Abstract
The present study was carried out to evaluate the protective effects of xylanase on the intestinal mucosal barrier in broiler chickens challenged with Clostridium perfringens in a 21-day experiment. A total of 336 1-day-old male broiler chicks (Ross 308) were assigned to four treatment groups. A 2×2 factorial arrangement of treatments was used in a randomized complete block design to study the effects of enzyme addition (with or without xylanase 5500 U/kg wheat-based diet), pathogen challenge (with or without C. perfringens challenge), and their interactions. Most C. perfringens-challenged birds had a congested mucosa and focal haemorrhagic lesions in the jejunum. Xylanase addition tended to reduce (P=0.09) the intestinal lesion score in the challenged birds. C. perfringens challenge resulted in decreased villus height/crypt depth ratio in the jejunum and ileum (P<0.05). Xylanase supplementation significantly increased this ratio in the jejunum (P<0.05) and also had the tendency to decrease crypt depth (P=0.065) and increase this ratio in the ileum (P=0.087). Xylanase addition significantly decreased the plasma endotoxin levels of the birds challenged with C. perfringens (P<0.05). Occludin mRNA expression in the jejunum and ileum was significantly decreased by C. perfringens challenge (P<0.05), but xylanase addition significantly increased its expression in the ileum. Xylanase supplementation also significantly increased MUC2 mRNA expression in the ileum (P<0.05). C. perfringens challenge resulted in a significant increase in apoptotic index in all three intestinal segments (P<0.05), but xylanase supplementation obviously decreased apoptotic index in the ileum (P<0.05). In conclusion, xylanase supplementation could alleviate the impairment of intestinal mucosal barrier induced by C. perfringens challenge.
Introduction
The intestinal mucosal barrier is composed primarily of epithelial cell membranes and a mucous gel layer covering the epithelium (Turner, Citation2006). Bacterial enteropathogens must traverse the mucous layer in order to approach and adhere to the mucosal epithelium cells (Gaudier et al., Citation2004). The epithelial cells form a continuous intact physical barrier with tight junctions (TJs) formed with each neighbouring cell. TJs are a major regulator of paracellular permeability, and their disruption results in increased permeability to luminal antigens and bacteria that promote release of pro-inflammatory cytokines (Ballard et al., Citation1995). The produced pro-inflammatory cytokines may induce apoptosis of intestinal epithelia, and alter the expression or cellular distribution of TJ proteins to further deteriorate mucosal barrier function (Clark & Coopersmith, Citation2007). An intact intestinal mucosal barrier is therefore of great importance for both preventing gut-related diseases and ensuring adequate provision of dietary nutrients to the whole body.
Necrotic enteritis (NE) is a common poultry disease (Engström et al., Citation2003), becoming increasingly prevalent with the removal of feed antibiotics (McDevitt et al., Citation2006). The disease causes severe necrosis of the intestinal tract, disrupts villus–crypt micro-architecture, and increases intestinal permeability and translocation of intestinal bacteria, thus compromising the integrity of mucosal barrier function (Collier et al., Citation2003, Citation2008; Liu et al., Citation2010; Golder et al., Citation2011) and lowering nutrient absorption and bird performance (Xu et al., Citation2003; Jia et al., Citation2009). The major factors for NE are the proliferation of the enteric bacterium Clostridium perfringens type A or C and production of exotoxins (Keyburn et al., Citation2008). However, many cofactors are usually required to promote overgrowth of C. perfringens in the intestinal tract and precipitate an outbreak of NE, including diet composition such as great amounts of wheat, barley, or rye (Kaldhusdal & Hofshagen, Citation1992; Riddell & Kong, Citation1992; Langhout, Citation1998). Numerous studies have shown that diets with high amounts of wheat and barley increase the intestinal content viscosity caused by water-soluble cell wall or non-starch polysaccharides, thereby resulting in a greater incidence of NE than corn-based diets (Kaldhusdal & Hofshagen, Citation1992; Kaldhusdal & Skjerve, Citation1996; Engberg et al., Citation2004; Jia et al., Citation2009). The mechanism underlying this effect has been suggested to be that high intestinal viscosity reduces nutrient absorption by the host bird, decreases the rate of feed passage (Choct & Annison, Citation1992; Almirall & Esteve-Garcia, Citation1994), and may enhance mucus production (Langhout et al., Citation1999; Piel et al., Citation2005; Ito et al., Citation2009), which could lead to increased numbers of anaerobic bacteria in the intestine, particularly C. perfringens (Hübener et al., Citation2002; Kocher, Citation2003; Engberg et al., Citation2004).
Exogenic enzymes such as xylanase added to wheat-based feed can reduce digesta viscosity, increase the digesta passage rate, and improve nutrient digestion and absorption, thereby reducing the bacterial population in the small intestine (Bedford & Apajalahti, Citation2001; Hübener et al., Citation2002). Moreover, the non-starch polysaccharide hydrolysis products may serve as prebiotics (Monsan & Paul, Citation1995; Bedford, Citation2000) and indirectly prohibit the growth of certain pathogenic species including C. perfringens (Gibson & Roberfroid, Citation1995; Engberg et al., Citation2004). In addition, xylanase supplementation in wheat-based diets was found to change the profile of mucin types in the small intestine of chicks (Sharma et al., Citation1997), which suggested that manipulating the mucin profile by enzyme addition in chicken diets may protect the intestinal mucosa from NE.
However, reports on the effects of enzyme supplementation on the growth of C. perfringens and the incidence of NE are scarce. Likewise, until now, no information has been available as to whether and in which way xylanase addition could alleviate the impairment of intestinal mucosal barrier for chickens with NE. We therefore conducted this study to explore the protective effects of xylanase on the intestinal mucosal barrier in C. perfringens-challenged broiler chickens. More specifically, effects on intestinal lesions, endotoxin translocation, epithelial cell apoptosis, mucin dynamics, and gene expression of TJ proteins (occludin and claudin-1) were assessed.
Materials and Methods
Birds, diets and experimental design
This study was approved by the China Agricultural University Animal Care and Use Committee. A total of 336 1-day-old male broiler chicks (Ross 308) were assigned to four treatment groups consisting of six replicates with two cages per replicate and seven chicks per cage. A 2×2 factorial arrangement of treatments was used in a randomized complete block design to study the effects of enzyme addition (with or without xylanase 5500 U/kg diet), pathogen challenge (with or without C. perfringens challenge), and their interactions. The whole feeding period lasted 21 days. The chickens had free access to feed and water and were housed in wire cages and maintained on a 23-h lighting programme. Antibiotic-free and coccidiostat-free wheat-based meal diets were formulated to meet or exceed National Research Council (1994) requirements. The enzyme xylanase was provided by Asiapac (Dongguan) Biotechnology Company (Guangdong, China). Composition of the diet and nutrient levels are presented in .
Table 1. Composition of the diet and nutrient levels.
C. perfringens challenge
C. perfringens challenge was based on the model originally developed by Dahiya et al. (Citation2005), with some modifications. Briefly, a chicken C. perfringens type A field strain isolated from a clinical case of NE was obtained from the China Veterinary Culture Collection Center (Beijing, China). The organism was cultured anaerobically on tryptose–sulphite–cycloserine for 18 h at 37°C, and then aseptically inoculated into cooked meat medium and incubated anaerobically overnight at 37°C. On days 14 to 20, all birds (except the unchallenged birds) were orally gavaged once per day with this actively growing culture of C. perfringens type A (7.0×107 colony-forming units/ml, 1.0 ml/bird).
Sample collection
On day 21, two birds per replicate (12 birds/treatment) were randomly selected and blood was collected aseptically from the wing vein into heparinized vacutainers, and then plasma was obtained and stored at −20°C for plasma endotoxin assay. The birds were killed by intracardial administration of sodium pentobarbital (30 mg/kg body weight) and jugular exsanguination on the morning of day 21. The small intestine was then removed and gently flushed with ice-cold saline. Samples (~3 cm) were taken from the midpoint of the duodenum, from the midpoint between the point of entry of the bile duct and Meckel's diverticulum (jejunum), and from midway between Meckel's diverticulum and the ileocaeca junction (ileum). Samples for mRNA determination were frozen in liquid nitrogen. Twelve birds per treatment were examined for intestinal lesions and histomorphological parameters. Six birds per treatment were examined for all other outcome variables.
Intestinal histomorphology and goblet cell histochemistry
All small intestinal segments were immediately fixed in 4% paraformaldehyde and then embedded in paraffin. Consecutive sections (5 µm) were stained with haematoxylin and eosin and were observed for histomorphology examination. The villus height and crypt depth were measured from 15 randomly selected villi and associated crypts on one section per chicken at 40×magnification. The villus height/crypt depth ratio was then calculated from these measurements. Differential staining techniques were used to distinguish between mucin types. Sections were stained with periodic acid–Schiff reaction for neutral mucin, with Alcian blue 8 GX at pH 2.5 for acid mucin, and with high iron–diamine reaction without prior oxidation for sulfomucin (with a slight modification: 40% instead of 10% FeCl3). Mucin-containing goblet cells along the villi were counted and the associated villus surface area was measured in 10 full-length villi. The density of goblet cells was calculated as the number of goblet cells per unit surface area (mm2). All examinations and measurements were performed with an Olympus optical microscope using ProgRes CapturePro software (version 2.7; Jenoptik, Jena, Germany).
Intestinal lesion score
The small intestine from each bird was opened and subjected to scoring for NE lesions on a scale from zero to four as described by Liu et al. (Citation2010): 0 = normal intestinal appearance; 0.5 = severely congested serosa and mesentery engorged with blood; 1 = thin-walled and friable intestines with small red petechiae; 2 = focal necrosis, grey appearance and small amounts of gas production; 3 = sizable patches of necrosis, gas-filled intestine and small flecks of blood; and 4 = severe extensive necrosis, marked haemorrhage, and large amounts of gas in the intestine.
Intestinal epithelial cell apoptosis
Sections of intestinal tissues fixed in 4% paraformaldehyde were stained with the terminal deoxynucleotidyl transferase-mediated dUTP nick-end labelling technique for quantification of apoptosis in situ. The fluorometric apoptosis detection kit (DeadEnd Fluorometric TUNEL System; Promega, Madison, Wisconsin, USA) was used and the staining procedure was performed according to the manufacturer's protocol. Apoptotic and total cells were quantified at 400× magnification using images of 10 separate fields. The apoptotic index was defined as the ratio of apoptotic cells, expressed as apoptotic bodies, to the total cell number within the crypt–villus axis per section.
Plasma endotoxin assay
The endotoxin level in the plasma samples was measured using a quantitative chromogenic substrate assay kit (Xiamen TAL Experimental Plant Co., Ltd, Fujian, China). Briefly, 0.1 ml plasma was incubated with 0.1 ml Limulus amebocyte lysate at 37°C for 45 min. After several subsequent reactions, the samples were read spectrophotometrically at 545 nm. The plasma endotoxin levels were calculated against a standard curve of endotoxin (Escherichia coli) concentrations.
RNA isolation and quantitative real-time polymerase chain reaction
Total RNA was isolated using Trizol reagent (Invitrogen Life Technologies, Carlsbad, California, USA) according to the manufacturer's instructions. The concentration and purity of total RNA was estimated by measuring its optical density at 260 and 280 nm. One microgram of total RNA was reverse transcribed by a reverse transcription kit (Invitrogen Life Technologies) according to the manufacturer's instructions. All of the cDNA preparations were stored frozen at −30°C until further use. A quantitative real-time polymerase chain reaction (PCR) assay was performed with the 7500 fluorescence detection system (Applied Biosystems, Foster City, California, USA) according to optimized PCR protocols using the SYBR-Green PCR kit (Applied Biosystems). The primer pairs for the amplification of claudin-1, occludin, mucin-2 and β-actin cDNA fragments, used as an endogenous reference gene, were used as listed in . The following PCR conditions were employed: an initial denaturation step at 95°C for 10 min, 40 cycles at 95°C for 30 sec, the annealing and extension temperature at 60°C for 1 min, and a final extension step of 72°C for 10 min. To confirm amplification specificity, the PCR products from each primer pair were subjected to a melting curve analysis and subsequent agarose gel electrophoresis. Gene expression was quantified using the comparative threshold cycle method (Heid et al., Citation1996) and the data were expressed as the relative value to the unchallenged group.
Table 2. Primers used for quantitative real-time PCR.
Statistical analysis
Statistical analysis of data was conducted with SPSS Version 12.0 (SPSS Inc, Chicago, Illinois, USA). Results are expressed as the mean±pooled standard error of the mean. For the intestinal lesion score and plasma endotoxin levels, an independent-samples t test was used between the two challenged groups. The other data were analysed by two-factorial analysis of variance. Individual treatment means were compared using Duncan's multiple comparison when significant interaction between the main effects was observed. P<0.05 was considered statistically significant.
Results
Intestinal lesion score
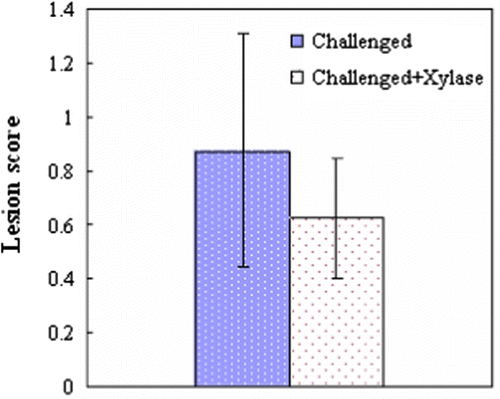
In the present study, no intestinal lesions were observed in the unchallenged birds. Most C. perfringens-challenged birds had a congested mucosa and focal haemorrhagic lesions in the jejunum. However, typical field-type lesions specific to NE (intestinal lesion score >2) were not found in any of the birds. The average lesion score tended to be lower (P=0.09) in the challenged birds fed xylanase-supplemented diets than in the challenged birds ().
Intestinal morphology
Neither C. perfringens challenge nor xylanase addition affected the villus height and crypt depth in the duodenum, jejunum or ileum (P>0.05). C. perfringens challenge did result in a decreased villus height/crypt depth ratio in the jejunum and ileum (P<0.05), intestinal epithelial cell apoptosis and xylanase supplementation significantly increased the villus height/crypt depth ratio in the jejunum (P<0.05). Xylanase supplementation also had the tendency to decrease the crypt depth (P=0.065) and increase the villus height/crypt depth ratio in the ileum (P=0.087) ().
Table 3. Histomorphological parameters of the small intestine from 21-day-old broilers, 1 day post C. perfringens challenge.a
Plasma endotoxin levels
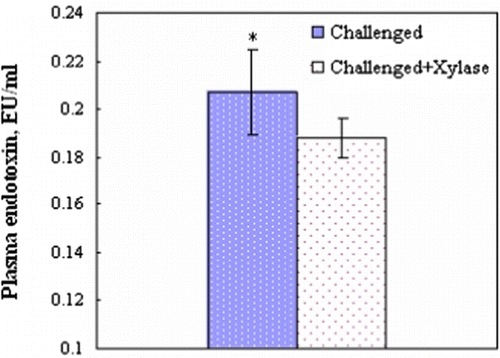
Endotoxin was not detectable (<0.01 Endotoxin unit/ml) in the plasma in any of the unchallenged birds by the Limulus amebocyte lysate test. Addition of xylanase in wheat-based diets significantly decreased the plasma endotoxin levels of the birds challenged with C. perfringens (P<0.05) ().
Claudin-1, occludin and MUC2 mRNA expression
The relative mRNA expression of claudin-1, occludin and MUC2 in the duodenum was not affected by C. perfringens challenge, xylanase supplementation or their interaction (P>0.05; data not shown). Occludin mRNA expression in the jejunum and ileum was significantly decreased by C. perfringens challenge (P<0.05), and xylanase addition resulted in a significant increase in occludin mRNA expression in the ileum (P<0.05) (). The effect of xylanase addition on claudin-1 mRNA expression in the ileum was significant and interacted with C. perfringens challenge (P<0.05). The birds in the unchallenged group had the lowest claudin-1 mRNA expression, and xylanase supplementation significantly increased (P<0.05) its expression in the unchallenged birds, but did not affect its expression in the challenged birds (P>0.05). MUC2 mRNA expression in the three intestinal segments was not affected by the pathogen challenge (P>0.05), and xylanase supplementation significantly decreased them in the ileum (P<0.05).
Table 4. Relative mRNA expressiona of claudin-1, occludin and MUC2 at 21 days of age, 1 day post C. perfringens challenge
Apoptosis
C. perfringens challenge resulted in a significant increase in the number of apoptotic bodies in all three segments of the small intestine (P<0.05) (). In the jejunum, xylanase supplementation did not affect the apoptotic index in the unchallenged birds (P>0.05) but obviously decreased (P<0.05) that in the C. perfringens-challenged birds, which resulted in a significant interaction between xylanase treatment and C. perfringens challenge (P<0.05). Xylanase supplementation significantly decreased the apoptotic index in the ileum (P<0.05).
Table 5. Apoptosis index in the small intestine of broiler chickens at 21 days of age, 1 day post C. perfringens challenge.a
Histochemistry for mucin subtypes
The number of neutral mucin-containing goblet cells in the three intestinal segments was not affected by C. perfringens challenge, xylanase supplementation or their interaction (P>0.05; data not shown). In the duodenum, xylanase addition resulted in a decrease in sulfomucin goblet cell counts in the unchallenged birds (P<0.05; ), whereas no difference was found among the C. perfringens-challenged birds (P>0.05), which resulted in a significant interaction between enzyme addition and C. perfringens challenge on the number of sulfomucin goblet cells (P<0.05). There was a similar interaction tendency on the number of acidomucin goblet cells in the duodenum (P=0.091). In the ileum, both C. perfringens challenge and xylanase supplementation significantly reduced acidomucin (P<0.05) and sulfomucin goblet cell counts (P<0.05).
Table 6. Numbers of goblet cells with neutral mucin, acidomucin, and sulfomucin of broiler chickens at 21 days of age, 1 day post C. perfringens challenge.a
Discussion
In the present trial, C. perfringens challenge did not result in characteristic intestinal NE lesions, but did induce histomorphological and functional changes in the intestine. C. perfringens challenge decreased the villus height/crypt depth ratio, an index of mucosal atrophy, in the jejunum and ileum. Intestinal lesions and mucosal atrophy can compromise eptithelial permeability and mucosal barrier function (Sun et al., Citation2006). In support of this view, it was observed that the plasma endotoxin levels were increased in the challenged birds. Although endotoxin is produced by Gram-negative bacteria and C. perfringens is Gram-positive, C. perfringens infection could cause the overgrowth of many Gram-negative bacteria, particularly E. coli, in the ileum (McReynolds et al., Citation2004; Liu et al., Citation2010) and bacteria translocation to the liver, spleen and blood (Collier et al., Citation2008; Liu et al., Citation2010), thereby leading to endotoxin translocation into the blood circulation.
Xylanase supplementation in wheat-based diets is known to enhance dietary nutrient utilization by the chickens, which may reduce microbial activity as a result of substrate limitation in the ileum (Choct et al., Citation1999). Our study showed that xylanase addition increased the apparent ileal digestibility of protein and gross energy of birds (unpublished data). Bedford & Apajalahti (Citation2001) demonstrated in birds fed wheat-based diets that addition of a xylanase-based enzyme preparation resulted in a 60% reduction in bacterial numbers, and research by Hübener et al. (Citation2002) supported this finding. Moreover, in the process of depolymerizing arabinoxylans, the major non-starch polysaccharide fraction in wheat, xylanases may produce xylose and xylo-oligomers (Christov et al., Citation1997; Valenzuela et al., Citation2010). These enzyme hydrolysis products are similar to prebiotics and may facilitate the proliferation of specific beneficial bacteria such as Bifidobacterium and Lactobacillus (Monsan & Paul, Citation1995; Bedford, Citation2000) to indirectly suppress proliferation of certain pathogenic species including C. perfringens (Gibson & Roberfroid, Citation1995), but no direct evidence about this was available. Regarding the effects of xylanase supplementation on NE incidence, few reports in the literature are found. Choct et al. (Citation2006) demonstrated that xylanase supplementation reduced C. perfringens numbers in the caeca of healthy broiler chickens fed wheat-based diets. Jia et al. (Citation2009) showed that enzyme supplementation did not affect the growth of C. perfringens, but it ameliorated the retarded growth by disease challenge through improving the feed conversion ratio in birds consuming the wheat-based diets. In this study, no C. perfringens enumeration was performed, but improved body weight and feed conversion ratio (unpublished data), reduced intestinal lesion scores and plasma endotoxin levels of birds supplemented with xylanase indicated that xylanase greatly attenuated the negative effects of C. perfringens challenge and improved the intestinal barrier function and growth performance of birds challenged with C. perfringens.
TJs create a paracellular permeability barrier and act as a fence preventing macromolecular transmission (Ballard et al., Citation1995). This barrier is comprised of several unique proteins including occludin, junctional adhesion molecule (JAM), claudins, and zonula occludens (ZO) (Schneeberger & Lynch, Citation2004). Occludin and claudin-1 are two of the most important components in the regulation of epithelial barrier function in the intestine (Fanning et al., Citation1998). However, the roles of TJ proteins for epithelial barrier function during C. perfringens challenge are not so far clear. The present work indicated that C. perfringens challenge decreased occludin mRNA expression in the jejunum and ileum, but had no impacts on claudin-1 mRNA expression in all intestinal segments. Xylanase addition up-regulated occludin mRNA expression in the ileum, irrespective of C. perfringens challenge, and claudin-1 mRNA expression only in the ileum of the unchallenged birds. These results suggested that regulation of occludin expression by xylanase may be involved in ameliorating increased intestinal permeability induced by C. perfringens challenge.
Apoptosis is a regulated process of cell death, and single epithelial cell apoptosis and extrusion without loss of barrier function are normal physiological events in the gastrointestinal tract (Madara, Citation1990; Abreu et al., Citation2000), but an increased epithelial apoptotic ratio might be another cause of relevant leaks in the epithelial barrier (Gitter et al, Citation2000). The present study demonstrated that epithelial apoptosis in all intestinal segments was considerably up-regulated in the challenged birds compared with the unchallenged birds, which suggested that C. perfringens challenge may disrupt epithelial barrier function by apoptosis-dependent mechanisms. The increased epithelial apoptosis might result from local inflammatory response in the small intestine caused by C. perfringens infection (Zhou et al., Citation2009). Previous studies showed that cytokine and chemokine responses appeared and pro-inflammatory cytokines such as tumour necrosis factor-α, interferon-γ and IL-1β expression were up-regulated in the intestinal tissues during C. perfringens challenge (Collier et al., Citation2008; Park et al., Citation2008; Zhou et al., Citation2009) . Epithelial cell apoptosis, rather than necrosis, may limit the host inflammatory response early after infection. On the other hand, deletion of infected epithelial cells by apoptosis may benefit the birds, since it allows maintenance of epithelial barrier integrity. However, Zhou et al. (Citation2009) reported that several key genes involved in the apoptosis pathway were down-regulated in chicken spleen after C. perfringens infection, which might indicate an inhibition of apoptosis process in chicken spleen at the early stage of C. perfringens infection. It can easily be explained that spleen was a secondary lymph organ for systemic immune response and the inhibition of apoptosis in the spleen might be needed for inducing and maintaining immune activity of immune cells. In this study, xylanase addition decreased the epithelial apoptosis index in the jejunum and ileum, implying that suppression of epithelial apoptosis by xylanase may contribute to alleviating the impairment of mucosal barrier function induced by C. perfringens challenge.
In this study, pronounced intestinal lesion and increased plasma endotoxin levels indicated that the mucous gel layer had been destroyed by C. perfringens challenge. The mucous layer consists mainly of mucin glycoproteins, which are synthesized and secreted by epithelial goblet cells. Goblet cells can be separated histologically into neutral or acidic mucin-producing types, and the latter can be divided into sulphated or sialylated mucin-producing subtype (Kiernan, Citation1990). There is evidence that acidomucins (primarily sulfomucins) restrict mucolysis because of their resistance to degradation by bacterial glycosidases and host proteases (Robertson & Wright, Citation1997). Therefore, it was hypothesized that the number of total acidic mucin-containing goblet cells may have been elevated in the C. perfringens-challenged chickens because of protective properties against pathogens of acidomucin (Conour et al., Citation2002), and high bacterial populations have been associated with increased secretion of sulphomucins (Robertson & Wright, Citation1997). However, C. perfringens was reported to possess 13 hydrolase families predicted to be involved in mucin glycoprotein breakdown (Ficko-Blean & Boraston, Citation2006) and had significant acidomucolytic potential and grew rapidly on mucin-containing medium (Deplancke et al., Citation2002), which suggested that C. perfringens may be particularly mucolytic and its growth would be favoured by the increased host mucous production (Collier et al., Citation2003, Citation2008). Our current study indicated C. perfringens challenge reduced acidomucin and sulfomucin goblet cell density in the ileum villi, which is not in accordance with other studies. Collier et al. (Citation2008) reported that neither C. perfringens infection nor Eimeria spp./C. perfringens co-infection influenced the number of sialomucin or sulfomucin goblet cells in villi or crypts of the ileum, but both of them elevated the number of total acidomucin goblet cells in crypt. Moreover, a recent study by Golder et al. (Citation2011) showed that Eimeria spp./C. perfringens co-infection did not appear to affect intestinal mucin profile. The discrepancy may be explained by differences in diet composition, challenge model systems, analysed units (villi, crypts or merged) and virulence factors.
In this study, xylanase supplementation in wheat-based diets reduced acidomucin, primarily sulfomucin, goblet cell density in the ileal villi, irrespective of C. perfringens challenge, but in the duodenum only in unchallenged birds. The precise mechanisms underlying the effects are not well understood, but appear to be related to the fact that xylanase addition can reduce intestinal content viscosity, which could enhance mucous production. Piel et al. (Citation2005) showed that ingestion of highly viscous carboxymethylcellulose increased the number of ileal acidomucin and sulfomucin goblet cells and luminal crude mucin in pigs. This is consistent with the results of Ito et al. (Citation2009), who found that viscous fibres up-regulated baseline secretion of small intestinal mucins by increasing the number of goblet cells in proportion to their own viscosities. The explanation responsible for the increase in goblet cells after ingestion of viscous water-soluble fibre was hypothesized to be associated with increased intestinal intraluminal pressure by viscous fibres (Ito et al., Citation2009). These observations suggested that intestinal content viscosity might be a contributing factor in small intestinal mucin secretion, thereby leading to increased numbers of C. perfringens. We therefore speculate that decreased acidomucin and sulfomucin goblet cell density by xylanase supplementation is due to considerable reduction in digesta viscosities and may serve as a mechanism for controlling the growth of C. perfringens and protecting birds against C. perfringens. However, the different responses in the three intestinal segments are not explained well, and may result from the difference in the density and protective properties of mucin subtypes, xylanase activity and the extent of mucosal lesions in these intestinal segments.
Mucin production is affected by both numbers of terminally differentiated goblet cells and mucin gene expression within these cells (Theodoropoulos & Carraway, Citation2007). In the small intestine, the major mucin gene is MUC2, which codes for the main secreted mucin and is confined to goblet cells (Van Klinken et al., Citation1995). In the present study, C. perfringens challenge did not affect the expression of MUC2 in any intestinal segments, and xylanase addition down-regulated its expression in the ileum, suggesting that mucin synthesis might be inhibited by xylanase but not by C. perfringens challenge, and the mucin production in the ileum might be reduced by xylanase addition due to a conjunct decrease of acidomucin goblet cells and mucin gene expression. Further investigation into mucin production and mucous layer thickness is needed.
In conclusion, xylanase supplementation of a wheat-based diet offered to C. perfringens-challenged broilers reduced intestinal permeability and epithelial apoptosis, altered the mucin profile and increased occludin mRNA expression. The protective action of xylanase on mucosal barrier might therefore, at least partly, be associated with these mechanisms of epithelial apoptosis, TJ expression and mucin dynamics in chickens with C. perfringens challenge.
Acknowledgements
This study was supported by the earmarked fund for China Agricultural Research Systems, and the China postdoctoral foundation (No. 20110490480). Dan Liu and Shuangshuang Guo contributed equally to this work.
References
- Abreu , M.T. , Palladino , A.A. , Arnold , E.T. , Kwon , R.S. and McRoberts , J.A. 2000 . Modulation of barrier function during Fas-mediated apoptosis in human intestinal epithelial cells . Gastroenterology , 119 : 1524 – 1536 .
- Almirall , M. and Esteve-Garcia , E. 1994 . Rate of passage of barley diets with chromium oxide: influence of age and poultry strain and effect of beta-glucanase supplementation . Poultry Science , 73 : 1433 – 1440 .
- Ballard , S.T. , Hunter , J.H. and Taylor , A.E. 1995 . Regulation of tight-junction permeability during nutrient absorption across the intestinal epithelium . Annual Review of Nutrition , 15 : 35 – 55 .
- Bedford , M.R. 2000 . Exogenous enzymes in monogastric nutrition-Their current value and future benefits . Animal Feed Science and Technology , 86 : 1 – 13 .
- Bedford , M.R. and Apajalahti , J. 2001 . “ Microbial interactions in the response to exogenous enzyme utilization ” . In Enzymes in Farm Animal Nutrition , Edited by: Patridge , M.R.B.A. 299 – 314 . Oxon : CABI Publishing .
- Choct , M. , Hughes , R.J. and Bedford , M.R. 1999 . Effects of a xylanase on individual bird variation, starch digestion throughout the intestine, and ileal and caecal volatile fatty acid production in chickens fed wheat . British Poultry Science , 40 : 419 – 422 .
- Choct , M. , Sinlae , M. , Al-Jassim , R.A.M. and Pettersson , D. 2006 . Effects of xylanase supplementation on between-bird variation in energy metabolism and the number of Clostridium perfringens in broilers fed a wheat-based diet . Australian Journal of Agricultural Research , 57 : 1017 – 1021 .
- Choct , M. and Annison , G. 1992 . The inhibition of nutrient digestion by wheat pentosans . British Journal of Nutrition , 67 : 123 – 132 .
- Christov , L.P. , Myburgh , J. , van Tonder , A. and Prior , B.A. 1997 . Hydrolysis of extracted and fibre-bound xylan with Aureobasidium pullulans enzymes . Journal of Biotechnology , 55 : 21 – 29 .
- Clark , J.A. and Coopersmith , C.M. 2007 . Intestinal crosstalk: a new paradigm for understanding the gut as the “motor” of critical illness . Shock , 28 : 384 – 393 .
- Collier , C.T. , Hofacre , C.L. , Payne , A.M. , Anderson , D.B. , Kaiser , P. , Mackie , R.I. and Gaskins , H.R. 2008 . Coccidia-induced mucogenesis promotes the onset of necrotic enteritis by supporting Clostridium perfringens growth . Veterinary Immunology and Immunopathology , 122 : 104 – 115 .
- Collier , C.T. , van der Klis , J.D. , Deplancke , B. , Anderson , D.B. and Gaskins , H.R. 2003 . Effects of tylosin on bacterial mucolysis, Clostridium perfringens colonization, and intestinal barrier function in a chick model of necrotic enteritis . Antimicrobial Agents and Chemotherapy , 47 : 3311 – 3317 .
- Conour , J.E. , Ganessunker , D. , Tappenden , K.A. , Donovan , S.M. and Gaskins , H.R. 2002 . Acidomucin goblet cell expansion induced by parenteral nutrition in the small intestine of piglets . American Journal of Physiology-Gastrointestinal and Liver Physiology , 283 : 1185 – 1196 .
- Dahiya , J.P. , Hoehler , D. , Wilkie , D.C. , Van Kessel , A.G. and Drew , M.D. 2005 . Dietary glycine concentration affects intestinal Clostridium perfringens and lactobacilli populations in broiler chickens . Poultry Science , 84 : 1875 – 1885 .
- Deplancke , B. , Vidal , O. , Ganessunker , D. , Donovan , S.M. , Mackie , R.I. and Gaskins , H.R. 2002 . Selective growth of mucolytic bacteria including Clostridium perfringens in a neonatal piglet model of total parenteral nutrition . American Journal of Clinical Nutrition , 76 : 1117 – 1125 .
- Engberg , R.M. , Hedemann , M.S. , Steenfeldt , S. and Jensen , B.B. 2004 . Influence of whole wheat and xylanase on broiler performance and microbial composition and activity in the digestive tract . Poultry Science , 83 : 925 – 938 .
- Engström , B.E. , Fermer , C. , Lindberg , A. , Saarinen , E. , Båverud , V. and Gunnarsson , A. 2003 . Molecular typing of isolates of Clostridium perfringens from healthy and diseased poultry . Veterinary Microbiology , 94 : 225 – 235 .
- Fanning , A.S. , Jameson , B.J. , Jesaitis , L.A. and Anderson , J.M. 1998 . The tight junction protein ZO-1 establishes a link between the transmembrane protein occludin and the actin cytoskeleton . Journal of Biological Chemistry , 273 : 29745 – 29753 .
- Ficko-Blean , E. and Boraston , A.B. 2006 . The interaction of a carbohydrate-binding module from a Clostridium perfringens N-acetyl-beta-hexosaminidase with its carbohydrate receptor . Journal of Biological Chemistry , 281 : 37748 – 37757 .
- Gaudier , E. , Jarry , A. , Blottiere , H.M. , de Coppet , P. , Buisine , M.P. , Aubert , J.P. , Cherbut , C. and Hoebler , C. 2004 . Butyrate specifically modulates MUC gene expression in intestinal epithelial goblet cells deprived of glucose . American Journal of Physiology-Gastrointestinal and Liver Physiology , 287 : G1168 – G1174 .
- Gibson , G.R. and Roberfroid , M.B. 1995 . Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics . Journal of Nutrition , 125 : 1401 – 1412 .
- Gitter , A.H. , Bendfeldt , K. , Schulzke , J.D. and Fromm , M. 2000 . Leaks in the epithelial barrier caused by spontaneous and TNF-alpha-induced single-cell apoptosis . The FASEB Journal , 14 : 1749 – 1753 .
- Golder , H.M. , Geier , M.S. , Forder , R.E. , Hynd , P.I. and Hughes , R.J. 2011 . Effects of necrotic enteritis challenge on intestinal micro-architecture and mucin profile . British Poultry Science , 52 : 500 – 506 .
- Heid , C.A. , Stevens , J. , Livak , K.J. and Williams , P.M. 1996 . Real time quantitative PCR . Genome Research , 6 : 986 – 994 .
- Hübener , K. , Vahjen , W. and Simon , O. 2002 . Bacterial responses to different dietary cereal types and xylanase supplementation in the intestine of broiler chicken . Archives of Animal Nutrition , 56 : 167 – 187 .
- Ito , H. , Satsukawa , M. , Arai , E. , Sugiyama , K. , Sonoyama , K. , Kiriyama , S. and Morita , T. 2009 . Soluble fiber viscosity affects both goblet cell number and small intestine mucin secretion in rats . Journal of Nutrition , 139 : 1640 – 1647 .
- Jia , W. , Slominski , B.A. , Bruce , H.L. , Blank , G. , Crow , G. and Jones , O. 2009 . Effects of diet type and enzyme addition on growth performance and gut health of broiler chickens during subclinical Clostridium perfringens challenge . Poultry Science , 88 : 132 – 140 .
- Kaldhusdal , M. and Hofshagen , M. 1992 . Barley inclusion and avoparcin supplementation in broiler diets. 2. Clinical, pathological, and bacteriological findings in a mild form of necrotic enteritis . Poultry Science , 71 : 1145 – 1153 .
- Kaldhusdal , M. and Skjerve , E. 1996 . Association between cereal contents in the diet and incidence of necrotic enteritis in broiler chickens in Norway . Preventive Veterinary Medicine , 28 : 1 – 16 .
- Keyburn , A.L. , Boyce , J.D. , Vaz , P. , Bannam , T.L. , Ford , M.E. , Parker , D. , Di Rubbo , A. , Rood , J.I. and Moore , D. 2008 . NetB, a new toxin that is associated with avian necrotic enteritis caused by Clostridium perfringens . PLoS Pathogens , 4 : e26
- Kiernan , J.A. 1990 . Carbohydrate histochemisrty . In Histological and Histochemical Methods: Theory and Practice , 2nd edn 170 – 197 Oxford : Pergamon Press .
- Kocher , A. 2003 . Nutritional manipulation of necrotic enteritis outbreak in broilers . Recent Advances in Animal Nutrition , 14 : 111 – 116 .
- Langhout , D.J. 1998 . The role of the intestinal flora as affected by non-starch polysaccharides in broiler chicks. PhD . Agricultural University of Wageningen , The Netherlands .
- Langhout , D.J. , Schutte , J.B. , Van Leeuwen , P. , Wiebenga , J. and Tamminga , S. 1999 . Effect of dietary high- and low-methylated citrus pectin on the activity of the ileal microflora and morphology of the small intestinal wall of broiler chicks . British Poultry Science , 40 : 340 – 347 .
- Liu , D. , Guo , Y. , Wang , Z. and Yuan , J. 2010 . Exogenous lysozyme influences Clostridium perfringens colonization and intestinal barrier function in broiler chickens . Avian Pathology , 39 : 17 – 24 .
- Madara , J.L. 1990 . Maintenance of the macromolecular barrier at cell extrusion sites in intestinal epithelium: physiological rearrangement of tight junctions . Journal of Membrane Biology , 116 : 177 – 184 .
- McDevitt , R.M. , Brooker , J.D. , Acamovic , T. and Sparks , N.H.C. 2006 . Necrotic enteritis; a continuing challenge for the poultry industry . World's Poultry Science Journal , 62 : 221 – 247 .
- McReynolds , J.L. , Byrd , J.A. , Anderson , R.C. , Moore , R.W. , Edrington , T.S. Genovese , K.J. 2004 . Evaluation of immunosuppressants and dietary mechanisms in an experimental disease model for necrotic enteritis . Poultry Science , 83 : 1948 – 1952 .
- Monsan , P.F. and Paul , F. 1995 . “ Oligosaccharide feed additive ” . In Biotechnology in Animal Feeds and Feeding , Edited by: Wallace , R.J. and Chesson , A. 233 – 245 . Weinheim : VCH Verlagsgesellschaft .
- Park , S.S. , Lillehoj , H.S. , Allen , P.C. , Park , D.W. , FitzCoy , S. , Bautista , D.A. and Lillehoj , E.P. 2008 . Immunopathology and cytokine responses in broiler chickens coinfected with Eimeria maxima and Clostridium perfringens with the use of an animal model of necrotic enteritis . Avian Diseases , 52 : 14 – 22 .
- Piel , C. , Montagne , L. , Sève , B. and Lallès , J.P. 2005 . Increasing digesta viscosity using carboxymethylcellulose in weaned piglets stimulates ileal goblet cell numbers and maturation . Journal of Nutrition , 135 : 86 – 91 .
- Riddell , C. and Kong , X.M. 1992 . The influence of diet on necrotic enteritis in broiler chickens . Avian Diseases , 36 : 499 – 503 .
- Robertson , A.M. and Wright , D.P. 1997 . Bacterial glycosulphatases and sulphomucin degradation . Canadian Journal of Gastroenterology , 11 : 361 – 366 .
- Schneeberger , E.E. and Lynch , R.D. 2004 . The tight junction: a multifunctional complex . American Journal of Physiology-Cell Physiology , 286 : C1213 – C1228 .
- Sharma , R. , Fernandez , F. , Hinton , M. and Schumacher , U. 1997 . The influence of diet on the mucin carbohydrates in the chick intestinal tract . Cellular and Molecular Life Sciences , 53 : 935 – 942 .
- Sun , X. , Spencer , A.U. , Yang , H. , Haxhija , E.Q. and Teitelbaum , D.H. 2006 . Impact of caloric intake on parenteral nutrition-associated intestinal morphology and mucosal barrier function . Journal of Parenteral and Enteral Nutrition , 30 : 474 – 479 .
- Theodoropoulos , G. and Carraway , K.L. 2007 . Molecular signaling in the regulation of mucins . Journal of Cellular Biochemistry , 102 : 1103 – 1116 .
- Turner , J.R. 2006 . Molecular basis of epithelial barrier regulation: from basic mechanisms to clinical application . American Journal of Pathology , 169 : 1901 – 1909 .
- Valenzuela , S.V. , Diaz , P. and Javier , P.F. 2010 . Recombinant expression of an alkali stable GH10 xylanase from Paenibacillus barcinonensis . Journal of Agricultural and Food Chemistry , 58 : 4814 – 4818 .
- Van Klinken , B.J. , Dekker , J. , Buller , H.A. and Einerhand , A.W. 1995 . Mucin gene structure and expression: protection vs. adhesion . American Journal of Physiology , 269 : G613 – G627 .
- Xu , Z.R. , Hu , C.H. , Xia , M.S. , Zhan , X.A. and Wang , M.Q. 2003 . Effects of dietary fructooligosaccharide on digestive enzyme activities, intestinal microflora and morphology of male broilers . Poultry Science , 82 : 1030 – 1036 .
- Zhou , H. , Gong , J. , Brisbin , J. , Yu , H. , Sarson , A.J. , Si , W. , Sharif , S. and Han , Y. 2009 . Transcriptional profiling analysis of host response to Clostridium perfringens infection in broilers . Poultry Science , 88 : 1023 – 1032 .