases while haemosiderin-laden macrophages were often observed. In the kidney, degeneration of the tubule epithelium and interstitial haemorrhage was observed. An interstitial mononuclear cell infiltrate was seen in most of the chronic cases. In the proventriculus, there were no changes in the acute cases whilst in the chronic cases focal cell infiltrates of mononuclear cells were observed. In the intestines of the chronic cases, infiltrations of mostly mononuclear cells were observed with lesser numbers of heterophils in the mucosa and submucosa (F). Lesions in the air sacs of the adult budgerigars were similar to those in the young budgerigars. In two cases, focal mononuclear cell infiltrates into the lung interstitium were noted.
Immunohistochemical findings
Immunohistochemical findings are summarized in .
Immunoperoxidase
In the young budgerigars, positivity showed both a diffuse and interstitial presentation. The most intense staining was observed in the areas of coagulative necrosis and in the cytoplasm of adjacent hepatocytes in the liver. Glisson's capsule stained quite intense and diffuse. In the heart, positive staining was present in cases with epicardial lesions. In the spleen, immunopositivity was observed on the capsule; and in cases with splenic necrosis, immunoreactivity was detected in the cytoplasm of macrophages. In the kidneys, immunostaining was noted in the sloughed-off or degenerate tubule epithelia (A). In some cases, positive immunoreactivity was observed within the necrotic material. In the serosa of the proventriculus and ventriculus, diffuse intense immunostaining was present. Positive immunoreactivity in the propria mucosa was more evident where severe haemorrhage was present. In all of the intestinal segments, immunostaining was present diffusely in the serosa and granularly in the propria mucosa. In the lungs, positive immunoreactivity was detected within the exudates present in some parabronchi.
Figure 3. 3A: Intense immunopositive reactions in the cytoplasm of the tubules in the kidney (arrows, young bird). Immunohistochemistry. Bar = 30 µm. 3B: Severe immunopositive reactions in the liver (arrows, adult bird). Immunohistochemistry. Bar = 30 µm.
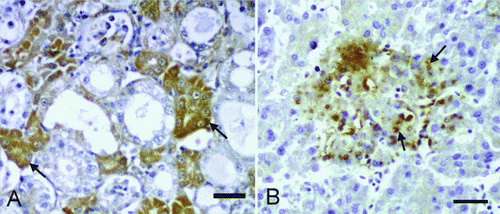
In adult budgerigars, the positive reactions were observed mostly in the liver (B). Positive immunostaining in cases with necrosis was observed in the cytoplasm of macrophages or free in the adjacent cell-free areas. Positive reactivity was also noted in the macrophage vacuoles within the areas of granulomatous inflammation. In the heart, a diffuse pattern of positivity was seen in the exudate covering the pericardium. In the spleen, immunostaining was present in the macrophages located both in the cortex and the medulla, and also the capsule. In the kidney, immunostaining was detected in the proximal tubule epithelia, sloughed-off epithelia and the macrophages located in the cortical interstitium. In the proventriculus, focal or diffuse immunostaining was present in the macrophages located in the propria mucosa. Similarly positive immunoreactivity was detected in the macrophages located in the mucosa, and to a lesser extent in the villus epithelium of the intestines. Positive reactivity was also observed in the serosal macrophages where peritonitis was observed.
Immunofluorescence
In the young budgerigars, positive staining was observed in the liver, heart, spleen, kidney and peritoneum. In the liver the necrotic areas fluoresced, while in heart a positive reaction was only observed in the cases with epicarditis. In the spleen, fluorescence was located in the capsule and to a lesser extent around the necrotic areas. In the kidneys, fluorescence was observed in the degenerate tubule epithelia (A). Little immunoreactivity was detected in the serous surface of the ventriculus and proventriculus. In the intestines, fluorescence was detected in the degenerate or sloughed-off villus epithelia and to a lesser extent in the serosa.
Figure 4. 4A: Immunolabelling of S. Gallinarum in cytoplasm of the tubules in the kidney (arrows, young bird) Immunofluorescence method. Bar = 20 µm. 4B: Immunopositive reactions in the liver (arrows, adult bird). Immunofluorescence method. Bar = 40 µm.
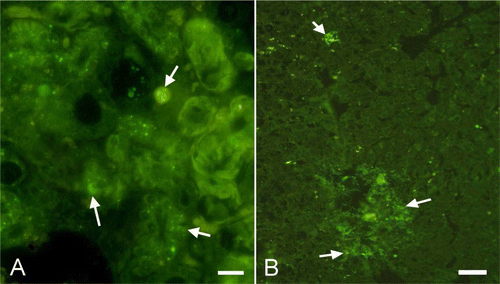
In adult budgerigars, positive immunoreactivity was seen mostly in the cytoplasm of the macrophages in the liver (B), heart, spleen, kidneys, proventriculus and intestine.
Bacteriological findings
S. Gallinarum was isolated from samples of the heart, liver, and spleen from young and adult infected budgerigars, and fertilized eggs, but not from the ovary, oviduct and testes. The bacteria were also detected on the cage materials and in the feed. S. Gallinarum was identified from 41 liver samples, 35 heart and spleen samples and 10 fertilized eggs. All isolates were confirmed with Polyvalent “O” Antiserum.
Discussion
Salmonellosis is an important zoonotic infection of poultry, and Salmonella-infected birds provide a great danger for the consumers (Yousuf et al., Citation2001; Sharifi-Mood et al., Citation2006; Shivaprasad & Barrow, Citation2008). Budgerigars, as pet birds, are commonly found in close association with people, thus making the infection more important. Moreover, no previous detailed reports describing pathologic, immunohistochemical and bacteriological findings in budgerigars naturally infected with S. Gallinarum were detected.
Poultry salmonellosis affects all age groups, although mortality rates are higher in the young compared with the adults (Waihenya et al., Citation2002; Oliveira et al., Citation2005; Parmer & Davies, Citation2007). In this study, mortality and morbidity rates were also found to be higher in the young budgerigars in the three different flocks. In addition, the mortality rate in budgerigars was observed to be higher compared with the other species, showing that budgerigars are highly susceptible to salmonellosis (Sato et al., Citation1997; Lee et al., Citation2001).
Infected birds, contaminated eggs, water, feed, and other materials play an important role in spreading the bacteria (Lowry et al., Citation1999; Berchieri et al., Citation2001; Kokosharov, Citation2001; Oliveira et al., Citation2005). In the present study, the continuous contamination of feed and the hatching eggs with faeces and other environmental material, the absence of an effective hatchery sanitation programme and the mixing of the outside adult budgerigars in all of the flocks probably played a major role in the occurrence and spread of S. Gallinarum in the young and adult budgerigars. In poultry salmonellosis, transovarian infection was also reported to be important (Smith & Tucker, Citation1980; Sato et al., Citation1997). In the current study, the absence of any pathological changes in the ovarium, oviduct and testes of budgerigars and no positive immunoreactivity and no bacterial isolation suggest that only horizontal spread was involved in these three flocks. The presence of infected fertilized eggs suggests environmental contamination rather than transovarial spread.
Pericarditis and myocarditis occur in poultry salmonellosis (Song et al., Citation2002; Msoffe et al., Citation2006; Freitas et al., Citation2007). The heart lesions in the present study were fibrinous pericarditis in the young birds and a mononuclear pericarditis and myocarditis in adults. With histochemistry, positive immunoreactivity against the bacterium was observed in the lesions, suggesting that the histopathological changes were associated with the bacterium. Heart muscle necrosis, as described by some investigators, was not detected in the current study (Kaushik et al., Citation1986; Msoffe et al., Citation2006).
Kidney lesions caused by S. Gallinarum in various poultry species have been described (Waihenya et al., Citation2002; Freitas et al., Citation2007). Similar findings, more severe in young budgerigars, were also observed in the present study (Waihenya et al., Citation2002; Freitas et al., Citation2007; Beyaz et al. Citation2010). In addition, positive immunoreactivity in the proximal renal tubules of young and adult budgerigars suggests that urine might therefore play a role in the spread of the infection among birds and also to humans.
Macrophages are integral in the pathogenesis of salmonellosis (Chen et al., Citation1996; Henderson et al., Citation1999; Chadfield et al., Citation2003). In the present study, the presence of agent antigen within the phagosomes of macrophages located in many organs of the adult, although less commonly in the young budgerigars, confirmed intracellular localization of the agent. Similar activity of the agent, as in other species (Henderson et al., Citation1999; Chadfield et al., Citation2003), is therefore most likely.
Microscopic findings in the spleen (i.e. vascular changes in the acute cases and mononuclear phagocytic cell hyperplasia in the chronic cases) were similar to those described in the literature (Berchieri et al., Citation2000; Song et al., Citation2002; Beyaz & Kutsal, Citation2003; Beyaz et al., Citation2010). Haemosiderosis in the peracute cases was suspected to be as a result of massive destruction of erythrocytes via Salmonella toxins (Assoku et al., Citation1970; Kokosharov, Citation2002).
Immunoperoxidase and IF techniques are effectively utilized in the diagnosis of many poultry diseases (Beyaz & Kutsal, Citation2003; Shivaprasad & Barrow, Citation2008; Beyaz et al. Citation2010). In the present study, antigenic localization of the agent differed between the two techniques used. With IF, the immunoreactivity observed in the tissue and organs was weak or absent; however, strong positive immunoreactivity was detected with immunoperoxidase. From this, the use of immunoperoxidase was found to be more useful in the diagnosis of the disease.
In conclusion, clinical and pathologic findings of the current investigation indicate that S. Gallinarum is an important bacterial disease of budgerigars, and should be included in the list of diseases for this species.
References
- Audisio , M.C. and Terzolo , H.R. 2002 . Virulence analysis of a Salmonella gallinarum strain by oral inoculation of 20-day-old chickens . Avian Diseases , 46 : 186 – 191 .
- Assoku , R.KG. , Penhale , W.J. and Buxton , A. 1970 . Haematological changes in acute experimental Salmonella Gallinarum infection in chickens . Journal of Comparative Pathology , 80 : 473 – 485 .
- Berchieri , Jr.A. , Oliveira , G.H. , Pinheiro , L.A.S. and Barrow , P.A. 2000 . Experimental Salmonella Gallinarum infection in light laying hen lines . Brazilian Journal of Microbiology , 31 : 50 – 52 .
- Berchieri , Jr.A. , Murphy , C.K. , Marston , K. and Barrow , P.A. 2001 . Observations on the persistence and vertical transmission of Salmonella enterica serovars Pullorum and Gallinarum in chickens: effect of bacterial and host genetic background . Avian Pathology , 30 : 221 – 231 .
- Beyaz , L. and Kutsal , O. 2003 . Pathological and immunohistochemical studies in experimental Salmonella gallinarum infection (fowl typhoid) in chickens . Veterinary Journal Ankara University , 50 : 219 – 227 .
- Beyaz , L. , Atasever , A. , Aydin , F. , Gümüsoy , K.S. and Abay , S. 2010 . Pathological and clinical findings and tissue distribution of Salmonella gallinarum infection in turkey poults . Turkish Journal of Veterinary and Animal Sciences , 34 : 101 – 110 .
- Chadfield , M.S. , Brown , D.J. , Aabo , S. , Christensen , J.P. and Olsen , J.E. 2003 . Comparison of intestinal invasion and macrophage response of Salmonella Gallinarum and other host-adapted Salmonella enterica serovars in the avian host . Veterinary Microbiology , 92 : 49 – 64 .
- Chen , L.M. , Kaniga , K. and Galan , J.E. 1996 . Salmonella spp. are cytotoxic for cultured macrophages . Molecular Microbiology , 21 : 1101 – 1115 .
- Freitas , N.O.C. , Arroyave , W. , Alessi , A.C. , Fagliari , J.J. and Berchieri , A. 2007 . Infection of commercial laying hens with Salmonella Gallinarum: clinical, anatomopathological and haematological studies . Brazilian Journal of Poultry Science , 9 : 133 – 141 .
- Henderson , S.C. , Bounous , D.I. and Lee , M.D. 1999 . Early pathogenesis of avian salmonellosis . Infection and Immunity , 67 : 3580 – 3586 .
- Holt , J.G. , Krieg , N.R. , Sneath , P.H.A. , Stanley , J.T. and Williams , T.S. 1994 . Bergey's Manual of Determinative Bacteriology. , 9th edn , 186 – 187 . Maryland , , USA : William and Wilkins Baltimore .
- Hossain , M.S. , Chowdhury , E.H. , Islam , M.M. , Haider , M.G. and Hossain , M.M. 2006 . Avian salmonella infection: isolation and identification of organisms and histopathological study . Bangladesh Journal of Veterinary Medicine , 4 : 7 – 12 .
- Kaushik , R.K. , Singh , J. , Kumar , S. and Kulshreshtla , R.C. 1986 . Fowl typhoid in a few poultry farms of Haryana state . Indian Journal of Animal Sciences , 56 : 511 – 514 .
- Kokosharov , T. 2001 . Some observations on the caecal microflora of the chickens during experimental acute fowl typhoid . Revue de Médecine Vétérinaire , 152 : 531 – 534 .
- Kokosharov , T. 2002 . Clinical and hematological effects of Salmonella gallinarum endotoxin in cockerels . Veterinarski Arhiv , 72 : 269 – 276 .
- Koneman , E.W. , Allen , S.D. , Janda , W.M. , Schreckenberger , P.C. and Winn , W.C.J. 1997 . Color Atlas and Textbook of Diagnostic Microbiology. , 5th edn , 190 – 191 . Philadelphia : Lippincott-Raven Publishers .
- Lee , Y-J. , Kang , M-S. , Woo , Y-K. , Mo , I-P. and Tak , R.B. 2001 . Competitive exclusion against Salmonella gallinarum of Salmonella enteritidis infected chickens . Journal of Veterinary Science , 2 : 33 – 36 .
- Lowry , V.K. , Tellez , G.I. , Nisbet , D.J , Garcia , G. , Urquiza , O. , Stanker , L.H. and Kogut , M.H. 1999 . Efficacy of Salmonella enteritidis-immune lymphokines on horizontal transmisson of S. arizonae in turkeys and S. gallinarum in chickens . International Journal of Food Microbiology , 48 : 139 – 148 .
- Msoffe , P.L.M. , Minga , U.M. , Mtambo , M.M.A. , Gwakisa , P.S. and Olsen , J.E. 2006 . Differences in resistance to Salmonella enterica serovar Gallinarum infection among indigenous local chicken ecotypes in Tanzania . Avian Pathology , 35 : 270 – 276 .
- Oliveira , G.H. , Berchieri , Jr.A. and Fernandes , A.C. 2005 . Experimental infection of laying hens with Salmonella enterica serovar Gallinarum . Brazilian Journal of Microbiology , 36 : 51 – 56 .
- Parmer , D. and Davies , R. 2007 . Fowl typoid in a small backyard laying flock . The Veterinary Record , 160 : 348
- Sato , Y. , Sato , G. , Tuchili , L. , Pandey , G.S. , Nakajima , A. , Chimana , H. and Sisungwe , H. 1997 . Status of Salmonella gallinarum-pullorum infections in poultry in Zambia . Avian Diseases , 41 : 490 – 495 .
- Sharifi-Mood , B. , Metanat , M. and Salehi , M. 2006 . Salmonella gallinarum empyema-the first case from Iran . Journal of Medical Science , 6 : 180 – 182 .
- Shivaprasad , H.L. and Barrow , P.A. 2008 . “ Pullorum Disease and Fowl Typhoid ” . In Diseases of Poultry , 12th edn , Edited by: Saif , Y.M. , Fadly , A.M. , Glisson , J.R. , McDougald , L.R. , Nolan , L.K. and Swayne , D.E. 620 – 636 . Ames : Iowa State Press .
- Smith , H.W. and Tucker , J.F. 1980 . The virulence of salmonella strains for chickens: their excretion by infected chickens . The Journal of Hygiene , 84 : 479 – 488 .
- Song , S.K. , Cho , S.W. , Lee , J.H. , Choi , Y.C. , Shin , Y.U. and Park , I.G. 2002 . Apoptosis in experimentally infected chicks with Salmonella gallinarum . Korean Journal of Veterinary Service , 25 : 357 – 370 .
- Waihenya , R.K. , Mtambo , M.M.A. , Nkwengulila , G. and Minga , U.M. 2002 . Efficacy of crude extract of Aloe secundiflora against Salmonella gallinarum in experimentally infected free-range chickens in Tanzania . Journal of Ethnopharmacology , 79 : 317 – 323 .
- Yousuf , M. , Nadeem , A. and Irfan , A. 2001 . Salmonella gallinarum septicaemia in humans . Pakistan Journal of Medical Sciences , 17 : 50 – 52 .