Abstract
Microfilariae are considered non-pathogenic in wild birds. The objective of the current communication is to report host reactions to microfilarial infection of unusual intensity in emaciated boreal owls (Aegolius funereus). An unusually large number of boreal owls (n = 21) were submitted to the Canadian Cooperative Wildlife Health Center—Quebec Region for post-mortem examination during the winter of 2009. Nineteen out of 21 birds were considered emaciated based on atrophy of adipose tissue and pectoral muscles and suboptimal weight. A microscopic examination of a subset of nine owls revealed the presence of microfilariae in six owls. Three of the birds with a heavy parasite burden had masses of larval nematodes obstructing large vessels of the lungs. The emaciated owls are believed to have died from starvation due to a cyclic decrease in prey abundance in the boreal forest. This cycle also drives winter movements of boreal owls to urbanized areas of southern Quebec, presumably accounting for the large number of birds submitted in 2009. In the most severely infected owls, the extreme microfilarial burden might have caused an alteration in circulatory dynamics, gaseous exchanges and also probably some metabolic cost. Consequently, microfilariae could have significantly contributed to the death of some of these owls.
Introduction
Microfilariae, the first-stage larvae of filarioid nematodes, are parasites of wild birds that can be found either in the blood or in the skin of their hosts (Bartlett, Citation2009). Microfilariae are regularly found on blood films from clinically healthy wild birds and they have been reported in almost 100 families of birds (Bartlett, Citation2009). Their lifecycle is indirect and involves transmission by arthropod vectors (Bartlett, Citation2009). Adult filarioid nematodes, which are found in various tissues, are usually considered non-pathogenic in birds (Bartlett, Citation2009). Nevertheless, some filarioid species have been associated with clinical diseases, generally resulting from adult parasites invading major organs (Bartlett, Citation2009). For example, adults of Chandlerella quiscali, which can be found in the brain of some North-American birds, have been reported to cause encephalitis in emus (Dromaius novaehollandiae) (Law et al., Citation1993). Similarly, the adults of Splendidofilaria eurycerca, which are found under the epicardium and in the myocardium of several species of swans and geese, can cause weakness and non-specific signs of chronic illness (Cole, Citation1999).
Reports of host reactions to microfilariae are rare and limited to cases in which the microfilariae are located outside the lumen of blood vessels. Microfilariae of Splendidofilaria caperata, which are usually blood borne, have been reported to elicit chronic inflammation in the walls of the pulmonary arteries of otherwise clinically healthy American crows (Corvus brachyrhynchos) (Bartlett et al., Citation1981; Bartlett, Citation2009). Microfilariae from an unidentified species of adult filarioids located in the lungs were reported to cause fatal pneumonia in red-billed blue magpies (Urocissa erythrorhyncha) (Simpson et al., Citation1996). To our knowledge, microfilariae located in blood vessel lumens have not been documented as pathogenic in birds.
Boreal owls (Aegolius funereus) are small holartic strigidae that live in the boreal coniferous forest. In Quebec, Canada, irruptions of this species in the southern part of the province follow a 4-year cycle driven by the cyclic low abundance of their rodent preys in the boreal forest (Cheveau et al., Citation2004).
The objective of the current communication is to report microfilarial infection of unusual intensity in emaciated boreal owls (A. funereus), which were submitted for post-mortem examination to the Université de Montréal, Quebec, Canada, in 2009.
Materials and Methods
The boreal owls described in this paper were sick, injured or dead when collected by wildlife officers of the Ministère des Ressources naturelles et de la Faune during winter 2008/09. They were subsequently shipped by courier to the Faculté de médecine vétérinaire of the Université de Montréal. The dead birds and those euthanized after treatment at the university raptor clinic were submitted to the Canadian Cooperative Wildlife Health Center—Quebec Region as part of a continuous wildlife disease surveillance effort. A complete gross examination was performed on each boreal owl. A histopathological examination was performed on a subset of nine owls. Selection was based on the state of carcass preservation and on the absence of an obvious cause of death on initial gross examination. The heart, liver, lung and kidney from these owls were consistently sampled. Other coelomic organs and brain were collected when well preserved. Tissues were fixed in 10% buffered formalin, embedded in paraffin, sectioned at 3 µm, stained with haematoxylin phloxine saffron (Luna, Citation1968) and examined by light microscopy. Additional samples were kept frozen (−20°C) for further reference.
The intensity of microfilarial infection on histological sections of lung observed under 400×magnification was graded using the following scale: 0, absence of microfilariae;+, 1 to 50 microfilariae present;++, 50 to 200 microfilariae present;+++, over 200 microfilariae present.
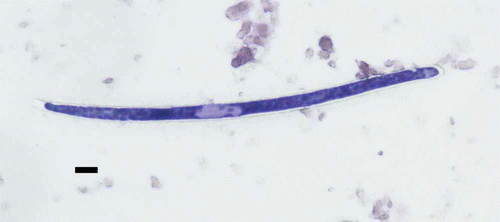
Lung impressions were made on a glass slide, using thawed frozen lung from one boreal owl, then stained with a commercial modified Wright–Giemsa stain (Dip Quick; Jorgensen Labs. Inc., Loveland, Colorado, USA). The microfilariae observed on the stained impressions were microphotographed (Nikon digital sight camera and NIS-Elements F 3.00 software; Nikon Instruments Inc., Melville, New York, USA) and measured in length and diameter using ImageTools software (UTHSCSA ImageTool 3.0; University of Texas Health Science Center at San Antonio, San Antonio, Texas, USA).
Results
A total of 21 boreal owls were submitted during winter 2008/09. The boreal owls’ weight ranged from 82 to 101 g for males and from 109 to 142 g for females. Nineteen of these 21 owls were considered emaciated based on the moderate to marked atrophy of the pectoral muscles, the marked atrophy of subcutaneous, coelomic and pericardial adipose tissue and the suboptimal weight for the species. One of the owls had multiple recent fractures and haemorrhages indicative of trauma.
The only unusual histopathological finding was the presence of variable amounts of microfilariae within the blood vessel lumens of different organs, including the lungs, liver, heart, and brain of six of the nine birds examined (). In the three most heavily infected birds, large numbers of microfilariae filled the lumen of blood vessels, especially in the lungs; over 300 sections of microfilariae were visible per microscopic field in the lumen of selected large vessels at a magnification of 400×(). Morphological changes associated with these microfilariae were limited to discreet accumulation of fibrinoid material within aggregates of nematode larvae (). Rare sections of 15 µm diameter unidentified nematode larvae, different from microfilariae, were also found embedded in localized granulomatous foci in the liver of two of the boreal owls with the highest microfilarial burden.
Figure 1. Extremely high microfilarial burden in the lung vessels of an emaciated boreal owl. Scale bar = 200 µm; haematoxylin phloxine saffron. Insert: numerous intertwined microfilariae have completely filled the vascular lumen.
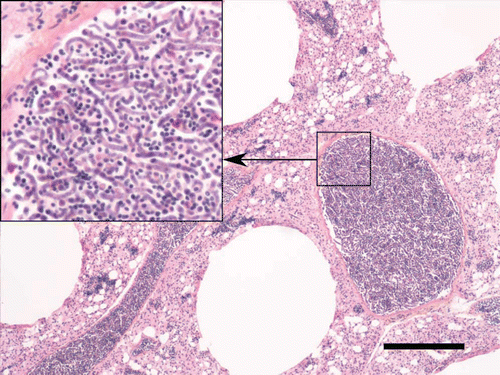
Figure 2. Aggregation of microfilariae (arrows) in a vein of the pericardium of an emaciated boreal owl. Some microfilariae are embedded in a fibrinoid material (arrowhead). Scale bar = 50 µm; haematoxylin phloxine saffron.
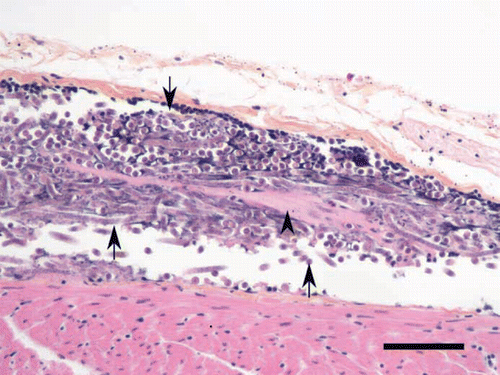
Table 1. Boreal owls submitted for histopathology to the Canadian Cooperative Wildlife Health Center—Quebec Region during winter 2008/09: intensity of microfilarial infection and summary of the morphological changes observed.
Direct microscopic examination of lung impressions revealed the presence of numerous sheathed, round-tailed, round-headed, 7 µm diameter microfilariae ranging from 140 to 280 µm in length (mean±standard deviation, 191±29 µm, n=130) ().
Discussion
Numerous species of filarioid nematodes have been reported in birds. However, very little is known about the occurrence of these parasites in owls (for example, Wood et al., Citation1943; Greiner et al., Citation1975; Bartlett et al., Citation1987; Gutiérrez, Citation1989; Murata, Citation2002; Bedin et al., Citation2007). Filarioids of the genera Pelecitus, Aproctella, Cardiofilaria, Splendidofilaria and Lemdana have been reported in Strigidae (Bartlett et al., Citation1987; Bartlett, Citation2009). Unidentified microfilaria species of undetermined significance have been reported in blood smears of live wild-captured boreal owls (Korpimaki et al., Citation1993). Scarce microfilariae were detected in one of the boreal owls submitted to the Canadian Cooperative Wildlife Health Center—Quebec Region between 2000 and 2008 (n=8 with histological examination; data not shown). Adult filarioid nematodes, which can be found in various locations including blood vessels, body cavities, viscera and subcutaneous spaces, should be examined for species identification (Bartlett, Citation2009). Since adults were not found in the present cases, a formal species identification was not possible. The adult filarioids might have been overlooked on gross examination or could have disappeared prior to the death of the birds, as they have a shorter lifespan than microfilariae (Bartlett, Citation2009). The parasite species from which the fragments of nematode larvae were observed in the liver of two owls could not be identified. The morphological characteristics of the microfilariae were similar to those of the genus Chandlerella (Bartlett, Citation2009), although this genus has not been reported in owls. Molecular techniques designed to identify the species of filarioid nematodes have been developed, but more studies are still warranted to obtain sequences of avian filarioids (Ferri et al., Citation2009; Czajka et al., Citation2012). Because of the impossibility to compare sequences, molecular analysis was not performed in the current cases.
The number of boreal owls submitted to our diagnostic facility in winter 2008/09 was substantially higher than usual. The mean number of yearly boreal owl submissions between 2000 and 2008 was 4.4 owls per year (standard deviation = 3.4). As the south of the province is far more densely inhabited than the north, the likelihood of boreal owl carcass recovery is higher in southern Québec during the winter irruptions. The 2008/09 winter was identified as an irruption winter (Savard, Citation2008), which is thought to account for the large number of boreal owls submitted in 2009. The poor nutritional condition observed in almost all boreal owls examined also coincides with a season of low prey abundance. The mean (±standard deviation) weight of North American boreal owls from Idaho has been documented as 117 (±9.8) g in males and 167 (±17.9) g in females (Dunning, Citation2007). The weight of the owls submitted for post-mortem analyses in 2009 were mildly to markedly low compared with this reference range. Consequently, starvation resulting from a shortage of food resources appears to have played a significant role in the death of the vast majority (18/21) of the boreal owls examined. The cause of mortality of the two least emaciated birds remains undetermined.
The synergy between parasites and stressors, such as detrimental weather conditions or low food abundance, can increase bird mortality (Howe, Citation1992; Daoust et al., Citation1998; Work et al., Citation2004). It is difficult to establish the extent to which the microfilariae contributed to the state of undernourishment observed. Microfilariae are generally considered a non-pathogenic agent in wild birds (Bartlett, Citation2009). Host reactions are only reported when the larval nematodes are located outside the vascular lumen (Bartlett, Citation2009). In the current most severely infected cases, fibrinoid aggregates suggestive of vascular stasis were occasionally observed in the lumen of blood vessels. Moreover, in the owls with the highest parasite burden, the presence of extremely large quantities of microfilariae circulating in the lumen of the vessels must have impaired normal circulatory dynamics and gaseous exchanges. This high parasite burden might also have had a metabolic cost for the host (Behnke et al., Citation1992; Wobeser, Citation2009). Taken together, these observations suggest that microfilariae played a role in the death of the boreal owls with the heaviest parasite burden. The current communication would therefore represent the first report of potential pathogenic effects of intraluminal microfilariae in wild birds of prey.
The reasons explaining the presence of such a large parasite load in some of these owls were not identified. The prevalence and intensity of parasitic infections in a given bird population could be influenced by variations in the abundance of the intermediate and definitive host species. Variations in arthropod populations may especially be driven by humidity and temperature variations. In the current climate change context, this may result in increased abundance of intermediate hosts, and thus in more severe infections (Laaksonen et al., Citation2010). It is also plausible that the intense parasitic infection observed was secondary to the poor nutritional state of these birds, which compromised the effectiveness of their immune system.
Complete post-mortem examination, including histological samples, of all free-ranging birds submitted in necropsy would be ideal for disease surveillance and gathering of information, but it is not always possible in practice (Wobeser, Citation2007). In the present case, the specimens sampled for histology were triaged based on their level of preservation. As large numbers of microfilariae may be recognized even in poorly preserved specimens, sampling at least the lungs of emaciated boreal owls for histology would be warranted in the future to increase knowledge on these parasites.
From a clinical point of view, wildlife rehabilitators dealing with emaciated boreal owls may want to look for circulating microfilariae. This can be performed with several methods, such as the modified Knott's test, the filtration test, the commercial enzyme-linked immunosorbent assay test for detection of Dirofilaria immitis in dogs, the quantitative buffy coat capillary tube method or direct blood smears (Echols et al., Citation2000; Mylonakis et al., Citation2004). Direct blood smear is considered insensitive for low levels of microfilaremia, sensitivity of which increases with the quantity of larvae present in peripheral blood (Mylonakis et al., Citation2004). Filtration and Knott's tests would require too much blood to be feasible in small birds (Kelly, Citation1973). The diagnostic value of the commercial enzyme-linked immunosorbent assay is not known in birds. Microscopic examination of the buffy coat extracted from a capillary tube necessitates a small amount of blood and allows for the visualization of larvae. It would thus seem the most appropriate method for boreal owls.
Whether infected owls should be treated or not is currently not known. The main concern is that the inflammation caused by dying adults following treatment may be more detrimental than non-intervention (Bartlett, Citation2009). Drugs reported to treat filarioid nematodes in birds include ivermectin and levamisole (Bartlett, Citation2009).
In a species subject to population cycles, an infectious agent that is non-pathogenic to healthy individuals, but which accelerates the death of debilitated animals during low prey abundance periods, would favour a return to balance between the number of predators and prey (Wobeser, Citation2009). In other words, the infectious agent would increase the resilience of a population to fluctuating abundance of food sources, which may benefit the species in the long term. Further studies are warranted to precisely identify the species of filarioids involved, to characterize more precisely vascular morphological changes as well as to better understand the metabolic cost of nematode infection in these small owls.
Acknowledgements
The authors would like to thank the Union Québécoise de réhabilitation des oiseaux de proie for their involvement in the transport, care, and submission of the owls presented in this communication.
References
- Bartlett , C.M. 2009 . “ Filarioid Nematodes ” . In Parasitic Diseases of Wild Birds , Edited by: Atkinson , C.T. , Thomas , N.J. and Hunter , D.B. 439 – 462 . Ames , IA : Wiley-Blackwell .
- Bartlett , C.M. and Anderson , R.C. 1981 . Occult filariasis in crows (Corvus brachyrhynchos brachyrhynchos Brehm) infected with Splendidofilaria caperata Hibler, 1964 (Nematoda: Filarioidea) . Journal of Wildlife Diseases , 17 : 69 – 77 .
- Bartlett , C.M. and Anderson , R.C. 1987 . Lemdana wernaarti n.sp. and other filarioid nematodes from Bubo virginianus and Asio otus (Strigiformes) in Ontario, Canada, with a revision of Lemdana and a key to avian filarioid genera . Canadian Journal of Zoology , 65 : 1100 – 1109 .
- Bedin , M. , Petterino , C. , Gallo , E. , Selleri , P. and Morgante , M. 2007 . Clinical pathological findings in an owl (Athene noctua) with microfilaraemia in Italy . Journal of Veterinary Medicine. A, Physiology, Pathology Clinical Medicine , 54 : 128 – 130 .
- Behnke , J.M. , Barnard , C.J. and Wakelin , D. 1992 . Understanding chronic nematode infections: evolutionary considerations, current hypotheses and the way forward . International Journal for Parasitology , 22 : 861 – 907 .
- Cheveau , M. , Drapeau , P. , Imbeau , L. and Bergeron , Y. 2004 . Owl winter irruptions as an indicator of small mammal population cycles in the boreal forest of eastern North America . Oikos , 107 : 190 – 198 .
- Cole , R.A. 1999 . Heartworm of swans and geese . In Field Manual of Wildlife Diseases: General Field Procedures and Diseases of Birds Information and Technology Report 1999–001 . Madison , WI : USGS National Wildlife Health Center, Biological Resources Division . 231 – 234
- Czajka , C. , Becker , N. , Poppert , S. , Jost , H. , Schmidt-Chanasit , J. and Krueger , A. 2012 . Molecular detection of Setaria tundra (Nematoda: Filarioidea) and an unidentified filarial species in mosquitoes in Germany . Parasites & Vectors , 5 : 14
- Daoust , P.Y. , Conboy , G. , McBurney , S. and Burgess , N. 1998 . Interactive mortality factors in common loons from Maritime Canada . Journal of Wildlife Diseases , 34 : 524 – 531 .
- Dunning , J.B. 2007 . CRC Handbook of Avian Body Masses , (2nd ed.) , 174 Boca Raton , FL : CRC .
- Echols , S.M. , Craig , T.M. and Speer , B.L. 2000 . Heartworm (Paronchocerca ciconarum) infection in 2 saddle-billed storks (Ephippiorhynchus senegalensis) . Journal of Avian Medicine and Surgery , 14 : 42 – 47 .
- Ferri , E. , Barbuto , M. , Bain , O. , Galimberti , A. , Uni , S. , Guerrero , R. , Ferté , H. , Bandi , C. , Martin , C. and Casiraghi , M. 2009 . Integrated taxonomy: traditional approach and DNA barcoding for the identification of filarioid worms and related parasites (Nematoda) . Frontiers in Zoology , 6 : 1
- Greiner , E.C. , Bennett , G.F. , White , E.M. and Coombs , R.F. 1975 . Distribution of the avian hematozoa of North America . Canadian Journal of Zoology , 53 : 1762 – 1787 .
- Gutiérrez , R.J. 1989 . Hematozoa from the spotted owl . Journal of Wildlife Diseases , 25 : 614 – 618 .
- Howe , F.P. 1992 . Effects of Protocalliphora braueri (Diptera: Calliphoridae) parasitism and inclement weather on nestling sage thrashers . Journal of Wildlife Diseases , 28 : 141 – 143 .
- Kelly , J.D. 1973 . Detection and differentiation of microfilariae in canine blood . Australian Veterinary Journal , 49 : 23 – 27 .
- Korpimaki , E. , Hakkarainen , H. and Bennett , G.F. 1993 . Blood parasites and reproductive success of Tengmalm's owls: detrimental effects on females but not on males? . Functional Ecology , 7 : 420 – 426 .
- Laaksonen , S. , Pusenius , J. , Kumpula , J. , Venäläinen , A. , Kortet , R. , Oksanen , A. and Hoberg , E. 2010 . Climate change promotes the emergence of serious disease outbreaks of filarioid nematodes . EcoHealth , 7 : 7 – 13 .
- Law , J.M. , Tully , T.N. and Stewart , T.B. 1993 . Verminous encephalitis apparently caused by the filarioid nematode Chandlerella quiscali in emus (Dromaius novaehollandiae) . Avian Diseases , 37 : 597 – 601 .
- Luna , L.G. 1968 . Manual of Histologic Staining Methods of the Armed Forces Institute of Pathology , 3rd ed , New York , NY : McGraw-Hill Book Company .
- Murata , K. 2002 . Prevalence of blood parasites in Japanese wild birds . The Journal of Veterinary Medical Science/The Japanese Society of Veterinary Science , 64 : 785 – 790 .
- Mylonakis , M.E. , Papadopoulos , E. , Koutinas , A.F. , Paitaki , C. and Leontides , L. 2004 . Comparative methodology for the detection and differentiation of circulating microfilariae of Dirofilaria immitis in the dog . Journal of Helminthology , 78 : 137 – 140 .
- Savard , M. 2008 . La Grande Visite: les incursions de chouettes nordiques dans les basses terres du Saguenay–Lac-Saint-Jean . Le Harfang , 31 : 20 – 29 .
- Simpson , V.R. , MacKenzie , G. and Harris , E.A. 1996 . Fatal microfilarial infection in red billed blue magpies (Urocissa erythrorhynchus) . The Veterinary Record , 138 : 522 – 523 .
- Wobeser , G.A. 2007 . Disease in Wild Animals: Investigation and Management , 2nd ed , Heidelberg , , Germany : Springer .
- Wobeser , A.G. 2009 . “ Parasitism: Costs and Effects ” . In Parasitic Diseases of Wild Birds , Edited by: Atkinson , C.T. , Thomas , N.J. and Hunter , D.B. 3 – 9 . Ames , IA : Wiley-Blackwell .
- Wood , S.F. and Herman , Carlton M. 1943 . The occurrence of blood parasites in birds from Southwestern United States . The Journal of Parasitology , 29 : 187 – 196 .
- Work , T.M. , Meteyer , C.U. and Cole , R.A. 2004 . Mortality in Laysan ducks (Anas laysanensis) by emaciation complicated by Echinuria uncinata on Laysan Island, Hawaii, 1993 . Journal of Wildlife Diseases , 40 : 110 – 114 .