Abstract
Previous studies have demonstrated the presence of multiple strains of Marek's disease virus simultaneously circulating within poultry flocks, leading to the assumption that individual birds are repeatedly exposed to a variety of virus strains in their lifetime. Virus competition within individual birds may be an important factor that influences the outcome of co-infection under field conditions, including the potential outcome of emergence or evolution of more virulent strains. A series of experiments was designed to evaluate virus competition within chickens following simultaneous challenge with two virulent serotype 1 Marek's disease virus strains, using either pathogenically similar (rMd5 and rMd5/pp38CVI) or dissimilar (JM/102W and rMd5/pp38CVI) virus pairs. Bursa of Fabricius, feather follicle epithelium, spleen, and tumour samples were collected at multiple time points to determine the frequency and distribution of each virus present using pyrosequencing, immunohistochemistry and virus isolation. In the similar pair, rMd5 appeared to have a competitive advantage over rMd5/pp38CVI, which in turn had a competitive advantage over the less virulent JM/102W in the dissimilar virus pair. Dominance of one strain over the other was not absolute for either virus pair, as the subordinate virus was rarely eliminated. Interestingly, competition between two viruses with either pair rarely ended in a draw. Further work is needed to identify factors that influence virus-specific dominance to better understand what characteristics favour emergence of one strain in chicken populations at the expense of other strains.
Introduction
Marek's disease (MD) is a lymphoproliferative disease in chickens caused by an alphaherpesvirus, Marek's disease virus (MDV). The disease is typically characterized by enlarged nerves and visceral tumours composed of transformed T cells. Although vaccination has been successfully used to prevent disease, the vaccines do not prevent infection and transmission of the virulent virus. Thus, as early as 2 weeks after inoculation or exposure, MDV is spread in the feather dust to other chickens as the keratinized layer of infected epithelial cells is sloughed and shed (Calnek, Citation1980). Shedding appears to continue indefinitely, peaking at approximately 3 to 5 weeks (Witter, Citation1971; Witter et al., Citation1971), and virus can remain infectious in a poultry house for months or years depending on environmental conditions (Kenzy, Citation1967; Calnek, Citation1980).
As a result of continual shedding and virus stability in the poultry house environment, it is assumed that most poultry houses are rich in infectious virus. Also, previous studies suggest that multiple strains of MDV can be present simultaneously within a flock (Biggs et al., Citation1972; Jackson et al., Citation1976). Thus, chickens raised in a commercial setting are probably exposed to MDV repeatedly and continuously over their lifetime. A study by Jackson et al. (Citation1976) confirmed the presence of multiple pathotypes circulating within the same flock, and demonstrated that the presence of apathogenic virus strains was associated with low incidence of MD and provided protection against pathogenic MDV challenge. In two flocks, Witter (Citation1997) evaluated the virulence of multiple isolates (two or three) and found little difference within flocks. These isolates were all from tumour-bearing birds, which may have created a bias towards isolation of more virulent strains. This also suggests, however, that a single virus strain may have the ability to become dominant.
Dominance in this study was defined as the inherent property of a particular MDV strain to become the exclusive or majority strain present in the tissue from a bird following dual MDV challenge. If a virus strain becomes dominant in an individual chicken, it must first compete successfully with other virus strains infecting that chicken. Similarly, for a virus strain to become dominant within the entire flock, it must compete successfully within individual chickens against other strains circulating in the flock. Whether referring to one chicken or to the entire flock, the model presumes the existence of multiple infections within individual chickens, which could occur either simultaneously or at separate time points (superinfection). Although superinfection may be more likely, if the virus load is high enough, newly placed chicks may be exposed to multiple strains virtually simultaneously.
Vaccine studies and commercial field isolates have shown that co-infection can occur in chickens with virulent and avirulent MDV strains. Serotype 1 MDV (GaHV-2) strains have been isolated following initial infection with serotype 2 and serotype 3 (GaHV-3, MeHV-1) avirulent MDV strains (Witter et al., Citation1976; Calnek et al., Citation1980). Another study reported evidence of co-infection with acute (Id-1) and mild (HN) MDV strains following simultaneous challenge, although HN was later classified as a non-pathogenic serotype 2 strain (Cho & Kenzy, Citation1972; Schat & Calnek, Citation1978; Witter, Citation1983). After contact exposure to both strains, the authors concluded that infection with one strain, whether acute or mild, did not exclude subsequent infection by the other strain (Cho & Kenzy, Citation1973). After simultaneous challenge by injection, Cho (Citation1975) reported that isolates from both strains were present in susceptible birds at 1 week continuing through termination at 8 weeks, with gradual reduction from six of 17 birds with both strains present at 2 weeks post inoculation down to one bird with both strains present at 8 weeks. The same author also reported the presence of both viruses in feather tip extracts following simultaneous challenge (Cho, Citation1977). Co-infection with attenuated serotype 1 strains and virulent serotype 1 strains has also been documented in laboratory studies and from Rispens-vaccinated birds in the field. Churchill et al. (Citation1969) infected chickens with live attenuated serotype 1 virus (HPRS-16/att) and demonstrated that subsequent challenge with more virulent serotype 1 strains can cause infection.
Our previous studies using recombinant DNA technology for virus differentiation have clearly and for the first time demonstrated co-infection with two virulent (unattenuated) strains of MDV strains (Dunn et al., Citation2010). Results from these studies indicated a strong influence of time interval between virus challenge on the susceptibility of chickens to the second inoculum (virus). Results also indicated that the virus strain may have been a factor influencing the ability for superinfection, but the effect of strain was confounded by difference in infection times. It was concluded that experiments where two viruses were administered simultaneously were needed to resolve the issue of dominance. Thus, the goal of these current studies was to determine whether virulent strains administered simultaneously led to a majority frequency of one particular strain.
Possible outcomes of simultaneous infection include independent growth of each strain (no dominance), total exclusion of a lesser strain (dominance), or competition without complete exclusion (dominance). If competition occurs between different strains, this may lead to decreased replication and/or transmission of the subordinate strain and may affect the contribution of each virus in tumours. The potential selection of highly competitive strains within individual chickens may be an important mechanism in the evolution of virus strains within a flock.
Materials and Methods
Chickens
MD-susceptible White Leghorn 15I5×71 chickens were used in these experiments (Bacon et al., Citation2000). Maternal antibody-negative (Ab−) chickens (Experiments 1 and 2) were from a specific pathogen free breeding flock with no MD vaccinations or exposure that tested negative for MDV antibodies by routine surveillance tests. Maternal antibody-positive (Ab+) chickens (Experiment 3), used to reduce early mortality and simulate field conditions, were reared from breeder hens vaccinated at hatch with 2000 plaque-forming units of turkey herpesvirus (HVT) and at 25 weeks with 2000 plaque-forming units of SB1 and Md11/75C viruses for exposure to all three serotypes. Both flocks were also negative by routine surveillance testing for exogenous avian leukosis virus and reticuloendotheliosis virus. All birds were housed in negative-pressure Horsfall–Bauer isolators. Experiments were approved by the Avian Disease and Oncology Laboratory Animal Care and Use Committee.
Viruses
Two virus pairs were used for simultaneous dual infection: JM/102W and rMd5/pp38CVI; and rMd5 and rMd5/pp38CVI. JM/102W is a pathotyped as a prototype virulent MDV (vMDV) strain frequently used in MD experiments (Sevoian et al., Citation1962; Witter, Citation1983, Citation1997). The recombinant rMd5 was generated using overlapping cosmid clones (Reddy et al., Citation2002) produced from wild-type Md5, a very virulent MDV (vvMDV) strain (Witter et al., Citation1980; Witter, Citation1983; Reddy et al., Citation2002). Compiled pathotyping data demonstrates that gonad and heart tumours are seen most commonly following challenge with JM/102W and Md5, respectively (R.L. Witter, unpublished data). The recombinant rMd5/pp38CVI (aka rMd5//38CVI) was produced using the rMd5 cosmid clones to substitute the pp38 gene, located in the IRL and UL region, from CVI988/Rispens (Lee et al., Citation2005). Thus, these two viruses share the same rMd5 backbone and are only different in the pp38 gene, which differ by two single nucleotide polymorphisms. The rMd5 and rMd5/pp38CVI virus strains were also chosen based on their similar pathogenicity, reported to have no differences in the frequency of lymphoproliferative lesions between the viruses at either 6 or 15 weeks post inoculation (Gimeno et al., Citation2005; Lee et al., Citation2005). JM/102W was chosen to pair with rMd5/pp38CVI to study dual infection of two MDV strains of differing pathotype. A total of 500 plaque-forming units of each virus (as determined by initial stock titre) were administered intra-abdominally, with half of each dose inoculated on each side of the abdomen to try and reduce the chance of inoculation failures. Viruses were mixed together prior to inoculation for dual-challenged groups.
Experimental design
Three experiments were designed to characterize co-infection in simultaneously challenged chickens. Data from each virus pair were analysed separately as parts A and B of each experiment for the dissimilar pathotype (JM/102W and rMd5/pp38CVI) or similar pathotype (rMd5 and rMd5/pp38CVI) virus pair, respectively. Single-challenged control birds were used to confirm the specificity of each assay and as a comparison for quantitative differences compared with dual infection.
Experiment 1
Twenty 15I5×71 Ab− chickens were challenged singly with each virus or challenged dually with both viruses at hatch, except one group of uninfected chickens used as controls. Bursa samples were collected from six birds that were removed and euthanized from each lot at 4 and 6 days post infection (d.p.i.). The remaining eight birds from each lot were euthanized at 21 d.p.i. and feathers and skin sections were collected from the subhumeral feather tract for DNA and immunohistochemistry (IHC) testing, respectively.
Experiments 2 and 3
These two experiments were identical except that Experiment 3 used Ab + chickens to reduce early mortality. Groups of 17 chickens were challenged singly with each virus or challenged dually with both viruses at hatch to increase the probability of acquiring visceral tumours. Surviving chickens were euthanized at 56 d.p.i. and samples were collected. One tumour from each tumour-bearing organ per chicken was collected for pyrosequencing and spleens were collected for virus isolation. In Experiment 2, only visceral tumours were collected for analysis. In Experiment 3, all chickens with visceral tumours were sampled plus enlarged nerves were randomly collected from additional chickens without visceral tumours in order to collect samples from a minimum of eight birds per lot. The inclusion of enlarged nerves in Experiment 3 was due to the insufficient number of visceral tumours present in Experiment 2, plus the even lower number of visceral tumours expected in Experiment 3 due to the presence of maternal antibodies.
Monoclonal antibodies
Antibodies H19 and T65 were used to discriminate between viruses in the above virus pairs, based on differences in nucleotides #320 and #326 of the pp38 open reading frame (Cui et al., Citation1991; Endoh et al., Citation1994). H19 was specific to glutamine at amino acid #107 due to adenine (A) at base pair #320 (i.e. JM/102W, rMd5), whereas T65 was specific to glycine at amino acid #109 due to guanine (G) at base pair #326 (rMd5/pp38CVI) (Cui et al., Citation2004).
Virus isolation and immunohistochemistry testing
Virus isolation and IHC were performed as previously described (Dunn et al., Citation2010). Briefly, spleen cell suspensions (~6.0×105 cells) from each bird were plated on two to four secondary plates of duck embryo fibroblasts, fixed at 7 days and later incubated with H19 or T65 followed by fluorescein-conjugated secondary antibody (MP Biomedicals, LLC, Solon, Ohio, USA). Plaques were counted using a fluorescent microscope. Spleen suspensions were stored in freezing media at −80°C for a prolonged period of time (>1 year) prior to virus isolation assays, which was probably responsible for the lower than usual observed plaque counts.
For IHC, two serial sections (5 µm) of frozen bursa and feathered skin were cut, fixed, incubated with H19 (1:3200) or T65 (1:2000) and stained using the Vectastain ABC kit, as described by the manufacturer (Vector Laboratories, Inc., Burlingame, California, USA). Slides were processed using a DakoCytomation Autostainer (Dako, Glostrup, Denmark). Attempts at dual staining using isotype-specific secondary antibodies and direct labelling of primary antibodies were both unsuccessful due to lost specificity, despite that H19 (IgG1) and T65 (IgG2a) are different IgG isotypes (data not shown).
Pyrosequencing assay
Pyrosequencing was used to determine the percentage of each nucleotide present in mixed samples at base pair 320 of the pp38 gene. This assay was performed with DNA isolated from the bursa, feather follicles, spleen and tumour samples using a PSQ 96MA system (Qiagen, Hilden, Germany), as previously described (Dunn et al., Citation2010). Within this report, the term feather follicle is used to describe specific follicles within tissue sections, and feather follicle DNA refers to DNA isolated from feather follicle epithelium within the respective follicles. Results were analysed from all samples in which sequence was detected. Samples that did not have adequate virus present (e.g. negative control samples) or in which there was either insufficient or excessive total DNA present resulted in assay failure and were noted in the results. There was a small sequencing bias evident in all samples from single-challenged control birds inoculated with JM/102W or rMd5 in which guanine (rMd5/pp38CVI) was erroneously reported as having frequency ranging from 1 to 4% (data not shown). IHC data support that rMd5/pp38CVI was not present in these tissue sections. As a result, pyrosequencing results were adjusted so any samples that were reported to have between 1 and 4% frequency of guanine were normalized to 0%, which affected 10 DNA samples from dual-challenged birds across Experiments 1 to 3. Our previous validation using a series of plasmid DNA stocks containing the Md5 or Rispens pp38 gene serially diluted in 2.5 ng/µl salmon sperm DNA indicated that 1000 virus copies/µl of pp38 plasmid DNA was needed for detection by pyrosequencing (using 2 µl diluted plasmid DNA in a 25µl pyrosequencing reaction) and in some cases at least 10% of the lower frequency virus was needed to detect both viruses in a sample (Dunn et al., Citation2010).
Data analysis
IHC results were analysed by comparing individual bursa and feather follicles from serial sections stained with H19 or T65 monoclonal antibodies. The percentage of follicles staining for each virus or for both was contrasted. Pyrosequencing results compared the frequency of each virus present within all bursa, feather follicle or tumour DNA samples that had passing results (sequence was detected). Differences in frequency between viruses in each experiment were analysed by a paired t-test. Virus isolation results were analysed using the Wilcoxon rank-sum test due to low numbers of samples in most groups. The analysis compared plaque counts for each virus in dual-challenged chickens with single-challenged chickens challenged with the same virus. P values less than 0.05 were considered significant. Data were analysed with the statistical program SAS 9.1 (SAS Institute Inc., Cary, North Carolina, USA).
Results
Bursa and feather follicles
Experiment 1 was designed to compare the differences between virus frequency and distribution for both virus pairs during early and late cytolytic infection in the bursa and feather follicles, respectively. Data were not included from day 4 bursa samples due to a lack of staining in all but one IHC sample and lack of virus sequence detection in all but four samples by pyrosequencing. The number of birds was reduced in several treatment groups for Experiments 1A and 1B due to early mortality. In Experiment 1A, rMd5/pp38CVI was present in significantly higher frequency compared with JM/102W by pyrosequencing in all bursa samples from dual-challenged birds (P<0.0001) whereas dominance of rMd5/pp38CVI was much less pronounced in feather follicle epithelium samples (P = 0.2801) (). IHC confirmed a much higher percentage of mixed infections within feather follicles compared with bursa follicles. A majority of individual feather follicles were found to stain for both viruses following dual challenge, and viruses were frequently present within the same region of the follicle ().
Figure 1. Immunohistochemistry staining of serial feather follicle sections 21 d.p.i. from a chicken simultaneously challenged with JM/102W and rMd5/pp38CVI. Sections on the left and right were stained with H19 and T65 monoclonal antibodies, respectively (Experiment 1A).
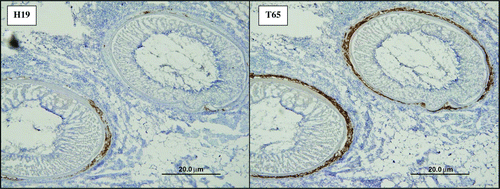
Table 1. Virus frequency results from bursa (6 d.p.i.) and feather follicle (21 d.p.i.) samples in single-challenged or dual-challenged chickens using a dissimilar pathotype virus pair (Experiment 1A).
In Experiment 1B, virus sequence was detected at day 6 from four of six bursa samples from chickens dual-challenged with rMd5 and rMd5/pp38CVI (). Mixtures were more common by pyrosequencing in bursa samples compared with the dissimilar virus pair, with higher frequency of rMd5 in three of four samples (P = 0.3317). In most cases, only one virus was detected within individual bursa follicles, although both viruses were detected in a small number of follicles (). In feather follicle DNA samples rMd5 was dominant in all seven samples (P<0.0001) by pyrosequencing, although IHC demonstrated that rMd5/pp38CVI was still present to a lesser degree in most feather follicles.
Figure 2. Immunohistochemistry staining of serial bursa sections 6 d.p.i. from a chicken simultaneously challenged with rMd5 and rMd5/pp38CVI. The sections on the left and right were stained with H19 and T65 monoclonal antibodies, respectively. Black circles surround follicles that stained for rMd5 virus only (H19 antibody), white circles stained for rMd5/pp38CVI virus only (T65 antibody), and white/black circles stained for both viruses (Experiment 1B).
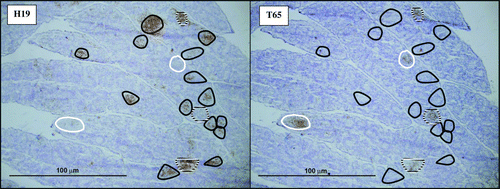
Table 2. Virus frequency results from bursa (6 d.p.i.) and FFE (21 d.p.i.) samples in single-challenged or dual-challenged chickens using similar pathotype virus pair (Experiment 1B).
Tumours
The purpose of Experiments 2 and 3 was to look for evidence of virus mixtures in tumours. Experiments 2 and 3 were identical except Ab+ birds were used in the latter experiment to increase survival until sampling. Virus sequence was detected from 14 tumours from eight birds challenged dually with JM/102W and rMd5/pp38CVI in Experiments 2A and 3A (). Of these 14 tumour samples, JM/102W was present in higher frequency in seven tumours whereas rMd5/pp38CVI was present in higher frequency from the other seven tumours. Ten of these 14 tumour samples were positive for both viruses. Seven tumours from five birds favoured JM/102W and seven tumours from five birds favoured rMd5/pp38CVI. Two of four birds (Birds Q1834 and Q1838) with multiple tumours had virus frequencies that were markedly different between tumours, and in fact different virus strains were dominant. In Bird Q1834, for example, the frequency of JM/102W was 100% in DNA isolated from the gonad tumour and 66% from the enlarged nerves, yet rMd5/pp38CVI was present in higher frequency (77%) in DNA from the heart tumour of the same bird. Virus plaque counts were unusually low from spleen cell suspensions, but of six samples that stained positive, two stained for only JM/102W and four stained only for rMd5/pp38CVI (). There were no significant differences between plaque counts for each virus in single-challenged versus dual-challenged chickens (P = 0.3152).
Table 3. Virus isolation and pyrosequencing results from single-challenged and dual-challenged chickens using a dissimilar pathotype virus pair: tumours or enlarged nerves were used for pyrosequencing, except negative controls that were spleen samples (Experiments 2A and 3A).
In chickens challenged dually with rMd5 and rMd5/pp38CVI in Experiments 2B and 3B, virus was detected in 17 tumour samples from 11 chickens (). Both viruses were detected in three samples from two birds, and rMd5 was favoured in all three. In the remaining 14 tumours, rMd5 was detected exclusively. More than one tumour was tested for five birds and, unlike with samples from virus pair 1, all frequencies were similar within individual birds. Virus plaques stained only for rMd5 from spleen cells isolated from six of seven chickens. The remaining chicken had plaques present from both viruses, although twice as many were positive for rMd5 (). There were no significant differences between plaque counts for each virus in single-challenged versus dual-challenged chickens (P = 0.2413). A summary of pyrosequencing averages compares the overall virus frequencies between tissue types in all of the preceding experiments ().
Table 4. Virus isolation and pyrosequencing results from single-challenged and dual-challenged chickens using a similar pathotype virus pair: tumours or enlarged nerves were used for pyrosequencing, except negative controls that were spleen samples (Experiments 2B and 3B).
Table 5. Pyrosequencing average virus frequency per group.
Discussion
Our previous work demonstrated that superinfection could occur with multiple virulent serotype 1 MDV strains (Dunn et al., Citation2010). Although the time interval between inoculations had the greatest effect on the detection of the second inoculated virus, superinfection also appeared influenced by the strain of the primary and secondary virus. Following the short (24-h) challenge interval, superinfection was much easier for vvMDV rMd5/pp38CVI (detected in 98% of birds when JM/102W was challenged first) than for vMDV JM/102W (detected in only 22% of birds when rMd5/pp38CVI was challenged first) (Dunn et al., Citation2010). A virus-specific advantage was less apparent within the virus pair of similar pathotype. With the removal of the time interval between inoculations, the current study allowed us to better analyse virus-specific effects that lead to dominance of a particular strain.
Dominance in this study was determined by the ability of a virus strain to out-compete another strain to achieve an exclusive or majority frequency in a sample following dual inoculation. The virus strain rMd5/pp38CVI was dominant during early and late cytolytic infection in most birds simultaneously challenged with JM/102W and rMd5/pp38CVI (Experiment 1A), as rMd5/pp38CVI was present in greater frequency than JM/102W in all bursa samples at 6 d.p.i. and in six of eight feather follicle DNA samples at 21 d.p.i. within this group . In birds simultaneously challenged with the similar pathotype virus pair, rMd5 was dominant compared with rMd5/pp38CVI in three of four bursa samples at 6 d.p.i. and in all seven feather follicle DNA samples at day 21 d.p.i. In either virus pair, dominance was not absolute and the subordinate virus was rarely if ever eliminated.
In tumours, dominance was less evident within the dissimilar pathotype dual-challenged group, although rMd5/pp38CVI was still present in a slightly higher average frequency compared with JM/102W (). In birds challenged with the similar pathotype virus pair, the frequency of rMd5 within tumours was significantly higher than rMd5/pp38CVI, similar to results from bursa and feather follicle DNA samples in Experiment 1B. Tumours, however, may be less appropriate for measuring virus fitness traits since they originate from rare individual transformation events. It was interesting that with the rMd5 and rMd5/pp38CVI virus pair, the frequency of viruses in tumours was very consistent between multiple tumours in individual birds, which was similar to the virus frequency by virus isolation and DNA isolated from bursa and feather follicle samples at earlier time points. On the other hand, the JM/102W and rMd5/pp38CVI virus pair led to some differences in the virus frequency between tumours in the same bird, suggesting multiple transforming events as seen in previous reports (Burgess & Davison, Citation2002; Silva et al., Citation2004; Cheng et al., Citation2006). As mentioned earlier, JM/102W and Md5 virus challenge are most commonly associated with gonad and heart tumours, respectively (R.L. Witter, unpublished data, 2011). In the JM/102W and rMd5/pp38CVI dual-challenged group, rMd5/pp38CVI was the dominant strain in three of four heart tumours and JM/102W was the dominant strain in two of five gonad tumours (). Interestingly, JM/102W was dominant in the gonad tumour and rMd5/pp38CVI was dominant in the heart tumour in Bird Q1834. This suggests that if both virus strains are present in adequate numbers during latency, then these strains may have the ability to individually target specific organs prior to transformation and tumour formation.
If dominance of a virus strain is related to virulence as defined by pathotype, we would expect rMd5/pp38CVI to be dominant compared with JM/102W. However, rMd5 and rMd5/pp38CVI are derived from the same vvMDV strain and should have similar virulence, as previously reported by comparison of gross lesions following challenge (Gimeno et al., Citation2005; Lee et al., Citation2005). The rMd5/pp38CVI recombinant strain was derived following an earlier study that constructed a recombinant CVI988 Rispens strain, CVI/rpp38, with the pp38 gene from MDV GA strain (Cui et al., Citation1999). This mutation delayed and significantly lowered the antibody response compared with inoculation with native CVI988 (Cui et al., Citation2004). The authors suspected the reverse mutation (placing the CVI988 pp38 gene into rMd5 [rMd5/pp38CVI]) may reduce pathogenicity of rMd5 as the CVI988 pp38 gene would probably increase antigenicity compared with native rMd5, leading to a stronger immune response against the virus. By combining rMd5 and rMd5/pp38CVI together in vivo, however, we may have been able to detect differences in virus fitness that were not able to be detected in single-challenged birds in previous experiments. In effect, this challenge model may allow us to amplify small differences in virus competitiveness between two similar virus strains. This theory could help explain the lower competitiveness of rMd5/pp38CVI compared with rMd5 in our experiments.
The presence of one virus strain within bursa and feather follicles apparently provided no exclusion from infection with the second virus as IHC confirmed the presence of both viruses within a few individual bursa follicles and most feather follicles. In feather follicles, a very high percentage of follicles stained for both viruses and many times the positive cells occupied the same proximity (). Given the fact that eukaryotic cell sizes range from 10 to 100 µm in linear diameter (Alberts et al., Citation1983) and our serial sections were 5 µm thick, this suggests that multiple viruses were able to simultaneously infect single cells. Successful dual staining of individual cells would provide more definitive evidence for this conclusion. In most cases, frequency of a particular virus within a chicken as evident by pyrosequencing results was consistent with proportions of each strain within individual bursa and feather follicles, detected by IHC (data not shown).
Replication potential and its relationship to virulence has been the topic of several recent publications. Following challenge with vMDV and vv+MDV strains in resistant and susceptible birds, the vMDV strain became latent at 6 d.p.i. but the vv+MDV strain never went into latency in either chicken line during 10 days (Yunis et al., Citation2004). The prolonged virus replication and presence of viral transcripts for the vv+MDV throughout 10 days could cause more severe damage and atrophy of lymphoid organs. Another study showed significantly higher levels of viral replication for the vv + MDV strain RK-1 in two chicken lines compared with the virulent strain JM-16 (Jarosinski et al., Citation2005). Calnek et al. (Citation1998) also demonstrated similar infection levels at 4 to 5 d.p.i. between three vv + MDV isolates (RK-1, 584A, 648A) and two vMDV isolates (JM16, GA5), but a prolonged phase of cytolytic infection at 7 to 8 d.p.i. as measured by immunofluorescence tests for the three vv+MDV isolates compared with the two vMDV isolates.
If virulence is partially defined by replication potential, this would give a clear advantage to whichever virus can replicate faster during co-infection. In Experiment 1A, JM/102W was present in very few bursa follicles at 6 days post challenge, but was present in a high percentage of feather follicles at 21 days. It would be a major advantage for rMd5/pp38CVI to replicate more efficiently in the bursa as our previous work demonstrated that the outcome of mixed infections is generally determined very early in infection (Dunn et al., Citation2010).
Virus-specific factors such as dominance may be an important factor that influences the outcome of MDV co-infection under field conditions. This effect may be especially important in the selection and propagation of more virulent strains within a poultry flock. Further work is needed to identify factors that influence virus-specific dominance during cytolytic, latent and transforming infections, and to elucidate the factors that favour emergence of one strain in chicken populations at the expense of other strains.
Acknowledgements
Thank you to all of the staff at the USDA Avian Disease and Oncology Laboratory who helped with sampling or in necropsy at any given time during the project, including Barry Coulson, Tom Goodwill, Noah Koller, Jody Mays, Lonnie Milam, Laurie Molitor, Melanie Flesberg, and Evelyn Young. Thank you to Matti Kiupel and Tom Wood at MSU DCPAH who graciously allowed us to use their equipment for automated immunohistochemistry staining.
References
- Alberts , B. , Bray , D. , Lewis , J. , Raff , M. , Roberts , K. and Watson , J.D. 1983 . Molecular Biology of the Cell , New York : Garland Publishing, Inc .
- Bacon , L.D. , Hunt , H.D. and Cheng , H.H. 2000 . A review of the development of chicken lines to resolve genes determining resistance to diseases . Poultry Science , 79 : 1082 – 1093 .
- Biggs , P.M. , Powell , D.G. , Churchill , A.E. and Chubb , R.C. 1972 . The epizootiology of Marek's disease: I. Incidence of antibody, viraemia and Marek's disease in six flocks . Avian Pathology , 1 : 5 – 25 .
- Burgess , S.C. and Davison , T.F. 2002 . Identification of the neoplastically transformed cells in Marek's disease herpesvirus-induced lymphomas: recognition by the monoclonal antibody AV37 . Journal of Virology , 76 : 7276 – 7292 .
- Calnek , B.W. 1980 . “ Marek's disease virus and lymphoma ” . In Oncogenic Herpesviruses , 1st edn , Edited by: Rapp , F. 103 – 143 . Boca Raton , FL : CRC Press .
- Calnek , B.W. , Harris , R.W. , Buscaglia , C. , Schat , K.A. and Lucio , B. 1998 . Relationship between the immunosuppressive potential and the pathotype of Marek's disease virus isolates . Avian Diseases , 42 : 124 – 132 .
- Calnek , B.W. , Schat , K.A. and Fabricant , J. 1980 . “ Modification of Marek's Disease Pathogenesis by In Ovo Infection or Prior Vaccination ” . In Viruses in Naturally Occurring Cancers , Edited by: Essex , M. , Todaro , G. and zur Hausen , H. 185 – 197 . Cold Spring Harbor Laboratory .
- Cheng , H.H. , Hunt , H. , & Delany , M. 2006 . Molecular Characterization of MD Tumors with Respect to Clonality, Loss of Heterozygosity, and MDV Integration . Paper presented at the 4th International Workshop on Molecular Pathogenesis of Marek's Disease Virus , Newark , DE .
- Cho , B.R. 1975 . Viremic responses of genetically susceptible and resistant chickens to experimental infection with acute, mild, or both strains of Marek's disease herpesvirus . Avian Diseases , 19 : 67 – 74 .
- Cho , B.R. 1977 . Dual virus maturation of both pathogenic and apathogenic Marek's disease herpesvirus (MDHV) in the feather follicles of dually infected chickens . Avian Diseases , 21 : 501 – 507 .
- Cho , B.R. and Kenzy , S.G. 1972 . Isolation and characterization of an isolate (HN) of Marek's disease virus with low pathogenicity . Applied Microbiology , 24 : 299 – 306 .
- Cho , B.R. and Kenzy , S.G. 1973 . Dual infection of chickens with acute and mild strains of Marek's disease herpesvirus . Avian Diseases , 17 : 390 – 395 .
- Churchill , A.E. , Payne , L.N. and Chubb , R.C. 1969 . Immunization against Marek's disease using a live attenuated virus . Nature , 221 : 744 – 747 .
- Cui , Z.-Z. , Lee , L.F. , Liu , J.L. and Kung , H.J. 1991 . Structural analysis and transcriptional mapping of the Marek's disease virus gene encoding pp38, an antigen associated with transformed cells . Journal of Virology , 65 : 6509 – 6515 .
- Cui , Z.Z. , Qin , A. , Lee , L.F. , Wu , P. and Kung , H.J. 1999 . Construction and characterization of a H19 epitope point mutant of Marek's disease virus CV1988/Rispens strain . Acta Virologica , 43 : 169 – 173 .
- Cui , Z.Z. , Zhang , Z. , Qin , A.J. and Lee , L.F. 2004 . Analyzing the H19- and T65-epitopes in 38 kd phosphorylated protein of Marek's disease viruses and comparing chicken immunological reactions to viruses point-mutated in the epitopes . Science in China. Series C, Life Sciences , 47 : 82 – 91 .
- Dunn , J.R. , Witter , R.L. , Silva , R.F. , Lee , L.F. , Finlay , J. , Marker , B.A. , Kaneene , J.B. , Fulton , R.M. , & Fitzgerald , S.D. , 2010 . The effect of the time interval between exposures on the susceptibility of chickens to superinfection with Marek's disease virus . Avian Diseases , 54 , 1038 – 1049 . doi: 10.1637/9348-033010-Reg.1
- Endoh , D. , Niikura , M. , Hirai , K. , Inagaki , N. , Hayashi , M. , Nakajima , K. and Sato , F. 1994 . Expression and purification of recombinant Marek's disease virus serotype 1 specific phosphorylated protein pp38 in E. coli . Journal of Veterinary Medical Science , 56 : 823 – 826 .
- Gimeno , I.M. , Witter , R.L. , Hunt , H.D. , Reddy , S.M. , Lee , L.F. and Silva , R.F. 2005 . The pp38 gene of Marek's disease virus (MDV) is necessary for cytolytic infection of B cells and maintenance of the transformed state but not for cytolytic infection of the feather follicle epithelium and horizontal spread of MDV . Journal of Virology , 79 : 4545 – 4549 .
- Jackson , C.A. , Biggs , P.M. , Bell , R.A. , Lancaster , F.M. and Milne , B.S. 1976 . The epizootiology of Marek's disease. 3. The interrelationship of virus pathogenicity, antibody and the incidence of Marek's disease . Avian Pathology , 5 : 105 – 123 .
- Jarosinski , K.W. , Njaa , B.L. , O'Connell , P.H. and Schat , K.A. 2005 . Pro-inflammatory responses in chicken spleen and brain tissues after infection with very virulent plus Marek's disease virus . Viral Immunology , 18 : 148 – 161 .
- Kenzy , S.G. 1967 . Excretion of the Marek's disease agent by infected chickens . The Veterinary Record , 80 : 565 – 568 .
- Lee , L.F. , Cui , X. , Cui , Z. , Gimeno , I. , Lupiani , B. and Reddy , S.M. 2005 . Characterization of a very virulent Marek's disease virus mutant expressing the pp38 protein from the Serotype 1 vaccine strain CVI988/Rispens . Virus Genes , 31 : 73 – 80 .
- Reddy , S.M. , Lupiani , B. , Gimeno , I.M. , Silva , R.F. , Lee , L.F. and Witter , R.L. 2002 . Rescue of a pathogenic Marek's disease virus with overlapping cosmid DNAs: use of a pp38 mutant to validate the technology for the study of gene function . Proceedings of the National Academy of Sciences, USA , 99 : 7054 – 7059 .
- Schat , K.A. and Calnek , B.W. 1978 . Characterization of an apparently nononcogenic Marek's disease virus . Journal of the National Cancer Institute , 60 : 1075 – 1082 .
- Sevoian , M. , Chamberlain , D.M. and Counter , F.T. 1962 . Avian lymphomatosis. I. Experimental reproduction of the neural and visceral forms . Veterinary Medicine , 57 : 500 – 501 .
- Silva , R.F. , Reddy , S.M. and Lupiani , B. 2004 . Expansion of a unique region in the Marek's disease virus genome occurs concomitantly with attenuation but is not sufficient to cause attenuation . Journal of Virology , 78 : 733 – 740 .
- Witter , R.L. 1971 . Epidemiology of Marek's disease—a review . In P.M. Biggs , G. de-The & L. N. Payne International Agency for Research on Cancer (pp. 111 – 122 ). Lyon .
- Witter , R.L. 1983 . Characteristics of Marek's disease viruses isolated from vaccinated commercial chicken flocks: association of viral pathotype with lymphoma frequency . Avian Diseases , 27 : 113 – 132 .
- Witter , R.L. 1997 . Increased virulence of Marek's disease virus field isolates . Avian Diseases , 41 : 149 – 163 .
- Witter , R.L. , Sharma , J.M. and Fadly , A.M. 1980 . Pathogenicity of variant Marek's disease virus isolates in vaccinated and unvaccinated chickens . Avian Diseases , 24 : 210 – 232 .
- Witter , R.L. , Sharma , J.M. and Offenbecker , L. 1976 . Turkey herpesvirus infection in chickens: induction of lymphoproliferative lesions and characterization of vaccinal immunity against Marek's disease . Avian Diseases , 20 : 676 – 692 .
- Witter , R.L. , Solomon , J.J. , Champion , L.R. and Nazerian , K. 1971 . Long-term studies of Marek's disease infection in individual chickens . Avian Diseases , 15 : 346 – 365 .
- Yunis , R. , Jarosinski , K.W. and Schat , K.A. 2004 . Association between rate of viral genome replication and virulence of Marek's disease herpesvirus strains . Virology , 328 : 142 – 150 .