Abstract
Technology for infectious agent detection continues to evolve, particularly molecular methods that first emerged in the mid-1970s. The goals of new technology in diagnostics, whether in humans or in animals, including poultry, are to achieve the highest sensitivity and specificity possible to accurately identify the infection status of an individual or flock in the shortest time possible. Ease of use, low cost and increased information from a single test (e.g. multiplexing) are also critical areas frequently targeted for improvement. New tests and modifications of current tests are reported often, and diagnostic tests are now commonly developed by commercial companies. As one would expect, most advances in diagnostic technology are applied first to human health, and then may be adapted to animal health if practical. In the present review the trends and novel innovative technologies in primarily viral diagnostics are reviewed and the practicality of these methods and application for poultry health are discussed briefly. Also, influenza will seem to be over-represented in viral diagnostics since it is frequently used as a proof-of-concept target for novel technology due to its importance for animal and public health. Finally, the review is intended to be a brief survey of some of the innovative diagnostic technologies reported in recent years. It is not entirely comprehensive of all technology and the author makes no claims or endorsements of any of the technology or products mentioned.
A Brief History of Diagnostics
The classical age
It is noteworthy how rapidly technology for viral diagnostics has evolved over the past century. However, some of the oldest classical or molecular methods are still in use, although with some modifications.
Viruses were first identified as filterable agents in 1898, when foot and mouth disease was discovered by Loeffler & Frosch (Citation1898). The ability to propagate viruses outside an animal host was an enormous advance for virology and was first reported in embryonated chicken eggs with Rous sarcoma virus in 1911 (Rous & Murphy, Citation1911); however, it was not until the 1930s, after it was reported that fowlpoxvirus could be propagated in embryonated chicken eggs (Woodruff & Goodpasture, Citation1931), that the method began to be used more widely. By the mid-1930s methods to propagate Variola virus, mumps and herpesviruses were reported (Goodpasture et al., Citation1931; Lazarus et al., Citation1937; Nelson, Citation1939). It was not until 1948 that cell culture was first reported when Weller and Enders developed cell culture for mumps and influenza (Weller & Enders, Citation1948).
Antibody-based assays constituted the first era in viral diagnostics. Complement fixation was described in 1929 for several viruses (Bedson & Bland, Citation1929) and a fluorescent antibody test for influenza was reported in 1956 (Liu, Citation1956). Electron microscopy using negative staining was first described for virus detection in 1959 (Brenner & Horne, Citation1959), and then a vast improvement in electron microscopy came about in 1967 when Best et al. described the use of specific antibody, in what is now known as immune electron microscopy, for rubella virus detection (Best et al., Citation1967). Monoclonal antibodies, first reported in 1975 (Kohler & Milstein, Citation1975), were the next great technological leap and led the way for improved antibody-based tests for virus detection.
Protein separation by electrophoresis was developed in the mid-1960s (Davis, Citation1964; Ornstein, Citation1964) and allowed for virus proteins to be identified by weight and, more specifically, by antibody once the western blot was established (Towbin et al., Citation1979). The original protein assays were primarily used in research settings, but western blotting has been used to diagnose virus infection by antibody detection for viruses that establish chronic infections such as human immunodeficiency virus (Burke et al., Citation1987) and even feline immunodeficiency virus (Egberink et al., Citation1991). Western blot and enzyme-linked immunosorbent assay technology evolved into lateral-flow antigen-detection immunoassays in the mid-1980s by utilizing the antibody in the solid phase and the antigen in the liquid phase (Gary et al., Citation1985; Rivera et al., Citation1986). Antigen-detection immunoassays were soon optimized to be substantially cheaper and easier to run. Faster, point-of-care commercial kits were marketed and validation studies began to be reported (Dascal et al., Citation1989) and are now among the most common commercial diagnostic test formats in human and veterinary medicine. For example, in North America at least five companies manufacture kits for seasonal influenza and six companies manufacture kits for respiratory syncytial virus.
The molecular era
The development of molecular biological methods represented a substantial change in microbiological research and diagnostics by making the genetic material of an organism accessible and identifiable. The initial advantage of molecular diagnostic tests was that they could achieve a level of specificity well beyond that of classical methods. As the technology has been refined, molecular tests are now able to exceed classical tests in sensitivity and most can provide additional specific relevant information about the target, such as type, subtype, lineage, drug resistance, serotype, and so forth.
During the mid-1970s, molecular methods began to emerge when Southern blotting was described. Material could now be probed with radio-labelled nucleic acids for specific DNA sequences (Southern, Citation1975). Related methods such as northern blotting (Alwine et al., Citation1977), restriction fragment-length polymorphism analysis (Mears et al., Citation1978) and refinements of the original methods soon followed. Although there were some reports of the application of this technology for the detection of infectious agents, Southern and northern blotting were never widely adopted by clinical diagnostic laboratories because they are slow, expensive and at that time were further complicated by necessity of using radionuclides to visualize the results. The potential advantages of molecular methods over classical methods thus did not offset the disadvantages.
The method of polymerase chain reaction (PCR) was first reported in 1985 (Saiki et al., Citation1985) for primer-mediated enzymatic amplification of specific target sequences in genomic DNA. The technique was revolutionary in that it enabled the rapid and accurate detection of specific DNA sequences in pathogenic organisms. At that time, PCR reactions had to be carried out by hand; tubes were manually moved between water baths at different temperatures to complete each cycle and fresh polymerase enzyme needed to be added after each cycle because thermostable polymerases had not yet been utilized. The method was greatly enhanced when thermostable polymerases were introduced in 1988 (Saiki et al., Citation1988), which also improved the specificity of PCR because higher cycling temperatures were possible. Another problem was resolved when reverse transcription (RT)-PCR was developed in 1988, making it possible to produce DNA copies of target RNA viruses (Rappolee et al., Citation1988). Soon after its development, PCR was applied to the detection of human papillomavirus (Shibata et al., Citation1988) and RT-PCR was applied to human immunodeficiency virus type 1 detection (Hart et al., Citation1988). A multiplex test for influenza A and influenza B followed a few years later (Fan et al., Citation1998). Real-time PCR, which visualizes the results as the reaction is running, was introduced in 1996 (Heid et al., Citation1996) and was first applied to viral diagnostics in 1999 when tests for SV40 and hepatitis B were first reported (Abe et al., Citation1999; Shi et al., Citation1999). However, as with earlier molecular tests, PCR-based methods were not picked up quickly by clinical diagnostic laboratories.
Not surprisingly, as molecular technologies have expanded, commercial companies producing tests have multiplied; in 2011 there were approximately 350 companies that manufacture in vitro molecular medical tests (Carlson, Citation2011). However, commercial molecular tests are primarily found only in the developed world, with 79% of the market being in the US and Europe (Carlson, Citation2011).
Molecular diagnostics for poultry diseases
The early 1990s was also the time when PCR methods were first introduced for the detection of poultry viruses with tests for chicken infectious anaemia virus, infectious bronchitis virus, infectious bursal disease virus, Marek's disease virus, infectious laryngotracheitis virus and avian leucosis viruses (Tham & Stanislawek, Citation1992; Todd et al., Citation1992; van Woensel et al., Citation1992; Williams et al., Citation1992; Kwon et al., Citation1993; Stauber et al., Citation1995; Jackwood et al., Citation1996). The first RT-PCR for avian influenza virus (AIV) was reported in 1995 (Horimoto & Kawaoka, Citation1995), but as a method to amplify the haemagglutinin for sequencing to evaluate pathotype rather than for initial virus detection. In addition to identifying infected flocks, some of the earlier applications of PCR methods in poultry health were aimed at detecting vaccine contaminants (Bruckner et al., Citation1996; Reimann & Werner, Citation1996). As with human medicine, research laboratories adopted the technology much earlier than diagnostic laboratories.
Current Capabilities
Current achievable detection limits are a single molecule: virion, protein, RNA or DNA. However, few tests with that level of sensitivity and specificity are available clinically. In general the adoption of new technology lags behind its development, so the state of the art does not necessarily correlate with routine practice. One reason is that there are numerous barriers to adoption that must be overcome for any new technology to be adopted by diagnosticians. These include: cost of new equipment; fit into workflow; new skills/training needed for personnel; and validation and regulatory approval.
Finally, because of wide variation in resources and needs among veterinary (and public health) laboratories worldwide, it is difficult to identify a “standard” method or technology. However, it is apparent that in both veterinary medicine and human health two of the most widely used methods for virus detection are PCR-based methods and virus culture, depending on the target.
It is also interesting to point out a difference between veterinary and human health laboratories, where the veterinary laboratories have the advantage in adopting new technology. Although veterinary diagnostic programmes, particularly for food animals, generally do not have the same resources as public health, less strict regulations can promote more rapid adoption of new technologies (and frequently there are grey areas as regulations often lag behind the emergent technology). In public health, in vitro diagnostic tests are typically commercial kits, which frequently require some form of licensing (e.g. approval by the Food and Drug Administration in the US and the CE-IVD mark in Europe). In veterinary medicine, in-house tests are used much more commonly as there are fewer commercial kits available, due to the smaller market (but note that commercial veterinary kits often do require licensing as well). However, this extra freedom has also meant that harmonization has been difficult to achieve. Some harmonization has been established since the World Organisation for Animal Health (OIE) has been able to recommend specific methods for agents that are listed diseases (OIE, Citation2011). An additional difference between human and animal diagnostic tests is that tests for animal diseases can be tested and validated using experimentally-generated specimens from the target host. This allows for better information on test performance, an easier paradigm for refining tests and better validation data.
In poultry health laboratories, in-house tests (i.e. any test that is not available as a commercial kit, including peer-reviewed published assays and unpublished assays) are not uncommon. Commercially produced test kits have become increasingly available for important animal diseases, including tests for poultry (). By far the most targeted agent by commercial kits is AIV due to its zoonotic potential and because it is an OIE-listed disease. In some cases the same kit for AIV (type A influenza) marketed for poultry has been validated and is available for humans, swine, horses, and so forth, and vice versa. An interesting illustration of this is shown in , which compares graphically the number of publications in the PubMed database for AIV PCR tests, Newcastle disease virus (NDV) PCR tests and Marek's disease virus PCR tests by year. Although not all papers are necessarily for diagnostic tests, this demonstrates the focus on AIV versus NDV and Marek's disease virus, both of which are historically more important for poultry production but are not zoonotic concerns; market potential has thus driven discovery to some degree. Commercialization of new kits for poultry diseases will probably be slow due to limited markets compared with public health. However, bacterial agents that are important for food safety (e.g. Salmonella enteriditis, Campylobacter jejuni) have also been targeted widely for commercial kits.
Table 1. Commercial test kits available for poultry diagnostics (availability varies regionally).
Figure 1. Number of publications in the PubMed database by year with “PCR” and “avian influenza”, “Newcastle disease” or “Marek's disease”.
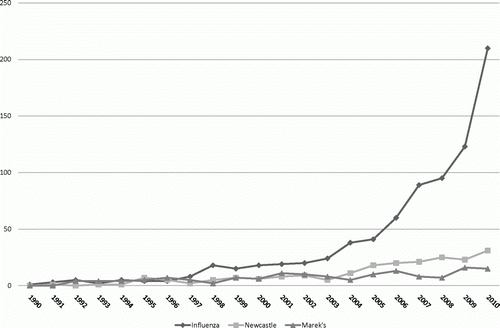
Among the in-house tests, real-time PCR-based methods are probably the assay of choice. In veterinary diagnostics, real-time PCR is the preferred test for numerous OIE-listed diseases (Hoffmann et al., Citation2009). Currently real-time RT-PCR is probably the most common test for AIV and NDV, where it is used as a screening test with virus isolation in embryonated chicken eggs being used as a confirmatory test. PCR-based tests (real-time or conventional) have been reported for detecting almost all poultry viral and bacterial diseases of poultry, and a few have been developed to further characterize isolates such as (not a comprehensive list): tests that differentiate NDV lineages (Wise et al., Citation2004; Farkas et al., Citation2009), AIV subtypes (Spackman et al., Citation2002; Das & Suarez, Citation2007) and infectious bursal disease virus pathotypes (Ghorashi et al., Citation2011; Hernandez et al., Citation2011) and that identify infectious bronchitis virus serotypes/genotypes (Jackwood et al., Citation1997; Keeler et al., Citation1998; Moscoso et al., Citation2005; Wang & Khan, Citation1999; Jones et al., Citation2011). Although PCR-based methods, including conventional and real time, are used widely, kits for few poultry agents have been commercialized.
Novel Technologies
In diagnostics there are limited numbers of targets to choose for agent detection: proteins from the target agent, the nucleic acid of the target agent, or the host's specific immune response to the target agent. Detection assays can either amplify the target or the signal to improve sensitivity, and refinements to these can substantially improve sensitivity and specificity of the established tests. We now have the technology to detect a single molecule of RNA, DNA or protein, including a single virion. Thus we have hit what is probably the limit for sensitivity and specificity, and now the trend in technology is to improve convenience (time, cost, ease of use, multiplexing, reusable or disposable), or to specialize for a specific situation (“personalized” medicine) in order to provide in-depth information.
PCR-based assays are the most common tests that target agent nucleic acids, and most protein detection assays utilize antibody for specific target detection. Of course improvements and modifications continue to be developed for each target type, which increase sensitivity, specificity, and information, and which can reduce cost and time to result. Overall trends in diagnostic assay improvements are presented in . To be viable, and also successful, technologies must involve improvements in numerous areas compared with any current tests. Instrumentation is a major area of development, and new assays frequently rely on test-kit-specific instruments (which is also due to commercialization).
Table 2. Trends in improving diagnostic tests and some common approaches.
Nucleic acid detection
There are numerous nucleic acid detection technologies available: PCR based, isothermic amplification, and microarray—but PCR-based assays are probably the most widely used. Assays to further characterize nucleic acids include sequencing, microarray and restriction fragment-length polymorphism, and are usually only utilized by reference laboratories. A full examination of nucleic acid detection technology is beyond the scope of this review, so the focus is on some of those that have had the most impact.
Isothermic amplification methods such as loop-mediated isothermal amplification and nucleic acid sequence-based amplification have been used for years, and have the advantage that thermo-cyclers are not necessary and the reactions can be incubated in a simple water bath. They have similar sensitivity and specificity to PCR, but can be more expensive to run due to the cost of the reagents.
Microarrays have been proposed as a diagnostic tool that can provide a high level of information, so not only can one identify the target agent but also crucial information such as strain, lineage, pathotype, and so forth, can be gained. Microarrays are similar to Southern blots as they both rely on DNA hybridization, but microarrays use a glass slide that has hundreds of DNA probes attached to it instead of a single probe in the liquid phase. The microarray slide is incubated with nucleic acid from a specimen, and if the nucleic acid in the sample matches (a complementary match) a probe sequence on the microarray slide it will bind, which produces a fluorescent signal. The signal can be detected and differentiated from that of all the other sequences on the slide, so the specific sequence that is bound can be identified from among the hundreds on the slide, thus providing specific sequence information on the target. If multiple sequences from the specimens bind they can be read separately, which can provide additional information by multiplexing. Unfortunately, however, microarrays have relatively poor sensitivity and require prior enrichment of the target; they also take more time to run than PCR tests, and cost substantially more per sample. If some of these problems can be resolved, microarrays would be a useful tool for reference laboratories to investigate new disease outbreaks (such as an index case), or even new variants of infectious agents. However, gene sequencing has become so fast and cheap that in-depth analysis of PCR products is common and can provide much of the same information that a microarray can, and more efficiently. Currently, microarrays have the most diagnostic utility for genetic conditions such as cancer, rather than for infectious disease.
Numerous modifications have been reported to improve PCR-based methods and many involve improving the sensitivity and specificity of the reaction. Real-time PCR probes can be constructed in many ways (hairpins, two probe systems, different dyes), all of which have different advantages and disadvantages for cost, sensitivity and specificity that can be optimized for a given test or instrument. The simplest modifications are those that optimize the temperature cycling parameters to exploit the characteristics of the enzyme and template. Some examples are linear-after-the-exponential PCR and touch-down PCR. Other modifications involve using molecularly altered primers (and sometimes probes with real-time PCR). An example is minor groove binding protein (Kutyavin et al., Citation2000), which enhances binding allowing for shorter probes by raising the melting temperature. Probes with minor groove binding protein have been applied to the detection of several viral diseases of poultry including AIV, infectious laryngotracheitis virus and NDV (Di Trani et al., Citation2006; Lu et al., Citation2008; Farkas, et al., Citation2009; McMenamy et al., Citation2011). This approach works well for highly variable targets where conserved regions are too short for a conventional probe.
Ultra-small particles such as nanoparticles, quantum dots and microspheres have also been utilized to modify PCR-based assays and have also been employed widely for antibody-based assays. Each of these has different features and needs to be used with specific instrumentation, but can be uniquely labelled and used to specifically identify a PCR amplification product. One of the most practical features of nanoparticles, microspheres and quantum dots is that there are many unique labels that can be used so, in theory, reactions can be highly multiplexed, even into hundreds. Of course there are other technical issues with interference and competition when PCR is highly multiplexed and the instrumentation is expensive, but as the technology improves it will become more practical.
Owing to advances in microfluidics in recent years, improved PCR instrumentation and reaction automation has been developed. One innovation is all-in-one/self-contained cartridges that extract the DNA or RNA and run the reaction. The cartridges are run in vendor-specific, dedicated instruments that analyse the results; for example, Gene Xpert (Cephied Inc., Sunnyvale, CA, USA) and Film Array (Idaho Technologies Inc., Salt Lake City, UT, USA). Microfluidics have also allowed sample volumes to decrease, which increases the sample capacity, decreases the cost per reaction and reduces the time needed to run the reaction; one example is the Integrated Cycler (3M, Focus and Simplexa, Focus Diagnostics, Inc. Cypress, CA, USA), which runs 96 samples in a small single-use carousel and heats with infrared radiation. Digital PCR (Applied Biosystems, Life Technologies Corp., Carlsbad, CA, USA) uses microfluidics to partition a PCR reaction into volumes so low that only a single molecule of analyte should be present, thus allowing absolute quantitation.
Biosensors
Biosensors are instruments that use everything from chemical reactions to electrical resistance to detect a wide range of analytes as diverse as chemicals, viruses and bacteria. Currently most commercially available biosensors are for chemicals, such as drugs and explosives, but perhaps the most common biosensors are blood glucose monitors. Biosensors have been reported for several viruses using DNA and viral proteins as targets (Wang et al., Citation1996; Inoue et al., Citation1999; Baeumner et al., Citation2002; Zhou et al., Citation2002; Xu et al., Citation2007), but none have been adopted clinically. If the technology can be validated for viruses, portable biosensors would provide an advantage over other technologies, as samples frequently require minimal processing, making them appropriate for point-of-care testing. Most are reusable or have minimal consumables; the costs of individual tests should therefore be low.
Reporter cell lines
Reporter cell lines are cell lines that have been modified to produce a measurable signal when they are infected by a target virus. For example, the enzyme-linked virus inducible system cell line (Proffitt & Schindler, Citation1995), which is now commercially available, is engineered to produce β-galactosidase when infected with herpes simplex virus. The β-galactosidase gene was inserted into the cell line and linked to a promoter that is controlled by viral proteins, so β-galactosidase is only produced when replicating herpes simplex virus is present. In some cases, cell lines that would not be naturally susceptible to infection with a virus can be engineered to be susceptible by inserting the appropriate receptor.
Although reporter cell lines are specific and sensitive, they take a relatively long time to yield results and require a high degree of technical skill and resources. Also, there is some limitation for the agents these cell lines can target because a high degree of detail of the virus replication cycle has to be known, and the disease in question needs to be important enough to merit the skill and resources required to make the tests practical and cost-effective.
Detection of a specific immune response
One novel approach is to detect the host-specific anamnestic immune response to a pathogen, instead of the pathogen itself. A commercially available kit using this technology is the “Quantiferon” kit (Cellestis Ltd., Melbourne, Australia), a tuberculosis test. A cytomegalovirus test is also available and is marketed for pre-organ transplant testing. Some of the details of the test are proprietary, but essentially blood from the patient is exposed to antigen from the target pathogen, and the test then evaluates the level of interferon induced from immune cells in the blood. The advantage of this format is that both active and latent infection can be detected in individuals while tests for the agent itself (including culture) or the agent's nucleic acid would be negative in a latent infection. In some ways Quantiferon is similar to the tube or plate agglutination tests used for avian mycoplasma, except that it detects active or latent infection instead of antibody. This format would probably not work well for acute infections and so far has not been applied to poultry infections.
Sample collection, transport and processing
One critical area, which has been largely ignored by diagnostic test developers, is sample collection and processing. Sampling methods for viruses (i.e. media, swab types, sample transport and storage) were originally based on bacterial methods and there are relatively few reports in veterinary or human medicine of validation studies of sample collection and transport. Similarly there are few reports of improvements in sample collection technology. Two relatively recent notable technologies are the use of flocked swabs and FTA cards (Whatman Inc. Whatman Plc, Maidstone, Kent UK). Flocked swabs are composed of a nylon material that is claimed to have superior capture and release characteristics compared with conventional swabs, thus improving sensitivity by increasing the amount of analyte in the sample (Daley et al., Citation2006; Dalmaso et al., Citation2008; Scansen et al., Citation2010). FTA cards have probably had the greatest impact on sample collection and transport of any technology in past decade. They can be used to collect any fluid that can be spotted on the card. Chemicals in the cards inactivate most pathogens and stabilize the nucleic acid, which can then be safely shipped at ambient temperatures and without International Air Transport Association classification as a live pathogen, which saves money. Nucleic acids are extracted from discs punched from the card at the destination laboratory and can be processed for any nucleic acid detection assay. The use of FTA cards has been reported for specimen collection, shipping and subsequent detection of AIV, infectious bronchitis virus, infectious bursal disease virus and NDV (Moscoso et al., Citation2005, Citation2006; Perozo et al., Citation2006; Purvis et al., Citation2006; Abdelwhab et al., Citation2011; Kraus et al., Citation2011).
Some sample processing and collection innovations are too new to have been widely adopted or are still in commercial development. A novel method for nucleic acid purification is syncronous coefficient of drag alternatives (Marziali et al., Citation2005), which uses the unique electro-chemical properties of DNA and RNA to purify them from cells and contaminants that could be inhibitory for PCR and other assays. This method has been described by its inventor, Andre Marziali, as “focusing DNA in a spot in a gel while all other molecules are excluded” due to their unique behaviour in an electric field (Roberts, Citation2011). Another approach to sample collection that is still in development is a mask to collect respiratory agents from coughs, which concentrates them in a buffered solution (Deton Corp., Pasadena, CA, USA) (Roberts, Citation2011). The latter will probably never be practical for poultry, but is a good example of emergent sample collection technology.
What Does the New Technology Mean for Poultry Health?
Regardless of technological capabilities the market will always determine what tests are implemented. Many of the above technologies were developed with human health in mind, where an individual is the target. In contrast, the approach to poultry and food animal health differs somewhat when the target is a population. Also, just because we can do something does not mean it is necessary or practical; for example, sensitivity to the point of single-molecule detection. Would detection of a single AIV virion or Mycoplasma gallisepticum cell really improve control or would it complicate control? Is detection of a single molecule clinically relevant? Is such detection necessary and can the cost be justified? The answers to these questions vary by agent and situation, and the utility and success of a test is heavily reliant on whether it is fit-for-purpose: the best test is the one that fulfils the needs of the situation. The needs vary among different compartments and regions, whether testing is for flock health versus food safety and trade, the importance of the test target, and so forth. For example, a test that works well for the broiler industry in Mexico may not be as practical for layers in the US. This leads to perhaps one of the most important questions when implementing a test, and one that should be considered during development: how will the results be used?
Looking at how new technology has been implemented in the past shows an interesting trend; molecular methods have not completely supplanted classical methods, such as culture. AIV and NDV are good examples where molecular tests have improved detection in the field, so that immediate action can be taken in the case of a positive flock, but culture is still required to obtain a live agent for regulatory action, to characterize the virus present, and for development of vaccines. This paradigm, where molecular tests and point-of-care tests are used as screening tests and then additional confirmatory tests (often culture) must be conducted, is utilized for numerous veterinary and human diseases. The new technology has added to our arsenal, but in many cases has not truly replaced the original test. This is likely to be true with the newest innovations; the molecular methods could be modified or replaced, but the classical methods, specifically culture, will not be abandoned.
In general, many novel technologies will not be easily adopted due to cost or an impractical format. But if one was to select one of the technologies outlined above as having the most promise for poultry diagnostics, the biosensor would probably be the best because it could make pen-side testing possible. However, biosensors are among those that are furthest from adoption (development is still nascent for virus and bacteria detection with biosensors). Microfluidics could also be useful for the diagnostic laboratory to increase capacity and decrease costs if the equipment is affordable. As some of the new technologies become cheaper the improvements may be able to justify the cost of adoption. At the very least there will definitely be improvements for poultry diagnostics from some of the simple modifications becoming available for nucleic acid detection assays even if substantial changes in format are unlikely in the near future.
Acknowledgements
This review was presented as the Houghton Lecture at the 17th Congress of the World Veterinary Poultry Association in Cancun, Mexico, 2011.
Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the US Department of Agriculture.
References
- Abdelwhab , E. M. , Lüschow , D. , Harder , T.C. and Hafez , H.M. 2011 . The use of FTA(R) filter papers for diagnosis of avian influenza virus . Journal of Virololgical Methods , 174 : 120 – 122 .
- Abe , A. , Inoue , K. , Tanaka , T. , Kato , J. , Kajiyama , N. , Kawaguchi , R. and Kohara , M. 1999 . Quantitation of hepatitis B virus genomic DNA by real-time detection PCR . Journal of Clinical Microbiology , 37 : 2899 – 2903 .
- Alwine , J.C. , Kemp , D.J. and Stark , G.R. 1977 . Method for detection of specific RNAs in agarose gels by transfer to diazobenzyloxymethyl-paper and hybridization with DNA probes . Proceedings of the National Academy of Sciences USA , 74 : 5350 – 5354 .
- Baeumner , A J. , Schlesinger , N.A. , Slutzki , N.S. , Romano , J. , Lee , E.M. and Montagna , R.A. 2002 . Biosensor for dengue virus detection: sensitive, rapid, and serotype specific . Analytical Chemistry , 74 : 1442 – 1448 .
- Bedson , S. and Bland , J. 1929 . Complement-fixation with filterable viruses and their antisera . British Journal of Experimental Pathology , 10 : 393 – 404 .
- Best , J. , Banatvala , J.E. , Almeida , J. and Waterson , A.P. 1967 . Morphological characteristics of rubella virus . The Lancet , 290 : 237 – 239 .
- Brenner , S. and Horne , R.W. 1959 . A negative staining method for high resolution electron microscopy of viruses . Biochimica et Biophysica Acta , 34 : 103 – 110 .
- Bruckner , L. , Stauber , N. , Brechtbuhl , K. and Hofmann , M. A. 1996 . Detection of extraneous agents in vaccines using the polymerase chain reaction of Newcastle disease virus in poultry biologicals . Developments in Biololgical Standards , 86 : 175 – 182 .
- Burke , D.S. , Brandt , B.L. , Redfield , R.R. , Lee , T.H. , Thorn , R.M. , Beltz , G.A. and Hung , C.H. 1987 . Diagnosis of human immunodeficiency virus infection by immunoassay using a molecularly cloned and expressed virus envelope polypeptide. Comparison to Western blot on 2707 consecutive serum samples . Annals of Internal Medicine , 106 : 671 – 676 .
- Carlson , B. 2011 . Molecular diagnostics: potential and reality . Genetic Engineering and Biotechnology News , 31 : 18 – 19 .
- Daley , P. , Castriciano , S. , Chernesky , M. and Smieja , M. 2006 . Comparison of flocked and rayon swabs for collection of respiratory epithelial cells from uninfected volunteers and symptomatic patients . Journal of Clinical Microbiology , 44 : 2265 – 2267 .
- Dalmaso , G. , Bini , M. , Paroni , R. and Ferrari , M. 2008 . Qualification of high-recovery, flocked swabs as compared to traditional rayon swabs for microbiological environmental monitoring of surfaces . PDA Journal of Pharmaceutical Science and Technology , 62 : 191 – 199 .
- Das , A. and Suarez , D.L. 2007 . Development and bench validation of real-time reverse transcription polymerase chain reaction protocols for rapid detection of the subtypes H6, H9, and H11 of avian influenza viruses in experimental samples . Journal of Veterinary Diagnostic Investigation , 19 : 625 – 634 .
- Dascal , A. , Chan-Thim , J. , Morahan , M. , Portnoy , J. and Mendelson , J. 1989 . Diagnosis of herpes simplex virus infection in a clinical setting by a direct antigen detection enzyme immunoassay kit . Journal of Clinical Microbiology , 27 : 700 – 704 .
- Davis , B.J. 1964 . Disc electrophoresis-II. Method and application to human serum proteins . Annals of the New York Academy of Sciences , 121 : 404 – 427 .
- Di Trani , L. , Bedini , B. , Donatelli , I. , Campitelli , L. , Chiappini , B. , De Marco , M. A. , Delogu , M. , Buonavoglia , C. and Vaccari , G. 2006 . A sensitive one-step real-time PCR for detection of avian influenza viruses using a MGB probe and an internal positive control . BMC Infectious Diseases , 6 : 87
- Egberink , H.F. , Lutz , H. and Horzinek , M.C. 1991 . Use of western blot and radioimmunoprecipitation for diagnosis of feline leukemia and feline immunodeficiency virus infections . Journal of the American Veterinary Medical Association , 199 : 1339 – 1342 .
- Fan , J. , Henrickson , K.J. and Savatski , L.L. 1998 . Rapid simultaneous diagnosis of infections with respiratory syncytial viruses A and B, influenza viruses A and B, and human parainfluenza virus types 1, 2, and 3 by multiplex quantitative reverse transcription-polymerase chain reaction-enzyme hybridization assay (Hexaplex) . Clinical Infectious Diseases , 26 : 1397 – 1402 .
- Farkas , T. , Szekely , E. , Belak , S. and Kiss , I. 2009 . Real-time PCR-based pathotyping of Newcastle disease virus by use of TaqMan minor groove binder probes . Journal of Clinical Microbiology , 47 : 2114 – 2123 .
- Gary , G.W. Jr. , Kaplan , J.E. , Stine , S.E. and Anderson , L.J. 1985 . Detection of Norwalk virus antibodies and antigen with a biotin-avidin immunoassay . Journal of Clinical Microbiology , 22 : 274 – 278 .
- Ghorashi , S.A. , O'Rourke , D. , Ignjatovic , J. and Noormohammadi , A.H. 2011 . Differentiation of infectious bursal disease virus strains using real-time RT-PCR and high resolution melt curve analysis . Journal of Virololgical Methods , 171 : 264 – 271 .
- Goodpasture , E.W. , Woodruff , A.M. and Buddingh , G.J. 1931 . The cultivation of vaccine and other viruses in the chorioallantoic membrane of chick embryos . Science , 74 : 371 – 372 .
- Hart , C. , Schochetman , G. , Spira , T. , Lifson , A. , Moore , J. , Galphin , J. , Sninsky , J. and Ou , C. Y. 1988 . Direct detection of HIV RNA expression in seropositive subjects . Lancet , 332 : 596 – 599 .
- Heid , C.A. , Stevens , J. , Livak , K.J. and Williams , P.M. 1996 . Real time quantitative PCR . Genome Research , 6 : 986 – 994 .
- Hernandez , M. , Tomas , G. , Hernandez , D. , Villegas , P. , Banda , A. , Maya , L. , Panzera , Y. and Perez , R. 2011 . Novel multiplex RT-PCR/RFLP diagnostic test to differentiate low- from high-pathogenic strains and to detect reassortant infectious bursal disease virus . Avian Diseases , 55 : 368 – 374 .
- Hoffmann , B. , Beer , M. , Reid , S.M. , Mertens , P. , Oura , C.A. , van Rijn , P.A. , Slomka , M J. , Banks , J. , Brown , I.H. , Alexander , D.J. and King , D.P. 2009 . A review of RT-PCR technologies used in veterinary virology and disease control: sensitive and specific diagnosis of five livestock diseases notifiable to the World Organisation for Animal Health . Veterinary Microbiology , 139 : 1 – 23 .
- Horimoto , T. and Kawaoka , Y. 1995 . Direct reverse transcriptase PCR to determine virulence potential of influenza A viruses in birds . Journal of Clinical Microbiology , 33 : 748 – 751 .
- Inoue , K. , Arai , T. and Aoyagi , M. 1999 . Sensitivity of real time viral detection by an optical biosensor system using a crude home-made antiserum against measles virus as a ligand . Biological and Pharmaceutical Bulletin , 22 : 210 – 213 .
- Jackwood , D.J. , Hanes , G. and Miller , S. H. 1996 . Infectious bursal disease viral RNA amplification using RT/PCR from bursa tissue following phenol:chloroform inactivation of the virus . Avian Diseases , 40 : 457 – 460 .
- Jackwood , M.W. , Yousef , N.M. and Hilt , D.A. 1997 . Further development and use of a molecular serotype identification test for infectious bronchitis virus . Avian Diseases , 41 : 105 – 110 .
- Jones , R.M. , Ellis , R.J. , Cox , W.J. , Errington , J. , Fuller , C. , Irvine , R.M. and Wakeley , P.R. 2011 . Development and validation of RT-PCR tests for the detection and S1 genotyping of infectious bronchitis virus and other closely related gammacoronaviruses within clinical samples . Transboundary and Emerging Diseases , 58 : 411 – 420 .
- Keeler , C.L. Jr. , Reed , K.L. , Nix , W.A. and Gelb , J. Jr. 1998 . Serotype identification of avian infectious bronchitis virus by RT-PCR of the peplomer (S-1) gene . Avian Diseases , 42 : 275 – 284 .
- Kohler , G. and Milstein , C. 1975 . Continuous cultures of fused cells secreting antibody of predefined specificity . Nature , 256 : 495 – 497 .
- Kraus , R.H. , van Hooft , P. , Waldenstrom , J. , Latorre-Margalef , N. , Ydenberg , R.C. and Prins , H.H. 2011 . Avian influenza surveillance with FTA cards: field methods, biosafety, and transportation issues solved . Journal of Visualized Experiments , 54 : e2832
- Kutyavin , I.V. , Afonina , I.A. , Mills , A. , Gorn , V.V. , Lukhtanov , E.A. , Belousov , E.S. , Singer , M.J. , Walburger , D.K. , Lokhov , S.G. , Gall , A.A. , Dempcy , R. , Reed , M.W. , Meyer , R.B. and Hedgpeth , J. 2000 . 3’-minor groove binder-DNA probes increase sequence specificity at PCR extension temperatures . Nucleic Acids Research , 28 : 655 – 661 .
- Kwon , H.M. , Jackwood , M.W. and Gelb , J. Jr. 1993 . Differentiation of infectious bronchitis virus serotypes using polymerase chain reaction and restriction fragment length polymorphism analysis . Avian Diseases , 37 : 194 – 202 .
- Lazarus , A S. , Eddie , B. and Meyer , K.F. 1937 . Propagation of variola virus in the developing egg . Proceedings of the Society of Experimental Biology and Medicine , 36 : 7 – 8 .
- Liu , C. 1956 . Rapid diagnosis of human influenza infection from nasal smears by means of fluorescein-labeled antibody . Proceedings of the Society of Experimental Biology and Medicine , 92 : 883 – 887 .
- Loeffler , F. and Frosch , P. 1898 . Berichte der Kommission zur Erforschung der Maul und Klauenseuche bei dem Institut für Infektionskrankheiten in Berlin . Zentralblatt für Bakteriologie, Parasitenkunde und Infektionskrankheiten , 23 : 371 – 391 .
- Lu , Y.Y. , Yan , J.Y. , Feng , Y. , Xu , C.P. , Shi , W. and Mao , H.Y. 2008 . Rapid detection of H5 avian influenza virus by TaqMan-MGB real-time RT-PCR . Letters in Applied Microbiology , 46 : 20 – 25 .
- Marziali , A. , Pel , J. , Bizzotto , D. and Whitehead , L.A. 2005 . Novel electrophoresis mechanism based on synchronous alternating drag perturbation . Electrophoresis , 26 : 82 – 90 .
- McMenamy , M.J. , McKillen , J. , Hjertner , B. , Kiss , I. , Yacoub , A. , Leijon , M. , Duffy , C. , Belak , S. , Welsh , M. and Allan , G. 2011 . Development and comparison of a Primer-Probe Energy Transfer based assay and a 5’ conjugated Minor Groove Binder assay for sensitive real-time PCR detection of infectious laryngotracheitis virus . Journal of Virological Methods , 175 : 149 – 155 .
- Mears , J.G. , Ramirez , F. , Leibowitz , D. , Nakamura , F. , Bloom , A. , Konotey-Ahulu , F. and Bank , A. 1978 . Changes in restricted human cellular DNA fragments containing globin gene sequences in thalassemias and related disorders . Proceedings of the National Academy of Sciences USA , 75 : 1222 – 1226 .
- Moscoso , H. , Alvarado , I. and Hofacre , C.L. 2006 . Molecular analysis of infectious bursal disease virus from bursal tissues collected on FTA filter paper . Avian Diseases , 50 : 391 – 396 .
- Moscoso , H. , Raybon , E.O. , Thayer , S.G. and Hofacre , C.L. 2005 . Molecular detection and serotyping of infectious bronchitis virus from FTA filter paper . Avian Diseases , 49 : 24 – 29 .
- Nelson , J. B. 1939 . The behavior of pox viruses in the respiratory tract. II. The response of mice to the nasal instillation of variola virus . Journal of Experimental Medicine , 70 : 107 – 116 .
- OIE . 2011 . Manual of Diagnostic Tests and Vaccines for Terrestrial Animals 2011 Retrieved November 14, 2011, from http://www.oie.int/index.php?id=170
- Ornstein , L. 1964 . Disc Electrophoresis. I. Background and Theory . Annals of the New York Academy of Sciences , 121 : 321 – 349 .
- Perozo , F. , Villegas , P. , Estevez , C. , Alvarado , I. and Purvis , L. B. 2006 . Use of FTA filter paper for the molecular detection of Newcastle disease virus . Avian Pathology , 35 : 93 – 98 .
- Proffitt , M.R. and Schindler , S. A. 1995 . Rapid detection of HSV with an enzyme-linked virus inducible system (ELVIS) employing a genetically modified cell line . Clinical and Diagnostic Virology , 4 : 175 – 182 .
- Purvis , L.B. , Villegas , P. and Perozo , F. 2006 . Evaluation of FTA paper and phenol for storage, extraction and molecular characterization of infectious bursal disease virus . Journal of Virololgical Methods , 138 : 66 – 69 .
- Rappolee , D.A. , Mark , D. , Banda , M.J. and Werb , Z. 1988 . Wound macrophages express TGF-alpha and other growth factors in vivo: analysis by mRNA phenotyping . Science , 241 : 708 – 712 .
- Reimann , I. and Werner , O. 1996 . Use of the polymerase chain reaction for the detection of reticuloendotheliosis virus in Marek's disease vaccines and chicken tissues . Zentralblatt für Veterinarmedizin B , 43 : 75 – 84 .
- Rivera , E. , Sjoland , L. and Karlsson , K.A. 1986 . A solid phase fluorescent immunoassay for the rapid detection of virus antigen or antibodies in fetuses infected with porcine parvovirus . Archives of Virology , 88 : 19 – 26 .
- Roberts , J. 2011 . Streamlining nucleic acid sample prep: advances in long-read technology, target enrichment, and collection reshape operations . Genetic Engineering and Biotechnology News , 31 : 26 – 28 .
- Rous , P. and Murphy , J. 1911 . Tumor implantations in the developing embryo . Journal of the American Medical Association , 56 : 741 – 742 .
- Saiki , R.K. , Gelfand , D.H. , Stoffel , S. , Scharf , S.J. , Higuchi , R. , Horn , G.T. , Mullis , K.B. and Erlich , H. A. 1988 . Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase . Science , 239 : 487 – 491 .
- Saiki , R.K. , Scharf , S. , Faloona , F. , Mullis , K.B. , Horn , G.T. , Erlich , H.A. and Arnheim , N. 1985 . Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia . Science , 230 : 1350 – 1354 .
- Scansen , K.A. , Bonsu , B.K. , Stoner , E. , Mack , K. , Salamon , D. , Leber , A. and Marcon , M.J. 2010 . Comparison of polyurethane foam to nylon flocked swabs for collection of secretions from the anterior nares in performance of a rapid influenza virus antigen test in a pediatric emergency department . Journal of Clinical Microbiology , 48 : 852 – 856 .
- Shi , L. , Ho , J. , Norling , L. A. , Roy , M. and Xu , Y. 1999 . A real time quantitative PCR-based method for the detection and quantification of simian virus 40 . Biologicals , 27 : 241 – 252 .
- Shibata , D.K. , Arnheim , N. and Martin , W. J. 1988 . Detection of human papilloma virus in paraffin-embedded tissue using the polymerase chain reaction . Journal of Experimental Medicine , 167 : 225 – 230 .
- Southern , E.M. 1975 . Detection of specific sequences among DNA fragments separated by gel electrophoresis . Journal of Molecular Biology , 98 : 503 – 517 .
- Spackman , E. , Senne , D.A. , Myers , T.J. , Bulaga , L.L. , Garber , L.P. , Perdue , M.L. , Lohman , K. , Daum , L.T. and Suarez , D.L. 2002 . Development of a real-time reverse transcriptase PCR assay for type A influenza virus and the avian H5 and H7 hemagglutinin subtypes . Journal of Clinical Microbiology , 40 : 3256 – 3260 .
- Stauber , N. , Brechtbuhl , K. , Bruckner , L. and Hofmann , M.A. 1995 . Detection of Newcastle disease virus in poultry vaccines using the polymerase chain reaction and direct sequencing of amplified cDNA . Vaccine , 13 : 360 – 364 .
- Tham , K.M. and Stanislawek , W.L. 1992 . Detection of chicken anaemia agent DNA sequences by the polymerase chain reaction . Archives of Virology , 127 : 245 – 255 .
- Todd , D. , Mawhinney , K.A. and McNulty , M.S. 1992 . Detection and differentiation of chicken anemia virus isolates by using the polymerase chain reaction . Journal of Clinical Microbiology , 30 : 1661 – 1666 .
- Towbin , H. , Staehelin , T. and Gordon , J. 1979 . Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications . Proceedings of the National Academy of Sciences USA , 76 : 4350 – 4354 .
- van Woensel , P.A. , van Blaaderen , A. , Moorman , R.J. and de Boer , G.F. 1992 . Detection of proviral DNA and viral RNA in various tissues early after avian leukosis virus infection . Leukemia , 6 ( Suppl 3 ) : 135S – 137S .
- Wang , J. , Cai , X. , Rivas , G. , Shiraishi , H. , Farias , P.A. and Dontha , N. 1996 . DNA electrochemical biosensor for the detection of short DNA sequences related to the human immunodeficiency virus . Analytical Chemistry , 68 : 2629 – 2634 .
- Wang , X. and Khan , M.I. 1999 . A multiplex PCR for Massachusetts and Arkansas serotypes of infectious bronchitis virus . Molecular and Cellular Probes , 13 : 1 – 7 .
- Weller , T.H. and Enders , J.F. 1948 . Production of hemagglutinin by mumps and influenza A viruses in suspended cell tissue cultures . Proceedings of the Society of Experimental Biology and Medicine , 69 : 124 – 128 .
- Williams , R.A. , Bennett , M. , Bradbury , J.M. , Gaskell , R.M. , Jones , R.C. and Jordan , F.T.W. 1992 . Demonstration of sites of latency of infectious laryngotracheitis virus using the polymerase chain reaction . Journal of General Virology , 73 : 2415 – 2420 .
- Wise , M.G. , Suarez , D.L. , Seal , B.S. , Pedersen , J.C. , Senne , D.A. , King , D.J. , Kapczynski , D.R. and Spackman , E. 2004 . Development of a real-time reverse-transcription PCR for detection of Newcastle disease virus RNA in clinical samples . Journal of Clinical Microbiology , 42 : 329 – 338 .
- Woodruff , A.M. and Goodpasture , E.W. 1931 . The susceptibility of the chorio-allantoic membrane of chick embryos to infection with the fowl-pox virus . American Journal of Pathology , 7 : 209 – 222 .
- Xu , J. , Suarez , D. and Gottfried , D.S. 2007 . Detection of avian influenza virus using an interferometric biosensor . Analytical and Bioanalyical Chemistry , 389 : 1193 – 1199 .
- Zhou , X. , Liu , L. , Hu , M. , Wang , L. and Hu , J. 2002 . Detection of hepatitis B virus by piezoelectric biosensor . Journal of Pharmaceutical and Biomedical Analysis , 27 : 341 – 345 .