Abstract
Stressors may influence chicken susceptibility to pathogens such as Salmonella enterica. Feed withdrawal stress can cause changes in normal intestinal epithelial structure and may lead to increased attachment and colonization of Salmonella. This study aimed to investigate modulatory effects of epigenetic modification by feed restriction on S. enterica serovar Enteritidis colonization in broiler chickens subjected to feed withdrawal stress. Chicks were divided into four groups: ad libitum feeding; ad libitum feeding with 24-h feed withdrawal on day 42; 60% feed restriction on days 4, 5, and 6; and 60% feed restriction on days 4, 5, and 6 with 24-h feed withdrawal on day 42. Attachment of S. Enteritidis to ileal tissue was determined using an ex vivo ileal loop assay, and heat shock protein 70 (Hsp70) expression was evaluated using sodium dodecyl sulphate-polyacrylamide gel electrophoresis and western blotting. Feed withdrawal stress increased S. Enteritidis attachment to ileal tissue. However, following feed withdrawal the epigenetically modified chickens had significantly lower attachment of S. Enteritidis than their control counterparts. A similar trend with a very positive correlation was observed for Hsp70 expression. It appears that epigenetic modification can enhance resistance to S. Enteritidis colonization later in life in chickens under stress conditions. The underlying mechanism could be associated with the lower Hsp70 expression in the epigenetically modified chickens.
Introduction
One strategy to cope with stress is epigenetic adaptation, defined as non-genetic changes that occur within short critical developmental phases during prenatal or early-postnatal ontogeny, and that affect gene expression and establish lifelong adaptation to an actual environment (de Kloet et al., Citation2005; Leventopoulos et al., Citation2007; Soleimani et al., Citation2011). The epigenetic response has been successfully modulated by early-age thermal manipulation (Yahav & Hurwitz, Citation1996) and feed restriction (Liew et al., Citation2003; Zulkifli et al., Citation2011). Neonatal feed restriction can improve resistance to marble spleen disease (Zulkifli et al., Citation1994) and infectious bursal disease (Liew et al., Citation2003) in heat-stressed chickens at market age. However, information on the link between neonatal stress and resistance to bacterial infections in chickens is lacking.
Salmonella enterica is considered a leading bacterial food-borne pathogen in humans (Lynch et al., Citation2006). Environmental stress has been shown to be a factor that may induce colonization of enteric pathogens, facilitate horizontal transmission of pathogens between animals, increase pathogen shedding, and contribute to carcass contamination during processing (Mulder, Citation1995; Poppe, Citation1999; Rychlik & Barrow, Citation2005). S. enterica serovar Enteritidis colonization and shedding is increased by short-term stresses attributed to heat, cold, feed and water restriction, and handling in layers (Nakamura et al., Citation1994). Moreover, acute heat stress and feed restriction can cause changes in the normal intestinal epithelial structure (Burkholder et al., Citation2008), which may lead to increased attachment and colonization of S. Enteritidis. Induction of heat shock proteins, as a conserved stress response to thermal and non-thermal stressors, may act as epithelial surface receptors for pathogen binding (Wampler et al., Citation2004). The expression of heat shock protein 70 (Hsp70) is a classical sign of stress in animals because it is the physical manifestation of specific genes that are induced to combat stressors (Craig & Gross, Citation1991; Kregel, Citation2002). There is a physiological and immunological cost for this response, and it is paid in reduced synthesis of structural proteins and suppressed immune response by suppressing the production of inflammatory mediators such as interleukin-8 (IL-8) (Malago et al., Citation2002, Citation2005). Following uptake of S. Enteritidis by macrophages, Hsp70 and heat shock protein 20 are two of the most strongly induced genes, with induction ratios more than five-fold (Eriksson et al., Citation2003). Hsp70 is also the most abundant stress protein (Craig & Gross, Citation1991) and thus might play an important role in alteration of immune response under stress conditions.
Our previous studies (Soleimani et al., Citation2011; Zulkifli et al., Citation2011) have characterized a resilient stress response in neonatally feed-restricted chickens, as documented by lower Hsp70 expression. On this basis, the objective of the following study was to investigate the possible role of neonatal feed restriction in conferring resistance to S. Enteritidis infection under stress conditions.
Materials and Methods
Ethical note
The study was undertaken following the guidelines of the Animal Care and Use Committee, Universiti Putra Malaysia on animal ethics (UPM/FPV/PS/3.2.1.551/AUP-R24).
Birds and housing
A total of 48 1-day-old broiler chicks were obtained from a local hatchery and individually wing-tagged, and were randomly assigned in groups of 12 to four pens in an environmentally controlled room. Chicks were subjected to either ad libitum feeding (C); ad libitum feeding with 24-h feed withdrawal on day 42 (CFR); 60% feed restriction on days 4, 5, and 6 (FR); or 60% feed restriction on days 4, 5, and 6 with 24-h feed withdrawal on day 42 (FRFR). Feed restriction was 60% of the feed intake of group C on the previous day. This feed restriction approach has been shown to have no influence on production parameters of broiler chickens at marketing age (Liew et al., Citation2003; Zulkifli et al., Citation2011).
Challenge microorganism
A primary poultry isolate of S. Enteritidis, phage type 13A, was obtained from the Department of Veterinary Pathology and Microbiology, Faculty of Veterinary Medicine, Universiti Putra Malaysia. This isolate was resistant to nalidixic acid (NA) (N8878; Sigma-Aldrich). The stock culture was grown in Luria Bertani (LB) broth containing 20 µg NA to inhibit the growth of other bacteria and was stored at −80 °C with 20% (v/v) added glycerol. Fresh cultures were grown statically overnight in LB-NA, transferred to fresh LB-NA and grown overnight for the challenge study. Bacterial cells were harvested by centrifugation at 6000×g at 4°C for 15 min and were washed three times in equal volumes of sterile phosphate-buffered saline (PBS). Cells were re-suspended in PBS and spectrophotometrically quantitated to 1.5×108 colony-forming units/ml. The inoculum was serially diluted and plated on an XLT-4 agar plate containing 20 µg NA to confirm the actual number of cells in the inoculum.
Ileal loop assay
Cloacal swabs were taken from all chickens on day 42 to ensure the absence of S. Enteritidis in the flock. Swab contents were directly enriched in Rappaport-Vassiliadis (CM0669; Oxoid, Hampshire, UK) at 41°C for 24 h, mixed thoroughly and plated on XLT-4 agar (CM1061; Oxoid) for 24 h at 37°C.
At day 42, chickens were killed at random by cervical dislocation and a 10-cm section of the ileum was taken, starting 3 cm proximal to the ileo-cecal junction, from each chicken for an ex vivo ileal challenge assay. Tissue sections were immediately placed in ice-cold Dulbecco's modified Eagle medium with l-glutamine (DMEM; Sigma-Aldrich, St Louis, Missouri, USA), kept on ice and used for the ex vivo S. Enteritidis challenge assay. The organ culture procedure of Burkholder et al. (2008) was used with some modifications. Briefly, the ileal sections were sealed using 35 mm dialysis clips at one end, and inoculated with 6 ml S. Enteritidis culture suspended in DMEM. The other open end of each section was also sealed, the exterior was rinsed with PBS and ileal loops were kept on ice. All ileal loops were incubated in 100 ml DMEM for 1 h at 41°C in a rotary water bath. Following incubation, ileal contents were removed, the interior and exterior of each section were rinsed with gentle flushing of PBS, and tissues were homogenized and serially diluted in buffered peptone water. One hundred microlitres of each dilution was placed on XLT-4 plates containing 20 µg/ml NA and incubated at 37°C for 24 h. Colonies were confirmed by biochemical tests on triple sugar–iron agar and lysine–iron agar and further identified as S. Enteritidis serologically using Salmonella O antiserum group D, factors 1, 9 and 12 (McReynolds et al., Citation2006). Another 10-cm section of the ileum was taken separately from each chicken as for the negative salmonella challenge assay and the same organ culture procedure was applied as mentioned earlier. These ileal loops were inoculated only with DMEM.
Heat shock protein 70 analysis
A portion of ileal tissue after incubation and rinsing by PBS was used for Hsp70 quantification using sodium dodecyl sulphate-polyacrylamide gel electrophoresis and western blotting. Ileal samples (0.3 g) were homogenized in a Potter Elvehjem tissue grinder (Sigma, St Louis, Missouri, USA) using 3 ml chilled Tris buffer (20 mM Tris, pH 7.5; 0.75 M NaCl; 2 mM 2-mercaptoethanol) with 10 µl/ml protease inhibitor cocktail (Sigma) and centrifuged at 23,000×g for 45 min at 4°C. The protein concentrations of the supernatants were quantified by the bicinchoninic acid protein assay kit (Sigma-Aldrich) with bovine serum albumin as the standard. Total protein (25 µg) was loaded and separated on 10% polyacrylamide gels containing sodium dodecyl sulphate using a mini gel apparatus. Gels were electrophoresed at 120 V until the tracking dye reached the base of the gel. The fractionated proteins were transferred to polyvinylidene difluoride membranes (MSI, Westborough, Massachusetts, USA) using a semi-dry electrophoretic transfer cell. The non-specific binding sites were blocked by 10 ml cold blocking buffer (Kirkegaard and Perry Labs Inc., Gaithersburg, Maryland, USA) for 60 min. The membranes were incubated for 1 h with 5 ml blocking buffer containing antiserum (monoclonal mouse antibody, catalogue number ab6535; Abcam, Cambridge, Massachusetts, USA) against Hsp70 in a 1:20,000 dilution. Following 1 h of incubation, the blots were washed three times (5 min each) with 10 ml cold Tris-buffered saline Tween 20. Blots were incubated in a horseradish-peroxidase-conjugated rabbit anti-mouse secondary antibody for 30 min in a 1:40,000 dilution (catalogue number ab6728; Abcam). After rinsing with cold Tris-buffered saline Tween 20 (three times, 5 min each), the blots were exposed to an enhanced chemiluminescent substrate (ChemiGlow; Alpha Innotech, San Leandro, California, USA). Visualization of bands was performed using a chemiluminescent imaging system (FluorChem 5500; Alpha Innotech) followed by quantification of the band summation density by Image-Pro Plus image processing and analysis software (Media Cybernetics, Silver Spring, Maryland, USA).
Statistical analysis
Data were analysed by one-way analysis of variance in a completely randomized design. Colony-forming units for S. Enteritidis were logarithmically transformed prior to analysis to achieve homogeneity of variance and were expressed as log10 colony-forming units. The relationship between Hsp70 expression and S. Enteritidis count was assessed using correlation analysis. Significant differences were further separated using Duncan's multiple-range tests.
Results
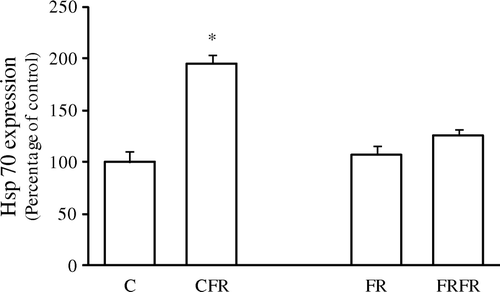
Feed withdrawal stress increased S. Enteritidis colonization in both ad libitum fed and neonatally feed restricted chickens compared with the control (). However, following 24-h feed withdrawal, the FRFR chickens had significantly lower colonization of S. Enteritidis than their CFR counterparts. Interestingly, a similar trend was observed in ileal Hsp70 expression (). Correlation analysis showed that there was a significant relationship between S. Enteritidis colonization and Hsp70 expression (P<0.0001). As for the negative salmonella challenge assay of this study, results showed that Hsp70 expression among the treatment groups was the same as for the salmonella challenged assay and all samples including cloacal swabs were negative for the presence of S. Enteritidis (data not shown).
Figure 1. Effect of 24-h feed withdrawal on in vitro S. Enteritidis attachment to ileal tissue of 42-day-old chickens. C, ad libitum feeding; CFR, ad libitum feeding with 24-h feed withdrawal on day 42; FR, 60% feed restriction on days 4, 5 and 6; FRFR, 60% feed restriction on days 4, 5 and 6 with 24-h feed withdrawal on day 42. a, b, cMean±standard error of the mean with no common letters differ at P<0.05. n=12.
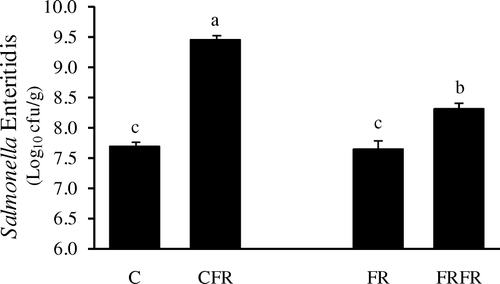
Figure 2. Effect of 24-h feed withdrawal on ileal Hsp70 expression upon in vitro S. Enteritidis challenge in 42-day-old chickens. C, ad libitum feeding; CFR, ad libitum feeding with 24-h feed withdrawal on day 42; FR, 60% feed restriction on days 4, 5 and 6; FRFR, 60% feed restriction on days 4, 5 and 6 with 24-h feed withdrawal on day 42. The data (mean±standard error of the mean) obtained by scanning and volume integration of Hsp70 bands are expressed as the percentage of ad libitum feeding control chickens. *Statistically significant differences, P<0.05. n=12.
Discussion
A striking finding of this study is that the altered level of colonization was accompanied by a lower Hsp70 expression. The Hsp70 functions in the protection and folding of newly synthesized protein and the refolding of recently denatured proteins (Lindquist & Craig, Citation1988). It also imparts an anti-inflammatory role by inhibiting IL-8 production from macrophages and intestinal epithelial cells (Malago et al., Citation2005; Nemeth et al., Citation2006). IL-8 is known as a chemokine that recruits polymorphonuclear leukocytes to inflammatory sites—a response that is vital to the inflammatory diarrhoea caused by salmonella infection (Lee et al., Citation2000). Apart from the physical barrier, the instant innate immune response following pathogen, including S. Enteritidis, invasion is inflammation through cytokine production. These mediators are aimed at eliminating the pathogens and protecting the cells, and their down-regulation before resolving the inflammation stress would tend to favour the Salmonella colonization. This down-regulation is mediated, at least in part, by way of Hsp70 synthesis (Yoo et al., Citation2000; Malago et al., Citation2005; Nemeth et al., Citation2006). This evidence reports a significant correlation between Hsp70 expression and S. Enteritidis colonization and supports our findings on the relationship between Hsp70 expression and S. Enteritidis count. It is therefore compelling to hypothesize that part of the mechanism imparted by feed withdrawal to increase the S. Enteritidis invasion involves down-regulation of IL-8 production, either directly via induction of Hsp70 synthesis or indirectly via glucocorticoid production and their immunosuppressive effect. Interestingly, as documented by the lower Hsp70 expression, feed withdrawal was less stressful in epigenetically modified chickens. This positive effect of epigenetic modification is in agreement with previous reports (Meaney et al., Citation1988; Yahav & Hurwitz, Citation1996; Champagne et al., Citation2008; Soleimani et al., Citation2012) and may last for life (Soleimani et al., Citation2011). These studies showed that epigenetic modification by feed restriction set a robust reactive response in young and senescent quails, allowing the development of adaptive, healthy and resilient phenotypes. In our study, it seems that the lower Hsp70 expression in epigenetically modified chickens enables them to have an efficient inflammatory response (probably by higher macrophages activity and IL-8 production) and consequently lower S. Enteritidis colonization. However, although epigenetic modification alleviated feed withdrawal stress, the direct link between production of IL-8 and reduction of S. Enteritidis colonization has not been established. In conclusion, it appears that an epigenetic approach using neonatal feed restriction can enhance resistance to S. Enteritidis colonization later in life in chickens under stress conditions. The underlying mechanism could be associated with the lower Hsp70 expression in the epigenetically modified chickens.
Acknowledgements
This work was supported by the Ministry of Higher Education, Malaysia under the Fundamental Research Grant Scheme.
References
- Burkholder , K.M. , Thompson , K.L. , Einstein , M.E. , Applegate , T.J. and Patterson , J.A. 2008 . Influence of stressors on normal intestinal microbiota, intestinal morphology, and susceptibility to Salmonella Enteritidis colonization in broilers . Poultry Science , 87 : 1734 – 1741 .
- Champagne , D.L. , Bagot , R.C. , van Hasselt , F. , Ramakers , G. , Meaney , M.J. , de Kloet , E.R. , Joels , M. and Krugers , H. 2008 . Maternal care and hippocampal plasticity: evidence for experience-dependent structural plasticity, altered synaptic functioning, and differential responsiveness to glucocorticoids and stress . The Journal of Neuroscience , 28 : 6037 – 6045 .
- Craig , E. and Gross , C.A. 1991 . Is hsp 70 the cellular thermometer? . Trends in Biochemical Sciences , 16 : 35 – 40 .
- de Kloet , E.R. , Sibug , R.M. , Helmerhorst , F.M. and Schmidt , M. 2005 . Stress, genes and the mechanism of programming the brain for later life . Neuroscience and Biobehavioral Reviews , 29 : 271 – 281 .
- Eriksson , S. , Lucchini , S. , Thompson , A. , Rhen , M. and Hinton , J.C. 2003 . Unravelling the biology of macrophage infection by gene expression profiling of intracellular Salmonella enterica . Molecular Microbiology , 47 : 103 – 118 .
- Kregel , K.C. 2002 . Heat shock proteins: modifying factors in physiological stress responses and acquired thermotolerance . Journal of Applied Physiology , 92 : 2177 – 2186 .
- Lee , C.A. , Silva , M. , Siber , A.M. , Kelly , A.J. , Galyov , E. and McCormick , B.A. 2000 . A secreted Salmonella protein induces a pro-inflammatory response in epithelial cells, which promotes neutrophil migration . Proceedings of the National Academy of Sciences USA , 97 : 12283 – 12288 .
- Leventopoulos , M. , Ruedi-Bettschen , D. , Knuesel , I. , Feldon , J. , Pryce , C.R. and Opacka-Juffry , J. 2007 . Long-term effects of early life deprivation on brain glia in Fischer rats . Brain Research , 1142 : 119 – 126 .
- Liew , P.K. , Zulkifli , I. , Hair-Bejo , M. , Omar , A.R. and Israf , D.A. 2003 . Effects of early age feed restriction and thermal conditioning on heat shock protein 70 expression, resistance to infectious bursal disease and growth in male broiler chickens subjected to chronic heat stress . Poultry Science , 82 : 1879 – 1885 .
- Lindquist , S. and Craig , E.A. 1988 . The heat-shock proteins . Annual Review of Genetics , 22 : 631 – 677 .
- Lynch , M. , Painter , J. , Woodruff , R. and Braden , C. 2006 . Surveillance for foodborne-disease outbreaks–United States, 1998–2002 . MMWR Surveillance Summaries , 55 : 1 – 42 .
- Malago , J.J. , Koninkx , J.F.J.G. and van Dijk , J.E. 2002 . The heat shock response and cytoprotection of the intestinal epithelium . Cell Stress and Chaperones , 7 : 191 – 199 .
- Malago , J.J. , Koninkx , J.F.J.G. , Tooten , P.C.J. , van Liere , E.A. and van Dijk , J.E. 2005 . Anti-inflammatory properties of heat shock protein 70 and butyrate on Salmonella-induced interleukin-8 secretion in enterocyte-like Caco-2 cells . Clinical & Experimental Immunology , 141 : 62 – 71 .
- Meaney , M.J. , Aitken , D.H. , van Berkel , C. , Bhatnagar , S. and Sapolsky , R.M. 1988 . Effect of neonatal handling on age-related impairments associated with the hippocampus . Science , 239 : 766 – 768 .
- McReynolds , J.L. , Moore , R.W. , Kubena , L.F. , Byrd , J.A. , Woodward , C.L. , Nisbet , D.J. and Ricke , S.C. 2006 . Effect of various combinations of alfalfa and standard layer diet on susceptibility of laying hens to Salmonella Enteritidis during forced molt . Poultry Science , 85 : 1123 – 1128 .
- Mulder , R.W.A.W. 1995 . Impact of transport and related stresses on the incidence and extent of human pathogens in pig meat and poultry . Journal of Food Safety , 15 : 239 – 246 .
- Nakamura , M. , Nagamine , N. , Takahashi , T. , Suzuki , S. , Kijima , M. , Tamura , Y. and Sato , S. 1994 . Horizontal transmission of Salmonella enteritidis and effect of stress on shedding in laying hens . Avian Disease , 38 : 282 – 288 .
- Nemeth , E. , Fajdiga , S. , Malago , J. , Koninkx , J. , Tooten , P. and van Dijk , J. 2006 . Inhibition of Salmonella-induced IL-8 synthesis and expression of Hsp70 in enterocyte-like Caco-2 cells after exposure to non-starter lactobacilli . International Journal of Food Microbiology , 112 : 266 – 274 .
- Poppe , C. 1999 . “ Epidemiology of Salmonella enterica serovar Enteritidis ” . In Salmonella enterica Serovar Enteritidis in Humans and Animals. Epidemiology, Pathogenesis and Control , Edited by: Saeed , A. 3 – 18 . Ames , IA : Iowa State University Press .
- Rychlik , I. and Barrow , P.A. 2005 . Salmonella stress management and its relevance to behaviour during intestinal colonisation and infection . FEMS Microbiology Reviews , 29 : 1021 – 1040 .
- Soleimani , A.F. , Zulkifli , I. , Omar , A.R. and Raha , A.R. 2011 . Neonatal feed restriction modulates circulating levels of corticosterone and expression of glucocorticoid receptor and heat shock protein 70 in aged Japanese quail exposed to acute heat stress . Poultry Science , 90 : 1427 – 1434 .
- Soleimani , A.F. , Zulkifli , I. , Hair-Bejo , M. , Omar , A.R. and Raha , A.R. 2012 . The role of heat shock protein 70 in resistance to Salmonella Enteritidis in broiler chickens subjected to neonatal feed restriction and thermal stress . Poultry Science , 91 : 340 – 345 .
- Wampler , J.L. , Kim , K.P. , Jaradat , Z. and Bhunia , A.K. 2004 . Heat shock protein 60 acts as a receptor for the Listeria adhesion protein in Caco-2 cells . Infection and Immunity , 72 : 931 – 936 .
- Yahav , S. and Hurwitz , S. 1996 . Induction of thermotolerance in male broiler chickens by temperature conditioning at an early age . Poultry Science , 75 : 402 – 406 .
- Yoo , C. , Lee , S. , Lee , C. , Kim , Y.M. , Han , S.K. and Shim , Y. 2000 . Anti-inflammatory effect of heat shock protein induction is related to stabilization of IκBα through preventing IκB kinase activation in respiratory epithelial cells . The Journal of Immunology , 164 : 5416 – 5423 .
- Zulkifli , I. , Dunnington , E.A. , Gross , W.B. and Siegel , P.B. 1994 . Food restriction early or later in life and its effect on adaptability, disease resistance, and immunocompetence of heat stressed dwarf and non-dwarf chickens . British Poultry Science , 35 : 203 – 214 .
- Zulkifli , I. , Soleimani , A.F. , Khalil , M. , Omar , A.R. and Raha , A.R. 2011 . Inhibition of adrenal steroidogenesis and heat shock protein 70 induction in neonatally feed restricted broiler chickens under heat stress condition . Archiv für Geflügelkunde , 75 : 246 – 252 .