Abstract
Red-legged partridges (Alectoris rufa) are a significant part of the culture, diet and income for many people in central and southern Spain. Due to declining populations in the wild, intensive farming is common and 4 million juvenile partridges are released each autumn. Intensive management and high densities result in high prevalence of enteric disease and the use of antimicrobials as preventive measures on partridge farms and prior to restocking in the wild. We determined the occurrence of avian pathogenic Escherichia coli (APEC), and screened phenotypic resistance of E. coli against enrofloxacin, gentamicin and cefotaxim in farmed, restocked and wild partridges. Prevalence of APEC in farmed and restocked red-legged partridges was significantly higher than in natural populations. Phenotypic resistance against both gentamicin and enrofloxacin was significantly more frequent in farmed (75%) and restocked (43%) partridges than in wild partridges, while most E. coli isolated from natural populations were susceptible to all three antimicrobials tested (65%). This indicates that farmed and restocked partridges carry APEC that could be a reason for disease outbreaks on farms, and that E. coli carried by farmed and restocked partridges can acquire resistance to frequently used antimicrobials, thus being a concern for the environment, wild birds and consumers. Management in farms and restocking procedures may create a hazard not only for spreading APEC, but also as a potential source of resistant E. coli in the environment.
Introduction
Game birds, and the red-legged partridge (Alectoris rufa) in particular, are a significant part of the culture, diet and income for many people in central and Southern Spain and are becoming increasingly popular in the rest of Europe. In the last two decades, natural populations of red-legged partridges have declined (Duarte & Vargas, Citation2004; Beja et al., Citation2009; Duarte et al., Citation2010). Due to the importance of the species in rural Spain, this has led to an intensive production of partridges on farms for restocking in nature, mostly for hunting purposes, and approximately 4 million juvenile partridges are released each autumn in Spain (Millán et al., Citation2004; Gortázar et al., Citation2007).
Recent studies have shown that farm-raised populations of red-legged partridges were positive for important avian and human pathogens such as Campylobacter spp., Salmonella spp. and Escherichia coli that are practically absent in the digestive tract of natural populations of these birds (Díaz-Sánchez et al., Citation2012).
E. coli is present in the microflora of the intestinal tract and environment of poultry (Delicato et al., Citation2003; Ewers et al., Citation2009a). Avian pathogenic E. coli (APEC) is the term commonly used to designate the aetiological agent of colibacillosis in poultry. In fact, this term includes all extra-intestinal pathogenic E. coli strains isolated from poultry, causing clinical signs of colibacillosis (van Bost et al., Citation2003; Rodriguez-Siek et al., Citation2005; Kwon, Citation2008) both as localized or systemic infections. Due to the importance of APEC, markers for consistent detection and identification of these isolates are under investigation. Several studies have revealed shared virulence genes among E. coli isolates obtained from colibacillosis cases (Allan et al., Citation1993; McPeake et al., Citation2005; Johnson et al., Citation2008a). Such virulence genes code for properties like adherence to the respiratory tract, resistance to immunological defence, multiplication under iron-restricted conditions and production of cytotoxic effects (Ewers et al., Citation2005; Johnson et al., Citation2008a). The importance and interaction of the specific virulence genes that determine pathogenicity of E. coli for chickens and the pathogenesis of APEC infections is still, however, poorly understood (Ewers et al., Citation2005; McPeake et al., Citation2005), and information on APEC in avian species other than domestic poultry is scarce (Camarda et al., Citation2007; Gibbs et al., Citation2007; Ewers et al., Citation2009a; Gaukler et al., Citation2009). Sequence analysis has also found evidence for similarity between certain human extra-intestinal pathogenic E. coli strains and APEC strains, suggesting a zoonotic potential of the latter through food-borne transmission (Johnson et al., Citation2008b).
The use of antimicrobials in poultry production, both to combat diseases such as colibacillosis and as growth promoters, has been associated with the appearance of antimicrobial resistances (Gyles, Citation2008). In the last two decades antimicrobial resistance has emerged in zoonotic enteropathogens (i.e. Salmonella spp., Campylobacter spp., pathogenic E. coli), commensal bacteria (i.e. E. coli, Enterococcus spp.), and bacterial animal pathogens (i.e. APEC, Pasteurella spp., Actinobacillus spp.) (Schwarz et al., Citation2001; Wagner et al., Citation2003; Segura et al., Citation2009; European Food Safety Authority, Citation2010). In an attempt to reduce the emergence of antimicrobial resistance in bacteria of concern for human and animal health, the European Union banned the use of antimicrobial growth promoters in poultry production in 2006, which resulted in an increase of therapeutic and preventive treatments (Castanon, Citation2007).
Thus, the potential zoonotic nature of APEC strains (Ewers et al., Citation2007; Tivendale et al., Citation2010) as well as the emergence of new mechanisms of antimicrobial resistance are veterinary as well as public health concerns well recognized in poultry production (Segura et al., Citation2009; European Food Safety Authority, Citation2010). However, little information is available on the prevalence and importance of APEC and multi-resistant bacteria in game bird production despite the growing importance of this sector. The degree of implication of APEC in so-termed colibacillosis outbreaks and the general prevalence of APEC in red-legged partridge farms remains unknown (La Ragione et al., Citation2004). This makes the control and prevention of mortality outbreaks on farms difficult and leads to the use of antimicrobials as a preventive measure, both on farms and prior to restocking in the wild. As no controls are enforced upon restocked partridges these practices can lead to an increase of disease transmission (Slota et al., Citation2011) and spread of resistant pathogenic bacteria to the environment, and potentially to the consumers of hunted partridges (Salyers, Citation1995).
We hypothesize that APEC strains exist in the microflora of farmed and restocked red-legged partridges, but not in natural populations of this species, and that resistance to antimicrobials would be more frequent in farmed and restocked red-legged partridges than in partridges from natural populations. Here we report the results obtained from screening farmed, restocked and free-living partridges for the presence of putative APEC strains and phenotypic antimicrobial resistance patterns to three antimicrobials commonly used on partridge farms.
Materials and Methods
Study area
The study area was located in south-central Spain within the Autonomic region of Castilla-La Mancha (UTM zone 30 S, 294,348 to 681,063 E, 4,208,706 to 4,575,340 N).
Samples
For the purpose of this study we distinguished three types of origin of red-legged partridges: farms, where red-legged partridges were bred intensively in open-air operations; natural populations, red-legged partridges sampled in hunting areas where no farmed partridges had been released during at least the past 5 years; and restocked populations, from areas in which releases of farmed partridges had been carried out regularly, mostly prior to or during the hunting season (from mid-October to mid-February) (). All samples were taken during 2009 to 2011 for the three types of origin, including all seasons, except for restocked locations, where samples were obtained only during the hunting season.
Table 1. Putative APEC prevalence in cloacal samples from partridges of farm, restocking and natural origin.
A total of 356 cloacal samples were taken from apparently healthy partridges from the three described origins: farm-reared (n = 143, from five farms), restocked populations (n = 112, from five hunting estates) and natural populations (n = 101 from eight hunting estates) ().
All procedures and handling of birds were carried out under the guidelines and animal welfare ethical code of the University of Castilla-La Mancha (6 March 2010, http://www.uclm.es/organos/vicinvestigacion/c_etica/pdf/st06106-re01.es10.pdf), and the Regional government (RD 223/1988 de 14 de marzo, Sobre protección de los animales utilizados para experimentación y otros fines científicos. Orden de 13 de octubre de 1989 del Ministerio de Agricultura, Pesca y Alimentación. Desarrolla el R.D. 223/1988). Captures were carried out under projects PII1I09-0271-5037, PAI06-0112 and PAII1C09-0227-0104 and under specific permits from the regional government.
Culture and APEC screening
Cloacal samples were collected with sterile cotton swabs into Amies transport medium (Deltalab, Barcelona, Spain), maintained at 4° C until arrival at the laboratory within 24 h of collection, plated directly onto MacConkey agar (Scharlab, Barcelona, Spain) and incubated at 37° C. Isolation and identification of E. coli were performed according to standard bacteriological methods.
E. coli growth from the first streaking area of the culture plate was tested by multiplex polymerase chain reaction (PCR), as previously described by Johnson et al. (Citation2008a), for the following virulence-associated genes: aerobactin siderophore receptor gene (iutA), the episomal increased serum survival gene (iss), the episomal outer membrane protease gene (ompT), the putative avian haemolysin gene (hlyF) and the salmochelin siderophore receptor gene (iroN). The genes targeted are frequently contained in plasmid-pathogenicity-associated islands and have been identified as being highly conserved among APEC isolates. They are also known to occur more widely among virulent avian strains than in commensal faecal E. coli strains (Johnson et al., Citation2008a). Briefly, a loopful of bacterial growth was suspended in 250 µl sterile distilled water, boiled for 5 min to release the DNA, and centrifuged at 10,000 r.p.m. for 5 min. The supernatant was added directly to the PCR mixture. Amplification of bacterial DNA was performed using a reaction mixture volume of 25 µl, containing 2 µl prepared sample supernatant, 0.3 µM each primer, 0.25 mM deoxynucleoside triphosphates (Biotools, Madrid, Spain), 4 mM magnesium chloride and 1 U Taq DNA polymerase. The reaction was performed according to the following cycling parameters using an Applied Biosystems 2720 thermal cycler (Applied Biosystems, Foster City, California, USA): 94°C for 2 min; 35 cycles of 94°C for 30 sec, 63°C for 30 sec, 72°C for 60 sec; and a final cycle of 72°C for 10 min. All samples were subjected to horizontal gel electrophoresis in 4% agarose, and amplicon sizes were determined by comparison with a 100-base-pair DNA marker (100 bp Ladder Marker, 0.5 mg/ml; Biotools, Madrid, Spain). Strains known to possess or lack the genes of interest were examined with each amplification procedure as positive and negative controls. Amplicons were stained with ethidium bromide and photographed under ultraviolet exposure (Gel Doc 2000; Bio-Rad, Hemel Hempstead, UK).
For each PCR-positive culture, 10 individual E. coli-like colonies obtained from MacConkey agar plates were tested in the same way as described above. E. coli-like colonies were considered as possible APEC if at least four of the five tested genes were detected by multiplex PCR. If no single colony was found to be positive among the first 10 colonies, at least 10 more were tested. The resulting suspected APEC isolates were confirmed biochemically as E. coli by the API 20E system (bioMérieux, Marcy L'Etoile, France).
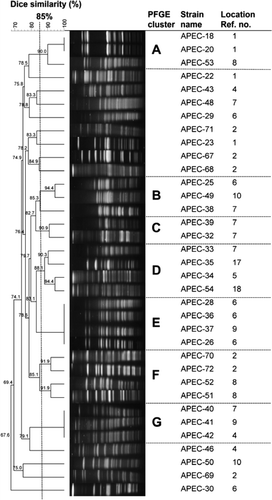
Macrorestriction analysis and pulsed-field gel electrophoresis
To reveal a possible clonal origin of E. coli strains considered to represent APEC, macrorestriction analysis was performed as previously described using a CHEF DRIII System (Bio-Rad, Munich, Germany) (Ewers et al., Citation2004). Pulsed-field gel electrophoresis (PFGE) profiles generated by restriction of chromosomal DNA with XbaI were compared digitally using Bionumerics software (version 6.6; Applied Maths, St.-Martens-Latem, Belgium). Cluster analysis of Dice similarity indices based on the unweighted pair group method with arithmetic means was applied to generate a dendrogram depicting the relationship among PFGE profiles. Isolates were considered to belong to a group of clonally related strains if the Dice similarity index of the PFGE pattern was ≥85%.
Testing for phenotypic antimicrobial susceptibility
A subset of 192 samples from the three husbandry groups—farmed (n=40), restocked (n=72), and natural (n = 74) red-legged partridges—was screened for phenotypic resistance patterns against three selected antimicrobial substances used frequently on red-legged partridge farms. For this, cloacal swab samples were first enriched in peptone water broth. Following overnight incubation at 37°C, a loop of the enrichment broth was streaked onto freshly prepared MacConkey agar supplemented with the estimated breakpoint concentration of either enrofloxacin (≥4 µg/ml), gentamicin (≥16 µg/ml) and cefotaxime (≥4 µg/ml) according to the microdilution method recommended by the National Antimicrobial Resistance Monitoring System (National Antimicrobial Susceptibility Monitoring Program, Citation1998). Based on the definition from National Antimicrobial Resistance Monitoring System, E. coli strains were considered resistant if growth occurred above antibiotic breakpoint concentrations.
Statistical analysis
The existence of statistically significant differences in occurrence of putative APEC and phenotypic resistance of E. coli against gentamicin, enrofloxacin and cefotaxime between the three origins of partridges, and also between individual farms and hunting estates, was tested using the chi-square test (χ2) for homogeneity. To test the effect of breeding, we analysed differences between husbandry groups separating natural populations in the breeding season out. In addition, we compared natural populations sampled in the breeding season with non-breeding populations. All analyses were carried out using SPSS Version 19.0 (IBM®, SPSS Inc., Chicago, Illinois, USA).
Results
Growth of E. coli on MacConkey agar plates as confirmed by morphological and biochemical characteristics was observed in 74.4% (265 out of 356) of the samples. In farmed (77%, 111 out of 143) and restocked (95%, 106 out of 112) red-legged partridges, detection rates of E. coli were significantly higher than in natural populations (48%, 48 out of 101; χ2=63.239, degrees of freedom [d.f.] = 2, P < 0.001; ).
Using the criteria described by Johnson et al. (Citation2008a), we differentiated 36 APEC isolates (10%) from 365 samples. In farmed (11%, 15 out of 143) and restocked (16%, 18 out of 112) red-legged partridges, prevalence of APEC isolates was significantly higher than in natural populations (3%, 3 out of 101; χ2=10.066, d.f.=2, P < 0.001; ).
Using PFGE we obtained seven clusters (A to G; see ), each containing clonally related strains according to the cut-off value defined. We detected clonal strains among APEC isolates but in most cases they were from different sampling locations, except for clusters A and E that contained two and three clonal strains from the same location respectively (). No association of clusters or clonal strains to management or location was observed. In addition, 12 singletons not grouped in any of the seven clusters were found.
Figure 1. Dendrogram showing the relationship of APEC strains based on XbaI-generated PFGE profiles (optimization, 1.0%; position tolerance, 1.5%). Closely related groups of strains (Dice similarity ≥85%) are indicated by bold letters (A to G). One strain (APEC-17) is not shown in the figure as it was not possible to recover the strain for further characterization. Location reference number is according to Table one..
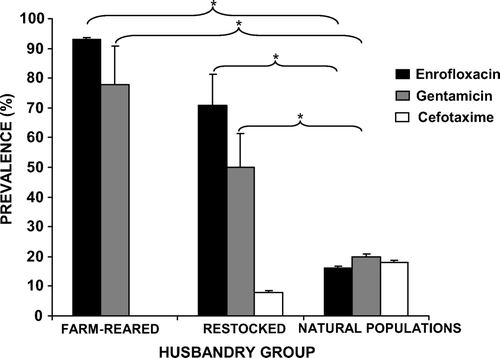
Significantly more samples contained E. coli with phenotypic resistance against gentamicin (44%, 85 out of 192) and enrofloxacin (53%, 101 out of 192) than against cefotaxime (10%, 19 out of 186; χ 2=84.403, d.f.=2, P<0.001). The number of samples that showed growth of E. coli above breakpoint concentrations for gentamicin or enrofloxacin was significantly higher in farmed (χ2=80.294, d.f.=2, P<0.001) and restocked (χ2=59.481, d.f.=2, P<0.001) red-legged partridges than in natural populations (). In contrast, growth of E. coli above breakpoint concentration of cefotaxime was very low and was only observed in the samples obtained from restocked red-legged partridges and from partridges from natural populations (6% and 13% respectively, ).
Figure 2. Prevalence of cloacal swab samples showing E. coli growth above breakpoint concentration of three different antimicrobials in farmed, restocked and wild red-legged partridges.
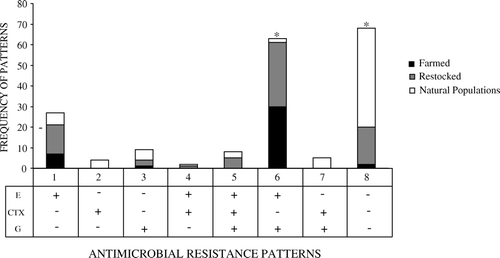
Finally, we observed that both in samples from farmed (χ2=162.138, d.f.=7, P<0.001) and restocked (χ2=128.000, d.f.=7, P < 0.001) red-legged partridges a pattern of growth above breakpoint concentration of both enrofloxacin and gentamicin was significantly more prevalent. In contrast, E. coli isolates from the digestive tract of red-legged partridges from natural populations most frequently exhibited a phenotype that was not able to grow above breakpoint concentrations of any of the three antibiotics tested (χ2=198.310, d.f.=7, P<0.001; ).
Figure 3. Frequency of different antimicrobial resistance patterns observed in faecal E. coli from farmed, restocked and wild red-legged partridge. E, enrofloxacin; CTX, cefotaxime; G, gentamicin; −, no growth at/above the breakpoint concentration of the corresponding antimicrobial; +, growth at/above breakpoint concentration of the corresponding antimicrobial. *Significantly higher frequency of resistance pattern for farmed and restocked partridges and for natural populations (P<0.001).
Discussion
This study showed that the occurrence of both putative APEC in healthy red-legged partridges and E. coli with resistance to antimicrobials was higher in farmed and restocked red-legged partridges than in the natural partridge populations. The overall prevalence of APEC (10%, 36 out of 356) detected in red-legged partridges was consistent with the report by Barnes et al. (Citation2003) on 10 to 15% of the coliform population in poultry belonging to pathogenic serotypes. Macrorestriction analysis revealed a large variability of PFGE profiles indicating a high diversity among APEC strains. Although we obtained clusters containing clonally related strains, only sequence-based methods such as multilocus sequence typing would enable discerning their true phylogenetic relationship. Currently, multilocus sequence typing is considered one of the best phylogenetic grouping methods for the investigation of the phylogenetic relationship between clinical pathogenic strains and reference strains of E. coli and thus to place APEC strains in the phylogenetic tree (Maiden et al., Citation1998; Moulin-Schouleur et al., Citation2007).
Based on PFGE profiles, association of clusters or clonal strains to management or sampling location was not observed. However, further analysis of a higher number of samples per location and using sequence-based methodology is necessary to determine whether farm-raising may favour the appearance of certain virulence gene combinations and clonal strains that could then be dispersed into the environment.
Prevalence of APEC was significantly higher in the intestinal microflora of farmed and restocked partridges than in birds from natural populations. Commensal pathogenic bacteria of the digestive tract such as APEC are known to be able to cause disease in hosts that have compromised immunity, such as, in this case, stressed farmed partridges after release (Johnson et al., Citation2008a; Ewers et al., Citation2009b). This could explain the higher prevalence of APEC in restocked partridges, and adds a potential cause for the low survival rate of restocked partridges (Gortázar et al., Citation2007). It also raises the question about the potential risks of spread of APEC from restocked partridges to natural populations.
Commensal E. coli that have acquired the ability to cause disease in avian hosts have been shown to be able to invade all host tissues, and become excreted via the intestinal tract, despite the disease process being primarily respiratory (Schubert et al., Citation1998; Giovanardi et al., Citation2005, Ewers et al., Citation2009b). In this way APEC may contaminate the environment and be transmitted to other avian hosts (McPeake et al., Citation2005; Ewers et al., Citation2009a). According to this, previous reports on the isolation of E. coli possessing traits commonly associated with pathogenesis in poultry from wild birds such as starlings (Turdus vulgaris) (Gaukler et al., Citation2009) or yellow-headed blackbirds (Xantocephalus xantocephalus) (Gibbs et al., Citation2007) have associated this presence with livestock and human activities. Wild birds that had acquired pathogenic bacteria from livestock could become a reservoir for strains potentially pathogenic for poultry and man (Gaukler et al., Citation2009). Other authors such as Camarda et al. (Citation2007) suspected that APEC, acquired by Adouin gulls (Larus audoinii), could pose a risk for the population if biological or environmental stress factors favoured outbreaks. An example of pathogen transmission from restocked to free-living red-legged partridges is a salmonellosis outbreak described by Lucientes (Citation1998) that was related to sharing of feeders between restocked and wild partridges.
The presence of APEC in the digestive tract flora suggests that they may be implicated in at least part of the disease outbreaks on partridge farms. To date the presence of APEC has been related to extra-intestinal disease in chicken, turkeys (Meleagris gallopa) and Japanese quail (Coturnix coturnix japonica) (Allan et al., Citation1993; Arenas et al., Citation1999; Ramírez et al., Citation2009; Salehi & Ghanbarpour, Citation2010), but not as yet in red-legged partridges. The only existing information on the implication of E. coli in disease in red-legged partridges consists of a report describing an outbreak of naturally occurring attaching and effacing E. coli in farmed red-legged partridges (La Ragione et al., Citation2004).
The comparative screening of two antimicrobial substances that are commonly employed on partridge farms but also in poultry (gentamicin and enrofloxacin) showed that growth of E. coli above breakpoint concentration occurred in approximately one-half of the cloacal swab samples tested from healthy farmed and restocked partridges, in contrast to results obtained in free-living red-legged partridges. Significantly less samples of E. coli grew above breakpoint concentration for cefotaxime (), a third-generation cephalosporine recognized as an important indicator for extended-spectrum β-lactamase resistance (Blanco et al., Citation1997). The agar method employed here serves as a qualitative screening test that allows us to explore whether natural populations of partridges reflect the resistance patterns observed for farmed birds. In this respect, we detected that the phenotypic E. coli resistance pattern was the same in farmed and restocked partridges, but was significantly different from natural populations, in which resistance to the three antimicrobials was nearly absent. While farmed and restocked partridges showed primarily combined phenotypic resistance to gentamicin and enrofloxacin, a low prevalence of E. coli with phenotypic resistance to cefotaxime was found in natural partridge populations (). Our results may reflect a misuse of gentamicin and enrofloxacin in partridge farms, as antibiotic misuse in combination with crowding, poor sanitation and intensive management has been considered responsible for the emergence of antimicrobial resistance in faecal commensal bacteria from poultry (van de Boogard & Stobberingh, Citation1999). An increase of infections with Campylobacter spp. that are resistant to fluoroquinolones has been reported in poultry (Luangtongkum et al., Citation2006; Nelson et al., Citation2007), while resistance of E. coli to gentamicin is described as common among poultry-associated enteric bacteria (Zhao et al., Citation2005).
The spread of bacteria possessing mechanisms of antimicrobial resistance from livestock to wild animals in areas close to farms has been described previously (Kozak et al., Citation2009). In concordance with these results, a recent study has found that E. coli isolated from wild birds displayed similar resistance patterns to those from domestic animals (Guenther et al., Citation2010). The acquisition of pathogenic bacteria carrying resistance genes by wild birds can cause these to later act as reservoirs for resistant bacteria and genetic determinants of antimicrobial resistance (Dolejska et al., Citation2007; Guenther et al., Citation2011). Also, Marrow et al. (Citation2009) suggested that avian predators can acquire resistant microorganisms via their prey. Being a key prey species in Mediterranean ecosystems, the red-legged partridge could thus be a significant source of antimicrobial-resistant bacteria for numerous avian predators in Spain, including the endangered Spanish imperial eagle (Aquila adalberti) (Martínez et al., Citation2002).
Growth of E. coli from restocked and natural populations above the breakpoint concentration of cefotaxime could reflect an environmental origin of resistance mechanisms as suggested by Martínez (Citation2008). Contact with contaminated water or acquisition via food (contaminated pasturelands) have been reported as important sources of resistant bacteria derived from human (sewage, fertilizers) or veterinary (livestock) activities (van de Boogard & Stobberingh, Citation1999; Chee-Sanford et al., Citation2001; Cole et al., Citation2005; Kozak et al., Citation2009).
In conclusion, farming and restocking of red-legged partridges are associated with higher prevalence of APEC and E. coli with phenotypic antimicrobial resistance. Thus, management on farms and restocking procedures in red-legged partridges may create a hazard not only for spreading APEC, but also because it could be an important source of antimicrobial-resistant E. coli in the environment and therefore to natural populations of partridges and other avian species. This also implies a potential risk for humans associated with the consumption of red-legged partridge meat, which is currently under no sanitary control. Further investigations should include molecular characterization of commensal APEC strains, the isolation and characterization of APEC strains from disease outbreaks on partridge farms, more broad antimicrobial susceptibility screening of both commensal and pathogenic E. coli, and detailed characterization of the resistance patterns and mechanisms of E. coli from farmed red-legged partridges.
Strict surveillance of sanitary conditions, drug use during production cycles on farms and restocking procedures and the investigation of alternative preventive and therapeutic strategies of treatment for enterobacterial disease in red-legged partridges could help to reduce risks in red-legged partridge farming and restocking procedures.
Acknowledgements
The authors thank V. Gutiérrez, V. Gamino and S. Ibarra for their assistance in the field, and Dr M.D. Vidal Roig for help in acquiring laboratory skills. This study was funded by Junta de Comunidades de Castilla-La Mancha (JCCM), Spain (project reference: PAC08-0296-7771) and is also a contribution to project Ag2008-02504GAN funded by the Spanish Ministry for Science and Innovation. Sandra Díaz-Sánchez holds a PhD research grant funded by JCCM (AG07). Dr S. Sánchez acknowledges the Consejería de Educación y Ciencia of the JCCM and Fondo Social Europeo for his research fellowship (09/02-C). The authors also gratefully acknowledge Dr S. Morabito (Istituto Superiore di Sanità, Rome, Italy) for providing E. coli control strains used in this study. Finally, the authors would like to acknowledge P. Krienke and M. Kuehl for their help in experimental analyses.
References
- Allan , B.J. , Vandenhurk , J.V. and Potter , A.A. 1993 . Characterization of Escherichia coli isolated from cases of avian colibacillosis . Canadian Journal of Veterinary Research , 57 : 146 – 151 .
- Arenas , A. , Vicente , S. , Luque , I. , Gomez-Villamandos , J.C. , Astorga , R. , Maldonado , A. and Tarradas , C. 1999 . Outbreak of septicaemic colibacillosis in Japanese quail (Coturnix coturnix japonica) . Journal of Veterinary Medicine , 46 : 399 – 404 .
- Barnes , H.J. , Vaillancourt , J.P. and Gross , W.B. 2003 . “ Colibacillosis ” . In Diseases of Poultry , 11th edn , Edited by: Saif , Y.M. , Barnes , H.J. , Glisson , J.R. , Fadly , A.M. , McDougald , L.R. and Swayne , D.E. 631 – 656 . Ames : Iowa State Press .
- Beja , P. , Gordinho , L. , Reino , L. , Loureiro , F. , Santos-Reis , M. and Borralho , R. 2009 . Predator abundance in relation to small game management in southern Portugal: conservation implications . European Journal of Wildlife Research , 55 : 227 – 238 .
- Blanco , J.E. , Blanco , M. , Mora , A. and Blanco , J. 1997 . Prevalence of bacterial resistance to quinolones and other antimicrobials among avian Escherichia coli strains isolated from septicemic and healthy chickens in Spain . Journal of Clinical Microbiology , 35 : 2184 – 2185 .
- Camarda , A. , Circella , E. , Giovanardi , D. , Pennelli , D. , Battista , P. , Campagnari , E. , Bruni , G. and Tagliabu , G. 2007 . Avian Pathogenic Escherichia coli in Audouin gulls (Larus audouinii). Could they affect the surviving of the bird colonies? . Italian Journal of Animal Science , 6 : 317 – 320 .
- Castanon , J.I.R. 2007 . History of the use of antibiotic as growth promoters in European Poultry Feeds . Poultry Science , 86 : 2466 – 2471 .
- Chee-Sanford , J.C. , Aminov , R.I. and Krapac , I.J. 2001 . Nucleotide occurrence and diversity of tetracycline resistance genes in lagoons and groundwater underlying two swine production facilities . Applied Environmental Microbiology , 67 : 1494 – 502 .
- Cole , D. , Drum , D.J. , Stalknecht , D.E. , White , D.G. , Lee , M.D. , Ayers , S. , Sobsey , M. and Maurer , J.J. 2005 . Free-living Canada geese and antimicrobial resistance . Emerging Infectious Diseases , 11 : 935 – 938 .
- Delicato , E.R. , de Brito , B.G. , Gaziri , L.C.J. and Vidotto , M.C. 2003 . Virulence-associated genes in Escherichia coli isolates from poultry with colibacillosis . Veterinary Microbiology , 94 : 97 – 103 .
- Díaz-Sánchez , S. , Moriones Mateo , A. , Casas Arenas , F. & Höfle , U. 2012 . Prevalence of Escherichia coli, Salmonella sp. and Campylobacter sp. in the intestinal flora of farm reared, restocked and wild red-legged partridges (Alectoris rufa): is restocking using farm-reared birds a risk? European Journal of Wildlife Research , 58 , 99 – 105 doi: 10.1007/s10344-011-0547-5 .
- Dolejska , M. , Cizek , A. and Literak , I. 2007 . High prevalence of antimicrobial-resistant genes and integrons in Escherichia coli isolates from black-headed gulls in the Czech Republic . Journal of Applied Microbiology , 103 : 11 – 19 .
- Duarte , J. and Vargas , J.M. 2004 . Field interbreeding of released farm reared partridges (Alectoris rufa) with wild ones . Game Wildlife Science , 21 : 55 – 61 .
- Duarte , J. , Farfán , M.A. & Vargas , J.M. 2010 . New data on mortality, home range and dispersal of red-legged partridges (Alectoris rufa) released in a mountain range . European Journal of Wildlife Research , 57 , 675 – 678 . doi: 10.1007/s10344-010-0467-9 .
- European Food Safety Authority . 2010 . The Community Summary Report on antimicrobial resistance in zoonotic and indicator bacteria from animals and food in the European Union in 2008 . EFSA Journal , 8 , 1658 . http://www.efsa.europa.eu/en/efsajournal/pub/1658.htm
- Ewers , C. , Janßen , T. , Kießling , S. , Philipp , H.C. and Wieler , L.H. 2004 . Molecular epidemiology of avian pathogenic Escherichia coli (APEC) isolated from colisepticemia in poultry . Veterinary Microbiology , 104 : 91 – 101 .
- Ewers , C. , Janssen , T. , Kiessling , S. and Wieler , L.H. 2005 . Rapid detection of virulence-associated genes in avian pathogenic Escherichia coli by multiplex polymerase chain reaction . Avian Diseases , 49 : 269 – 273 .
- Ewers , C. , Li , G. , Wilking , H. , Kieling , S. , Alt , K. , Antáo , E.M. , Laturnus , C. , Diehl , I. , Glodde , S. , Homeier , T. , Böhnke , U. , Steinrück , H. , Philipp , H.C. and Wieler , L.H. 2007 . Avian pathogenic, uropathogenic, and newborn meningitis-causing Escherichia coli: how closely related are they? . International Journal of Medical Microbiology , 297 : 163 – 176 .
- Ewers , C. , Guenther , S. , Wieler , L.H. and Schierack , P. 2009a . Mallard ducks—a waterfowl species with high risk of distributing Escherichia coli pathogenic for humans . Environmental Microbiology Reports , 1 : 510 – 517 .
- Ewers , C. , Antão , E.M. , Diehl , I. , Philipp , H.-C. and Wieler , L.H. 2009b . Intestine and environment of the chicken as reservoirs for extraintestinal pathogenic Escherichia coli strains with zoonotic potential . Applied and Environmental Microbiology , 75 : 184 – 192 .
- Gaukler , S.M. , Linz , G.M. , Sherwood , J.S. , Dyer , N.W. , Bleier , W.J. , Wannemuehler , Y.M. , Nolan , L.K. and Logue , C.M. 2009 . Escherichia coli, Salmonella, and Mycobacterium avium subsp paratuberculosis in wild European starlings at a Kansas cattle feedlot . Avian Diseases , 53 : 544 – 551 .
- Gibbs , P.S. , Kasa , R. , Newbrey , J.L. , Petermann , S.R. , Wooley , R.E. , Vinson , H.M. and Reed , W. 2007 . Identification antimicrobial resistance profiles and virulence of members from the Family Enterobacteriaceae from the feces of Yellow-Headed Blackbirds (Xanthocephalus xanthocephalus) in North Dakota . Avian Diseases , 51 : 649 – 655 .
- Giovanardi , D. , Campagnari , E. , Sperati Ruffoni , L. , Pesente , P. , Ortali , G. and Furlattini , V. 2005 . Avian pathogenic Escherichia coli transmission from broiler breeders to their progeny in an integrated poultry production chain . Avian Pathology , 34 : 313 – 318 .
- Gortázar , C. , Ferroglio , E. , Hofle , U. , Frolich , K. & Vicente , J. 2007 . Diseases shared between wildlife and livestock: a European perspective . European Journal of Wildlife Research , 53 , 241 – 256 , doi: 10.1007/s10344-007-0098-y .
- Guenther , S. , Grobbel , M. , Lübke-Becker , A. , Goedecke , A. , Friedrich , N.D. , Wieler , L.H. and Ewers , C. 2010 . Antimicrobial resistance profiles of E. coli from common European wild bird species . Veterinary Microbiology , 144 : 219 – 225 .
- Guenther , S. , Ewers , C. , Wieler , L.H. 2011 . Extended-spectrum beta-lactamases producing E. coli in wildlife, yet another form of environmental pollution? Frontiers in Microbiology 2 , 246 . doi: 10.3389/fmicb.2011.00246 .
- Gyles , C.L. 2008 . Antimicrobial resistance in selected bacteria from poultry . Animal Health Research Reviews , 9 : 149 – 158 .
- Johnson , T.J. , Wannemuehler , Y. , Doetkott , C. , Johnson , S.J. , Rosenberger , S.C. and Nolan , L.K. 2008a . Identification of minimal predictors of avian pathogenic Escherichia coli virulence for use as a rapid diagnostic tool . Journal of Clinical Microbiology , 46 : 3987 – 3996 .
- Johnson , T.J. , Wannemuehler , Y. , Johnson , S.J. , Stell , A.L. , Doetkott , C. , Johnson , J.R. , Kim , K.S. , Spanjaard , L. and Nolan , L.K. 2008b . Comparison of extraintestinal pathogenic Escherichia coli strains from human and avian sources reveals a mixed subset representing potential zoonotic pathogens . Applied and Environmental Microbiology , 74 : 7043 – 7050 .
- Kozak , G.K. , Boerlin , P. , Janecko , N. , Reid-Smith , R.J. and Jardine , C. 2009 . Antimicrobial resistance in Escherichia coli isolates from swine and wild small mammals in the proximity of swine farms and in natural environments in Ontario, Canada . Applied Environmental Microbiology , 75 : 559 – 566 .
- Kwon , S.G. 2008 . Epidemiological prevalence of Avian Pathogenic Escherichia coli differentiated by Multiplex PCR from commercial chickens and hatchery in Korea . Journal of Bacteriology and Virology , 38 : 179 – 188 .
- La Ragione , R.M. , Cooley , W.A. , Parmar , D.D.G. and Ainsworth , H.L. 2004 . Attaching and effacing Escherichia coli O103: K+:H− in red-legged partridges . The Veterinary Record , 155 : 397 – 398 .
- Luangtongkum , T. , Morishita , T.Y. , Ison , A.J. , Huang , S. , McDermott , P.F. and Zhang , Q. 2006 . Effect of conventional and organic production practices on the prevalence and antimicrobial resistance of Campylobacter spp. in poultry . Applied of Environmental Microbiology , 72 : 3600 – 3607 .
- Lucientes , J. 1998 . Las principales patologías de la perdiz roja silvestre . In FEDENCA/ V . La perdiz roja , I, Spain : Madrid .
- Maiden , M.C.J. , Buygraves , J.A. , Fei , E. , Morelli ., Russel , J.E. , Urwin , R. , Zhang , Q. , Zhou , J. , Zurth , K. , Caugant , D.A. , Feavers , I.M. , Achtman , M. & Spratt , B.G. 1998 . Multilocus sequence typing: a portable approach to the identification of clones within populations of pathogenic microorganisms . Proceedings of the National Academy of Sciences of the United States of América , 95 , 3140 – 3145 .
- Marrow , J. , Whittington , J.K. , Mitchell , M. , Hoyer , L.L. and Maddox , C. 2009 . Prevalence and antibiotic-resistance characteristics of Enterococcus spp. isolated from free-living and captive raptors in central Illinois . Journal of Wildlife Diseases , 45 : 302 – 313 .
- Martínez , J. , Viñuela , J. & Villafuerte , R. 2002 . Socioeconomic and cultural aspects of gamebird hunting . REGHAB project , European Commission , Brussels, Belgium .
- Martínez , J.L. 2008 . Antibiotics and antibiotic resistance genes in natural environments . Science , 321 , 365 – 367 , doi: 10.1126/science.1159483
- McPeake , S.J.W. , Smyth , J.A. and Ball , H.J. 2005 . Characterisation of Avian Pathogenic Escherichia coli (APEC) associated with colisepticaemia compared to faecal isolates from healthy birds . Veterinary Microbiology , 110 : 245 – 253 .
- Millán , J. , Gortázar , C. , Martin-Mateo , M.P. and Villafuerte , R. 2004 . Comparative survey of the ectoparasite fauna of wild and farm-reared red-legged partridges (Alectoris rufa), with an ecological study in wild populations . Parasitology Research , 93 : 605 – 611 .
- Moulin-Schouleur , M. , Répérant , M. , Laurent , S. , Brée , A. , Mignon-Grasteau , S. , Germon , P. , Rasschaert , D. and Schouler , C. 2007 . Extraintestinal Pathogenic E. coli strains of avian and human origin: link between phylogenetic relationships and common virulence patterns . Journal of Clinical Microbiology , 40 : 3366 – 3376 .
- National Antimicrobial Susceptibility Monitoring Program . 1998 . Veterinary isolates . Athens , , Georgia : FDA/USDSA/CDC . http://www.ars.usda.gov/Main/docs.htm?docid=6750&page=3 .
- Nelson , J. , Chiller , T.M. , Powers , J.H. and Angulo , F.J. 2007 . Fluoroquinolone-resistant Campylobacter species and the withdrawal of fluoroquinolones from use in poultry: a public health success story . Clinical Infectious Diseases , 44 : 977 – 80 .
- Ramírez , R.M. , Almanza , Y. , García , S. & Heredia , N. 2009 . Adherence and invasion of avian pathogenic Escherichia coli to avian tracheal epithelial cells . World Journal of Microbiology and Biotechnology . doi: 10.007/S11274-009-9978-5 .
- Rodriguez-Siek , K.E. , Giddings , C.W. , Doetkott , C. , Johnson , T.J. and Nolan , L.S. 2005 . Characterizing the APEC pathothype . Veterinary Research , 36 : 241 – 256 .
- Salehi , M. and Ghanbarpour , R. 2010 . Phenotypic and genotypic properties of Escherichia coli isolated from colisepticemic cases of Japanese quail (Coturnix coturnix japonica) . Tropical Animal Health and Production , 42 : 1497 – 1504 .
- Salyers , A.A. 1995 . “ Out of the ivory tower: bacterial gene transfer in the real world ” . In Antibiotic Resistance Transfer in the Mammalian Intestinal Tract: Implications for Human Health, Food Safety and Biotechnology , Edited by: Salyers , A.A . 109 – 136 . Berlin , , Germany : Springer-Verlag .
- Schwarz , S. , Kehrenberg , C. and Walsh , T.R. 2001 . Use of antimicrobial agents in veterinary medicine and food animal production . International Journal of Antimicrobial Agents , 17 : 431 – 437 .
- Schubert , S. , Rakin , A. , Karch , H. , Carniel , E. and Heesemann , J. 1998 . Prevalence of the “High Pathogenicity Island” of Yersinia species among Escherichia coli strains that are pathogenic to humans . Infection and Immunity , 66 : 480 – 485 .
- Segura , P.A. , François , M. , Gagnon , C. and Sauve , S. 2009 . Review of the occurrence of anti-infectives in contaminated wastewaters and natural and drinking waters . Environmental Health Perspectives , 117 : 675 – 684 .
- Slota , K.E. , Hill , A.E. , Keefe , T.J. , Bowen , R.A. and Pabilonia , K.L. 2011 . Biosecurity and bird movement practices in upland game bird facilities in the United States . Avian Diseases , 55 : 180 – 186 .
- Tivendale , K.A. , Logue , C.M. , Kariyawasam , S. , Jordan , D. , Hussein , A. , Li , G. , Wannemuehler , Y. and Nolan , L.K. 2010 . Avian-Pathogenic Escherichia coli strains are similar to neonatal meningitis E. coli strains and are able to cause meningitis in the rat model of human disease . Infection and Immunity , 78 : 3412 – 3419 .
- van Bost , S. , Jacquemin , E. , Oswald , E. and Mainil , J. 2003 . Multiplex PCRs for identification of necrotoxigenic Escherichia coli . Journal of Clinical Microbiology , 41 : 4480 – 4482 .
- van de Boogard , A.E and Stobberingh , E.E. 1999 . Antibiotic usage in animals-impact on bacterial resistance and public health . Drugs , 58 : 589 – 607 .
- Wagner , B.A. , Salman , M.D. , Dargatz , D.A. , Morley , P.S. , Wittum , T.E. and Keefe , T. 2003 . Factor analysis of minimum-inhibitory concentrations for Escherichia coli isolated from feedlot cattle to model relationships among antimicrobial-resistance outcomes . Preventive Veterinary Medicine , 57 : 127 – 13 .
- Zhao , S. , Maurer , J.J. , Hubert , S. , de Villena , J.F. , McDermott , P.F. and Meng , J. 2005 . Antimicrobial susceptibility and molecular characterization of avian pathogenic Escherichia coli isolates . Veterinary Microbiology , 107 : 215 – 224 .