Abstract
The aerial parts of the plant Artemisia annua contain essential oils having antimicrobial properties against Clostridium perfringens Type A, the causal agent for necrotic enteritis in broilers. In two experiments, the influence of increasing dietary concentrations of dried A. annua leaves (0, 5, 10 and 20 g/kg) and n-hexane extract from fresh A. annua leaves (0, 125, 250 and 500 mg/kg) on broiler performance was investigated. Dried plant material decreased feed intake and body weight in a dose-dependent manner, and 10 and 20 g/kg diet tended to improve the feed conversion ratio. The n-hexane extract also reduced feed intake, but broiler weight tended to decrease only at the highest dietary concentration. The feed conversion ratio tended to improve when birds received 250 and 500 mg/kg n-hexane extract. In a third experiment, a necrotic enteritis disease model was applied to investigate the effect of the dietary addition of dried A. annua leaves (10 g/kg on top) or n-hexane extract of A. annua (250 mg/kg) on the severity of the disease in broilers. The addition of n-hexane extract reduced the intestinal C. perfringens numbers and the severity of the disease-related small intestinal lesions. Over the infection period from day 17 to day 27, birds supplemented with the n-hexane extract gained more weight than both the challenged control birds and birds receiving dried plant material. The results indicate that n-hexane extracts derived from A. annua can modulate the course of necrotic enteritis and compensate to a certain extent for the disease-associated weight losses.
Introduction
Necrotic enteritis is a severe gastrointestinal disease in broiler chickens caused by toxin producing Clostridium perfringens Type A. The ability of the C. perfringens strain to produce alpha-toxin, and in particular NetB, seems crucial for triggering necrotic enteritis (Abildgaard, Citation2010; Keyburn et al., Citation2010). Acute disease outbreaks can result in significant production losses due to increased mortality. The subclinical form of the disease is characterized by reduced broiler growth, poor feed utilization and wet litter causing skin burns and food pad lesions, thus impairing bird welfare. Parasite infection with Eimeria spp. disrupting the integrity of the intestinal wall during formation, as well as high dietary concentrations of animal protein (e.g. fish meal), are important predisposing factors that support intestinal C. perfringens proliferation (Dahya et al., Citation2006; Gholamiandehkordi et al., Citation2007; Pedersen et al., Citation2008).
Many ionophore antibiotics added to the feed as anticoccidials are not only effective in the control of coccidiosis in broilers but also have an antibacterial effect against certain Gram-positive bacteria including C. perfringens, making these substances suitable for protection against necrotic enteritis (Lanckriet et al., Citation2010). The ban of ionophores as feed additives has been considered in the European Union as of 2012, but a final decision on this matter has been postponed due to an obvious lack of alternative disease control strategies.
Artemisia annua (Sweet Wormwood) belongs to the plant family of Asteraceae and has long been known to provide a number of different natural products being of major interest for human and veterinary medicine. Important constituents of A. annua are essential oil components, which are present in leaves in concentrations between 0.04 and 1.9% on a dry matter basis. Among the major components in the oil are camphor, 1,8-cineole and artemisa ketone (Tzenkova et al., Citation2010). Essential oils often show strong antimicrobial properties in vitro, which is the reason why these compounds have gained much attention regarding their potential as alternatives to antibiotic growth promoters (Botsoglou et al., Citation2002; Lee et al., Citation2004; Brenes & Roura, Citation2010; Franz et al., Citation2010). Essential oil blends have shown promising results with respect to reduction of C. perfringens colonization and proliferation (Mitsch et al., 2004; Ivarsen et al., Citation2010; Timbermont et al., Citation2010). Another important constituent of A. annua is the sesquiterpene lactone artemisinin. Through the last decade, artemisinin and its derivatives have been used as key components in the combination therapies against human malaria, caused by protozoan blood parasites (Plasmodium falciparum, Plasmodium vivax) belonging to the class of sporozoa (Douglas et al., Citation2010, Nosten & White, Citation2007; White, Citation2008). Eimeria spp. causing coccidiosis in poultry belong to the same protozoan class, and the use of dried leaf parts of A. annua as well as artemisinin in protection against and therapy for this disease has been reported (Allen et al., Citation1997; Allen et al., Citation1998; Arab et al., Citation2006; Brisibe et al., Citation2008; Del Cacho et al., Citation2010). The results of these studies generally indicate that the dietary addition of artemisinin and dried leaves protects against weight gain losses and reduces oocyst output and lesion scores after oral infection with coccidia. An advantage of artemisinin is a high margin of safety; even at high oral single doses, artemisinin seems to have only few adverse side effects in broilers (Arab et al., Citation2009).
Bearing the potential effect of A. annua against bacteria and parasites in mind, dry plant material or plant extract could possibly be used in the control of necrotic enteritis. The aim of the present study was to investigate whether dried leaves of A. annua and plant extracts (dichloromethane or n-hexane) could be applied as feed additives, taking their effect on broiler production results, on the composition and activity of the intestinal microbiota, and on the course of necrotic enteritis in a broiler disease model into consideration.
Materials and Methods
Plant material and extracts
Seeds of A. annua (cv. Artemis, F2 seeds; Mediplant, Switzerland) were raised as transplants in a greenhouse and 6 weeks later (June 2009 and 2010) were planted in a sandy loam field at Aarslev Research Station, Denmark (55°18′ N, 10° 27′ E). The soil was fertilized with 100 kg N/ha before planting. No artificial irrigation was performed, and weeding was done manually. Leaves for feeding and extract experiments were harvested in September. The leaves were air dried at 25°C, threshed from the main stems and stored in airtight plastic bags until feed preparation. The leaves for extraction were harvested at the same time and deep frozen at −20°C until extraction. For in vitro testing, fresh frozen leaves (100 g) of A. annua were extracted at room temperature by maceration with 300 ml n-hexane, dichloromethane or methanol for 24 h. After filtering, the extracts were subsequently taken to dryness at 40°C under reduced pressure to obtain the crude extracts. Additionally, 27 kg fresh frozen aerial parts of A. annua were extracted at room temperature by maceration with n-hexane for 8 h. The shorter extraction time, compared with the 24-h extraction used in the in vitro experiment, was for practical reasons. Chemical analysis of the in vitro and the in vivo n-hexane extracts revealed that the metabolite profiles were the same in both extracts. In total, approximately 200 l n-hexane were used for extraction, which after drying finally resulted in 200 g crude extract for the broiler experiment. The concentration of artemisinin in the n-hexane extract was determined by ultra-high-performance liquid chromatography, and the content of essential oil components was determined by gas chromatography (GC)–mass spectrometry and GC as described below.
Analysis of A. annua extracts for essential components
A Thermo Scientific DSQ II mass spectrometer operated at an ionization potential of 70 eV coupled to a Trace GC Ultra gas chromatograph fitted with a Phenomenex ZB-WAXplus column (30 m×0.25 mm×0.25 µm, Phenomenex, Værløse, Denmark) was used for the separation and identification of essential oil components. The oven temperature was programmed from 45 to 60°C at 4°C/min, from 60 to 64°C at 2°C/min, from 64 to 90°C at 2.6°C/min, from 90 to 110°C at 4°C/min, from 110 to 150°C at 1.50°C/min with an isothermal step at 150°C for 2 min, and finally from 150 to 230°C at 3°C/min with an isothermal step at 230°C for 15 min. Helium was applied as a carrier gas at a flow rate of 1.4 ml/min. The injection volume was 1 µl with a split ratio of 50:1. Identification of compounds was based on the comparison with mass spectra suggested by the National Institute of Standards and Technology mass spectrometry database and/or verified by comparison of GC retention indices and mass spectra of authentic reference compounds. For quantification of essential oil components, a Hewlett-Packard 6890 Series Plus GC (Agilent, Hørsholm, Denmark) equipped with a flame ionization detector operating at 230°C was used. The analytical conditions (column, carrier gas flow, temperature programme, injection volume) were the same as described above for the GC–mass spectrometry analysis. Essential oil components were quantified from the flame ionization detector peak areas relative to that of the internal standard (4-methyl-1-pentanol). The response factor was set to 1 for all compounds.
Quantification of artemisinin in A. annua extracts
Artemisinin was quantified using ultra-high-performance liquid chromatography coupled with a charged aerosol detector on a Dionex UltiMate 3000 high-performance liquid chromatography system. Separations were carried out on an Agilent eclipse C18 column (5 µm particle size; 150 mm×4.6 mm i.d., Agilent, Hørsholm, Denmark). The column temperature was 35°C and the mobile phases consisted of solvent A (water with 0.05% formic acid) and solvent B (acetonitrile with 0.05% formic acid). Separations were performed using the following solvent gradient: 0 to 3 min 1% B, 9 min 17% B, 10 min 17% B, 16 min 21% B, 18 min 21% B, 25 min 26% B, 29 min 26% B, 41 min 42% B, 44 min 47% B, 46 min 46% B, 54 min 54% B, 62 min 99% B, 66 min 99% B, 72 min 1% B and 76 min 1% B. The flow rate was 0.8 ml/min and the injection volume was 10 µl. The concentration of artemisinin was determined by an external calibration curve made from a stock solution of an authentic standard of artemisinin dissolved in acetonitrile.
In vitro antimicrobial activity of A. annua extracts against C. perfringens
To find the most potent extract in terms of C. perfringens growth inhibition, the in vitro antimicrobial activity of the extracts towards C. perfringens strain 200302-1-1-Ba (Abildgaard et al., Citation2010) was evaluated by assessing their minimal inhibitory concentrations (MIC) at pH 6.8. This particular microbial strain had been isolated from diseased broilers (PFGE type 48; Nauerby et al., Citation2003) and was found to produce both alpha-toxin and NetB toxin (Abildgaard et al., Citation2010). The plant extracts were initially dissolved in dimethyl sulphoxide, and serial two-fold dilutions were made in Anaerobe Basal Broth (CM0957; Oxoid, Roskilde, Denmark) using 96-well microplates. Concentrations ranged from 0.005 to 0.66 mg/ml for the dichloromethane extract, from 0.012 to 0.74 mg/ml for the n-hexane extract, and from 0.007 to 0.67 mg/ml for the methanol extract. Twenty microlitres of an overnight culture of C. perfringens strain 200302-1-1-Ba in Anaerobe Basal Broth (107 bacteria/ml) were added to 250 µl medium in the wells of the microplates, which were incubated in an anaerobic cabinet at 38°C. After 24 h of incubation, microbial growth was inspected visually and recorded spectrophotometrically by measuring absorbance at 650 nm. The analyses were carried out in triplicate.
Effect of A. annua feed supplements on broiler performance and intestinal microbiota
Two broiler feeding experiments with identical design were carried out over periods of 35 days to investigate the dose-dependent growth response to increasing amounts of dried A. annua leaves (Experiment 1) or a plant extract (Experiment 2). Each experiment involved 160 1-day-old female broilers (Ross 308). The birds were housed in floor pens with a floor area of 1.7 m2 covered with wood shavings. Temperature and humidity in the stable were managed according to the practical standard. Feed and water were provided ad libitum. During the first 7 days, the chickens received a standard compound mash diet () without any plant supplementation.
Table 1. Ingredients and calculated composition of the basic diet.
The first experiment included four experimental groups (four replicates with 10 broilers per replicate) receiving ground dried plant leaves (particle size 2 mm) in concentrations of 0, 5, 10 and 20 g/kg on top of the standard diet. Two samples of dried plant material harvested in two consecutive years from the same area were analysed for their content of dry matter, ash, fat nitrogen, starch as well as soluble and insoluble non-starch polysaccharides and lignin. The dry matter content was determined by drying at 103°C for 20 h. Ash was analysed according to method 923.03 (AOAC, Citation1990b), and fat (hydrochloric acid–fat) was extracted with diethyl ether after acid hydrolysis (Stoldt, Citation1952). Protein (N×6.25) was determined by the Kjeldahl method 984.13 (AOAC, Citation1990a) using a Kjell-Foss 16200 autoanalyser Foss, Hillerød, Denmark), and gross energy was determined by a LECO AC 300 automated calorimeter system 789-500 (LECO, St. Joseph, Michigan, USA). Starch was analysed by the enzymatic-colorimetric method of Bach Knudsen (Citation1997). Soluble and insoluble non-starch polysaccharides as well as lignin were determined as described by Bach Knudsen (Citation1997). The results are shown in .
Table 2. Chemical composition of dried ground A. annua leaves.
In the second experiment, the basic diet was supplemented with 0, 150, 250 and 500 mg n-hexane extract/kg feed for the four experimental groups, respectively. The n-hexane extract showed the highest in vitro activity against C. perfringens (see Results section) and was therefore chosen for the in vivo experiment. The n-hexane extract was added to the dietary soy oil fraction and extensively mixed before incorporation into the diet.
The birds were individually weighed at 1 day old and at 35 days and the feed intake was recorded. At day 35, three chickens from each pen were killed by cervical dislocation and the contents of ileum and caecum were sampled and pooled by intestinal segment. Coliform bacteria, lactose-negative enterobacteria, lactic acid bacteria, C. perfringens and total anaerobic bacteria were enumerated as described by Engberg et al. (Citation2004).
Necrotic enteritis disease model
The necrotic enteritis experiment (Experiment 3) was carried out with a total of 320 male broilers (Ross 308) housed in floor pens (20 birds/pen) with a floor area of 1.7 m2 covered with wood shavings. The birds were wing banded and divided into four experimental groups (four replicate pens/group) including a non-challenged control, a challenged control group and two challenged groups supplemented with either dried A. annua leaves (10 g/kg on top of the diet) or n-hexane extract (250 mg/kg). As described by Gholamiandehkordi et al. (Citation2007) and Petersen et al. (Citation2008), the infection model involved a feed shift (see further details below) to a feed providing high dietary fish meal concentrations, an overdose of a live attenuated coccidiosis vaccine, as well as an inoculation of the feed and of the individual bird with C. perfringens by direct crop instillation. Until day 7, all birds received the non-supplemented control diet (), and were then offered the respective experimental diets. From day 17 to day 21, all birds received the experimental diets where the soybean meal fraction had been substituted by fish meal. The challenged groups received the fish meal diets after addition of 200 ml of an overnight culture of C. perfringens, strain 200302-1-1-Ba, in Anaerobe Basal Broth (CM0957; Oxoid) at a final concentration of 106 bacteria/g feed on days 17, 18, 19, and 20, whereas the diet of the non-challenged control group was supplemented with 200 ml pure Anaerobe Basal Broth. On day 18, the challenged groups received a 10-fold overdose of a live attenuated coccidiosis vaccine (Paracox®-5 vet.; MSD Animal Health, Milton Keynes, UK), which was given orally in a volume of 0.5 ml. The non-challenged control group received the same volume of tap water orally. On day 21, the challenged groups received 1 ml of an overnight culture of C. perfringens (107/ml Anaerobe Basal Broth), whereas the non-challenged group received 1 ml pure Anaerobe Basal Broth. On day 22, all birds shifted again to the respective experimental soy bean diets, which were used throughout the further trial.
On each of days 22, 24 and 27, five birds were randomly selected from each pen and killed by cervical dislocation. Small intestinal lesions were scored according to the method of Keyburn et al. (Citation2006) on a scale from 0 (no pathological changes) to 6 (severe diffuse necrosis). In the ileal and caecal contents (pooled samples of five birds), the numbers of C. perfringens were counted on tryptose sulphite cycloserine agar incubated anaerobically for 24 h at 38°C. On day 27, the pH of pooled intestinal samples from five birds was measured and the numbers of coliform bacteria, lactose-negative enterobacteria, lactic acid bacteria and total anaerobic bacteria were enumerated as described by Engberg et al. (Citation2004). The concentrations of organic acids in intestinal samples were measured using gas chromatography as described in detail by Canibe et al. (Citation2007).
Individual body weights of 40 birds/dietary group were registered on day 1, on day 17 before feed shift and on day 27 when recovering from infection.
The described bird experiments complied with the guidelines of the Danish Ministry of Justice with respect to animal experimentation and care of animals under study.
Statistical methods
Mean values for each pen and each day were used for the statistical analyses of all response variables.
The effect of diet on performance as well as bacterial numbers measured only once per bird or pen was analysed using the general linear models procedure of SAS® (SAS Institute, Citation1990) according to the following general model:
where Y is the observed response, µ is the overall mean, α is the effect of diet, i=1, 2, 3, 4, and ϵ is the residual error. Results obtained from measurements in the intestinal content of the ileum and caeca were analysed separately for each segment.
The effect of diet on lesion score and C. perfringens counts over a range of days was analysed according to the following normal mixed model:
where Y=observed response, µ=overall mean, α=effect of diet, i=1, 2, 3, 4; γ=effect of day, j=1, 2, 3; (αγ)=effect of the interaction between diet and day; U=variance component that accounts for the correlation between measurements made on the same pen (p=pen) on days 22, 24 and 27, ~N (0, ), and ϵ=the residual error, ~N (0,
) represents the unexplained random error, which means that a compound symmetry variance structure was assumed. Results are reported as least-square means with their standard error. When there was an overall effect of diet at an alpha of P≤0.05, differences between means were compared pair-wise using a t-test.
Results
Antimicrobial activity of A. annua extracts against C. perfringens
The MIC values of A. annua plant extracts based on extraction with n-hexane and dichloromethane were 0.185 mg/ml and 0.270 mg/ml, respectively. The methanol extract showed the poorest antimicrobial activity with a MIC value above 0.670 mg/ml. The n-hexane extract had the strongest in vitro antimicrobial activity against C. perfringens strain 200302-1-1-Ba and was therefore chosen as feed additive in the subsequent broiler experiments, where this extract was included at a concentration of 250 mg/kg feed.
The artemisinin concentration of the applied n-hexane extract was 75 g/kg and essential oil components were present in concentrations of 900 g/kg. Camphor constituted with 60 g/kg the major essential oil fraction of the extract (results not shown).
Artemisia annua feed supplements, broiler performance and intestinal microbiota
The dietary supplementation of dried plant material resulted in a dose-related decrease in body weight (). Birds receiving 10 g and 20 g/kg feed weighed respectively 103 g and 165 g less than the non-supplemented control group (P diet = 0.013). The addition of 5 g/kg feed did not result in growth depression. The feed intake of birds receiving 10 g and 20 g/kg feed () was 322 g and 410 g lower compared with the feed intake of the non-supplemented group and the group receiving 5 g/kg feed (P diet = 0.009). There was a trend towards an improved feed conversion ratio (P diet = 0.099), when broilers received 10 g and 20 g dried plant material/kg feed ().
Table 3. Production results of broilers receiving increasing dietary concentrations of dried and ground A. annua leaves (Experiment 1) or n-hexane extract (Experiment 2).
The dietary addition of n-hexane extract in concentrations of 500 mg/kg feed tended to decrease the broiler weights () in the range of 70 to 127 g compared with the other groups (P diet = 0.105). The feed intake of the broilers decreased approximately 100 to 200 g each time the plant extract concentrations in the diet were doubled (P diet = 0.011). There was a trend towards an improved feed conversion ratio when birds were fed with 250 and 500 mg/kg n-hexane extract (P diet = 0.069).
The counts of lactic acid bacteria, coliform bacteria, lactose-negative enterobacteria, enterococci and anaerobe bacteria in the contents of the ileum and caeca (data not shown) were similar to earlier observations and close to the levels observed for the non-challenged control group in Experiment 3 (see ). The numbers of C. perfringens were 4.1 to 5.8 log colony-forming units/g digesta. The influence of Artemisia plant supplements on these dominant bacterial groups was generally limited and the addition of dried plant material to the broiler feed had no significant influence on the bacterial counts (P>0.05). The supplementation of 500 mg/kg n-hexane extract reduced the numbers of anaerobic bacteria in the caecal content (P diet = 0.004), and the numbers of C. perfringens were reduced in caecal contents of broiler chickens supplemented with 125 mg/kg plant extract (P=0.044).
Table 4. Numbers of selected bacteria in the ileum and caeca contents (log colony-forming units/g digesta) of C. perfringens challenged broilers on day 27.
Artemisia annua feed supplements and necrotic enteritis
Birds developed small intestinal lesions of varying severity when applying the described disease model (). However, broiler mortality in relation to the infection was low. A total of five chickens died after infection, two chickens of the non-challenged control, two of the group supplemented with dry plant material and one chicken of the group supplemented with n-hexane extract. None of the feed additives could prevent the development of necrotic enteritis in the challenged birds. Small intestinal lesion scores increased from day 22 to day 24 and then decreased again at day 27 (). Throughout the experiment, no intestinal lesions were observed in the non-challenged control group. The dietary supplementation of 250 mg/kg n-hexane extract resulted in the lowest lesion scores, especially on days 22 and 27 (P day < 0.001). In all groups, the highest numbers of C. perfringens in intestinal content were found on day 24. The intestinal C. perfringens numbers were lowest in the non-challenged control group (P<0.001). In the ileal content, the C. perfringens numbers were lower in the group supplemented with the n-hexane extract as compared with the challenged control group. In the caecal content, lower C. perfringens numbers were found in birds receiving the n-hexane extract compared with both challenged groups. Statistical analysis using the contrast statement showed a tendency to lower the C. perfringens counts in the ileum (P=0.10) and caeca (P=0.11) of both groups supplemented with Artemisia compared with the challenged control group (data not shown). The numbers of coliform bacteria in the ileal content at 27 days were between 370 and 540 times higher (P diet = 0.011) in challenged birds as compared with the non-challenged birds (). There was no effect of the Artemisia supplements on lactic acid bacteria, coliform bacteria, lactose-negative enterobacteria (P>0.05) and anaerobic bacteria in the ileal contents. The highest numbers of lactic acid bacteria (P diet = 0.025) were counted in caecal contents of challenged non-supplemented birds (). The dietary addition of the n-hexane extract reduced the numbers of lactic acid bacteria to the level of the non-challenged control group. Although the numbers of coliform bacteria were over 10 times lower in caecal contents of birds receiving the Artemisia supplements, significant results were not achieved (P=0.368). There was no difference (P>0.05) between the groups regarding pH (6.6 to 6.8), and the concentration of formic acid (3.0 to 4.2 mmol/kg) and acetic acid (6.7 to 9.9 mmol/kg) in the ileal content (data not shown). Isovaleric acid (0.3 to 0.4 mmol/kg) was only detected in the ileum of challenged birds. Ileal lactic acid concentrations (17.1 to 26.6 mmol/kg) were numerically higher (P=0.178) in birds receiving the supplementation of dried plant as compared with the other groups (27 vs. 18 mmol/kg). No differences between groups (P>0.05) were observed with respect to pH (6.4 to 6.6), acetic acid (58.2 to 70.6 mmol/kg) and propionic acid (3.9 to 4.8 mmol/kg) in the caecal content (data not shown). Butyric acid concentrations (11.7 to 20.1 mmol/kg) tended to be lower in birds receiving the plant supplements (12.2 vs. 18.2, P=0.059). Lactic acid (1.1 to 5.4 mmol/kg) was only detected in the caecal content of challenged birds (data not shown).
Table 5. Small intestinal lesion scores and numbers of C. perfringens in ileum and caeca of broilers following challenge with C. perfringens strain 200302-1-1-Ba.
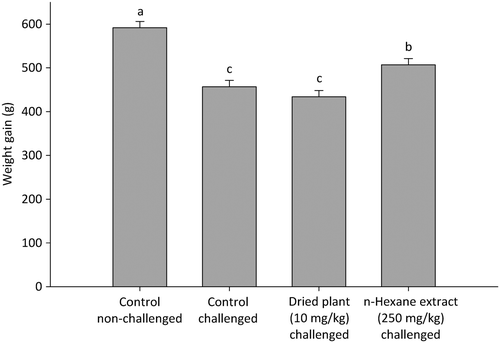
The infection caused a severe depression of the body weight gain (). In the period from day 17 to day 27, the non-challenged group gained ~130 g more weight compared with the challenged control group and the challenged group receiving the dry plant. Challenged birds supplemented with the n-hexane extract had a higher weight gain than the other challenged groups (P<0.05).
Discussion
Choice of A. annua extract
It has previously been shown that a number of essential oils possess both in vitro and in vivo antimicrobial activity against C. perfringens (Si et al., Citation2009; Timbermont et al., Citation2010), and A. annua is particularly rich in camphor showing a strong growth inhibition of C. perfringens specifically (Si et al., Citation2009).When comparing the MIC values of the n-hexane, dichloromethane and methanol extracts from the leaves of A. annua, it is obvious that the choice of extraction solvent has a strong influence on the quality and quantity of the extracted bioactive compounds. The n-hexane (chosen for in vivo broiler trials) and dichloromethane extracts were superior to the methanol extract with respect to their growth inhibition of C. perfringens, which may be explained by the stronger polarity of methanol, thus resulting in lower concentrations of non-polar constituents in the extract.
Dried A. annua material, broiler performance and intestinal microbiota
The maximum concentration of dried plant material (20 g/kg diet) was defined based on the chemical composition (), revealing significant amounts of non-soluble fibre and lignin that are expected to affect broiler performance negatively. The observation that feeding dried A. annua leaves at 10 and 20 g/kg diet suppressed broiler weight and feed intake and tended to improve the feed conversion ratio does not agree with the findings of Brisibe et al. (Citation2008), who observed an increased body weight gain and feed consumption in broilers when the dried plant was given in concentrations as high as 100 and 200 g/kg diet. However, the protein content of the plant material in that study was found to be considerably higher than that of the plant material used in the present study (197 g vs. 30 g/kg dry matter). In another study, the supplementation of a broiler starter diet with dried A. annua in a concentration of up to 50 g/kg did not result in adverse effects on broiler performance during the first week (Allen et al., Citation1997). The observation that dried A. annua material had no influence on the commensal intestinal microbiota has to our knowledge not been reported by others.
Artemisia annua n-hexane extract, broiler performance and intestinal microbiota
Regarding the influence of A. annua extracts on broiler performance, the literature is scarce. Different plant-derived essential oils have antimicrobial and antioxidant properties and their application as feed additives in broiler nutrition has been studied for some time (Lee et al., Citation2003; Lee et al., Citation2004; Brenes & Roura, Citation2010; Tiihonen et al., Citation2010). It is reasonable to assume that the effects of essential oils on broiler performance vary depending on the type of the oil as well as the quality and quantity of the active components (Tiihonen et al., Citation2010). The majority of experimental results indicate a reduced feed intake at largely unchanged body weight gain or final body weight, thus resulting in an improved feed conversion ratio, when feeding essential oils (Brenes & Roura, Citation2010). Our results generally confirm these findings when the plant extract was fed in concentrations of 250 mg/kg feed (). However, the dietary addition of 500 mg/kg n-hexane extract resulted in a strong reduction of the feed intake and the body weight as compared with the control, which may be due to the very distinct smell and bitter taste of the plant extract. Due to the content of antimicrobial components, it was expected that the dietary addition of A. annua would influence the composition of the intestinal microbiota of the ileum and caeca. There is not much information from the literature on the effect of essential oils on the composition of the intestinal microbiota. However, most studies investigating the antimicrobial activity of different essential oils against different bacteria agree that essential oils are slightly more active against Gram-positive bacteria than against Gram-negative bacteria (Brenes & Roura, Citation2010). However, in the present experiment it was found that the effect of A. annua was relatively limited when the infection pressure was low. Only the n-hexane extract at concentrations of 500 mg/kg feed reduced the numbers of anaerobic bacteria in caecal contents.
Artemisia annua feed supplements and necrotic enteritis
The applied necrotic enteritis disease model was chosen taking the published models of Gholamiandehkordi et al. (Citation2007) and Petersen et al. (Citation2008) into consideration and combining individual oral inoculation and infection via the feed. We generally observed higher small intestinal lesion scores than reported by the above-mentioned authors; however, although the observed lesions were quite severe, the disease-related mortality was still low.
The most severe small intestinal lesions and the highest numbers of C. perfringens in the ileal and caecal contents were observed on day 24, whereafter the birds recovered. This pattern was not influenced when using the dried A. annua material. However, throughout the experimental period, birds supplemented with the n-hexane extract had lower small intestinal lesion scores and lower ileal and caecal C. perfringens numbers, indicating that the dietary addition of A. annua extract modulates the severity and course of the disease in terms of a later disease onset and a faster recovery of the birds.
The disease challenge was associated with a change in the intestinal microbial composition on day 27 (). The observed post-challenge increase in ileal Escherichia coli numbers is probably due to the increased amount of mucus and intestinal epithelial cell debris serving as a substrate for these bacteria. Further, the challenged birds seemed to harbour higher numbers of lactic acid bacteria in the caeca as compared with the non-challenged control (), which may be explained by impaired digestive function of the small intestine in relation to the disease, allowing higher amounts of undigested carbohydrates to enter the caeca. The n-hexane extract decreased the numbers of lactic acid bacteria in the caeca to the level of those found in the non-challenged group. This supports the idea that the A. annua extract helps to maintain the ileal digestive functions after the challenge and agrees with the lower small intestinal lesion scores found in these birds. Further, these results generally indicate that the A. annua extract acts against Gram-positive bacteria (e.g. C. perfringens and lactic acid bacteria) rather than Gram-negative bacteria (e.g. E. coli), in accordance with the conclusions of Brenes & Roura (Citation2010). Changes of the intestinal microbiota are also indicated by the pattern of organic acids found in the ileum and caeca. The branch-chained isovaleric acid is a fermentation product originating from the degradation of protein and was only observed in the ileum of challenged birds, indicating the extensive bacterial protein hydrolysation in relation to necrotic enteritis.
Butyric acid is a short-chain fatty acid produced by different strictly anaerobic bacteria (including clostridia) living in the caeca, and a number of beneficial properties in relation to gastrointestinal health have been reported (Hamer et al., Citation2008) including reduced colonization and shedding of Salmonella (Van Immerseel et al., Citation2005). However, whether A. annua interferes in this respect via growth inhibition of beneficial butyrate producers, as indicated by our results, remains a matter for further investigation.
Finally, the fact that dietary supplementation with the n-hexane extract counteracted the severe weight gain depression, observed for the other challenged groups (P<0.05), strongly indicates that the n-hexane extract of A. annua leaves can to a certain extent compensate for production losses related to necrotic enteritis.
In conclusion, it can be stated that dried leaves of A. annua depressed broiler growth and feed intake even at low dietary concentrations (1 to 2%) and did not beneficially contribute to the health condition of broilers exposed to a necrotic enteritis challenge. The dietary addition of an n-hexane A. annua extract (250 mg/kg feed) depressed feed intake and improved feed conversion efficiency. Applying the described necrotic enteritis disease model, the A. annua extract showed a positive influence on the course of the disease and compensated to a certain extent for disease-associated weight losses.
Acknowledgements
The authors kindly acknowledge the project funding of the Danish Council for Strategic Research. The authors wish to express their gratitude to Karin Durup, Trine Poulsen, Mona Dinsen, Thomas Rebsdorf and Kirsten Lund Balthzersen for skillful technical assistance.
References
- Abildgaard , L. , Sondergaard , T.E. , Engberg , R.M. , Schramm , A. and Hojberg , O. 2010 . In vitro production of necrotic enteritis toxin B, NetB, by netB-positive and net B-negative Clostridium perfringens originating from healthy and diseased broiler chickens . Veterinary Microbiology , 144 : 231 – 235 .
- Allen , P.C. , Lydon , J. and Danforth , H.D. 1997 . Effects of components of A. annua on coccidia infections in chickens . Poultry Science , 76 : 1156 – 1163 .
- Allen , P. C. , Danforth , H.D. and Augustine , P.C. 1998 . Dietary modulation of avian coccidiosis . International Journal for Parasitology , 28 : 1131 – 1140 .
- AOAC (Association of official analytical chemists) 1990 . Method No. 923.03. Chapter 32 , p. 777 Official Methods of Analysis, , 15th ed . Arlington , VA : AOAC .
- Arab , H.A. , Rahbari , S. , Rassouli , A. , Moslemi , M.H. and Khosravirad , F. 2006 . Determination of artemisinin in Artemisia sieberi and anticoccidial effects of the plant extract in broiler chickens . Tropical Animal Health and Production , 38 : 497 – 503 .
- Arab , H.A. , Mardjanmehr , S.H. , Shahbazfar , A. , Rassouli , A. , Abdollahi , M. and Nekouie , O. 2009 . Toxicopathologic effects of artemisinin in broiler chickens following a single oral dose: an LD50 study . International Journal of Poultry Science , 8 : 808 – 812 .
- Bach Knudsen , K.E. 1997 . Carbohydrate and lignin contents of plant materials used in animal feeding . Animal Feed Science and Technology , 6 : 319 – 338 .
- Botsoglou , N.A. , Florou-Paner , P. , Christaki , E. , Fletouris , D.J. and Spais , A.B. 2002 . Effect of dietary oregano essential oil on performance of chickens and on iron-induced lipid oxidation of breast, thigh and abdominal fat tissues . British Poultry Science , 43 : 223 – 230 .
- Brenes , A. and Roura , E. 2010 . Essential oils in poultry nutrition: main effects and modes of action . Animal Feed Science and Technology , 158 : 1 – 14 .
- Brisibe , E.A. , Umoren , U.E. , Owai , P.U. and Brisibe , F. 2008 . Dietary inclusion of dried A. annua leaves for management of coccidiosis and growth enhancement in chickens . African Journal of Biotechnology , 7 : 4083 – 4092 .
- Canibe , N. , Højberg , O. , Badsberg , J.H. and Jensen , B.B. 2007 . Effect of feeding fermented liquid feed and fermented grain on gastrointestinal ecology and growth performance in piglets . Journal of Animal Science , 85 : 2959 – 2971 .
- Dahiya , J.P. , Wilkie , D.C. , Van Kessel , A.G. and Drew , M.D. 2006 . Potential strategies for controlling necrotic enteritis in broiler chickens in post-antibiotic era . Animal Feed Science and Technology , 129 : 60 – 88 .
- Del Cacho , E. , Gallego , M. , Francesch , M. , Quílez , J. and Sánchez-Acedo , C. 2010 . Effect of artemisinin on oocyst wall formation and sporulation during Eimeria tenella infection . Parasitology International , 59 : 506 – 511 .
- Douglas , N.M. , Anstey , N.M. , Angus , B.J. , Nosten , F. and Price , R.N. 2010 . Artemisinin combination therapy for vivax malaria . The Lancet Infectious Diseases , 10 : 405 – 416 .
- Franz , C. , Baser , K.H.C. and Windisch , W. 2010 . Essential oils and aromatic plants in animal feeding-a European perspective. A review . Flavour and Fragrance Journal , 25 : 327 – 340 .
- Engberg , R.M. , Steenfeldt , S. , Hedemann , M.S. and Jensen , B.B. 2004 . The influence of whole wheat and xylanase on broiler performance and microbial composition and activity in the digestive tract . Poultry Science , 83 : 925 – 938 .
- Gholamiandehkordi , A.R. , Timbermont , L. , Lanckriet , A. , Van Den Broeck , W. , Petersen , K. , Dewulf , J. , Pasmans , F. , Haesebrouck , F. , Ducatelle , R. and Van Immerseel , F. 2007 . Quantification of gut lesions in a subclinical necrotic enteritis model . Avian Pathology , 36 : 375 – 382 .
- Hamer , H.M. , Jonkers , D. , Venema , K. , Vanhoutvin , S. , Troost , FJ. and Brummer , R.J. 2008 . Review article: the role of butyrate on colonic function . Alimentary and Pharmacology & Therapeutics , 27 : 104 – 119 .
- Ivarsen , E. , Fretté , X.C. , Christensen , K.B. , Engberg , R.M. , Kjaer , A. , Jensen , M. , Grevsen , K. & Christensen , L.P. 2010 . Evaluation of the efficiency of essential oil components from A. annua as an antimicrobial against Clostridium perfringens in poultry . Abstracts Book of 6th Conference on Medicinal and Aromatic Plants of Southeast European Countries (CMAPSEEC) ; Antalya , Turkey . Phcog. Mag . 6 (Suppl.) : p. 126 .
- Keyburn , A.L. , Sheedy , S.A , Ford , M.E. , Williamson , M.M. , Awad , M.M. , Rood , J.I. and Moore , R.J. 2006 . Alpha-toxin of Clostridium perfringens is not an essential virulence factor in necrotic enteritis in chickens . Infection and Immunity , 74 : 6496 – 6500 .
- Keyburn , A.L. , Yang , X. , Bannam , T.L. , Van Immerseel , F. , Rood , J.I. and Moore , R.J. 2010 . Association between avian necrotic enteritis and Clostridium perfringens strains expressing NetB toxin . Veterinary Research , 41 : 21
- Keyburn , A.L. , Bannam , T. , Moore , R.J. and Rood , J.I. 2010 . NetB, a pore-forming toxin from necrotic enteritis strains of Clostridium perfringens . Toxins , 2 : 1913 – 1927 .
- Lanckriet , A. , Timbermont , L. , De Gussem , M. , Marien , M. , Vancraeynest , D. , Haesebrouck , F. , Ducatelle , R. and Van Immerseel , F. 2010 . The effect of commonly used anticoccidials and antibiotics in a subclinical necrotic enteritis model . Avian Pathology , 39 : 63 – 68 .
- Lee , K.-W. , Everts , H. , Kappert , H.J. , Yeom , K.-H. and Beynen , A.C. 2003 . Dietary carvacrol lowers body weight gain but improves feed conversion in female broiler chickens . Journal of Applied Poultry Research , 12 : 394 – 399 .
- Lee , K.-W. , Everts , H. and Beynen , A.C. 2004 . Essential oils in broiler nutrition . International Journal of Poultry Science , 3 : 738 – 752 .
- Mcdevitt , R.M. , Brooker , J.D. , Acamovic , T. and Sparks , N.H.C. 2006 . Necrotic enteritis; a continuing challenge for the poultry industry . World′s Poultry Science Journal , 62 : 221 – 247 .
- Nauerby , B. , Pedersen , K. and Madsen , M. 2003 . Analysis by pulsed-field gel electrophoresis of the genetic diversity among Clostridium perfringens isolates from chickens . Veterinary Microbiology , 94 : 257 – 266 .
- Nosten , F. and White , N. J. 2007 . Artemisinin-based combination treatment of Falciparum malaria . American Journal of Tropical Medicine and Hygiene , 77 ( suppl 6 ) : 181 – 192 .
- Pedersen , K , Friis-Holm , L.B , Heuer , O.E. , Wong , D.L.F. and Nauerby , B. 2008 . Reproducible infection model for Clostridium perfringens in broiler chickens . Avian Diseases , 52 : 34 – 39 .
- Sas Institute 1990 SAS/STAT Guide for Personal Computers , Cary , NC : SAS Institute Inc .
- Si , W. , Ni , X , Gong , J. , Yu , H. , Tsao , R. , Han , Y. and Chambers , J.R. 2009 . Antimicrobial activity of essential oils and structurally related synthetic food additives towards Clostridium perfringens . Journal of Applied Microbiology , 106 : 213 – 220 .
- Stoldt , W. 1952 . Vorschlag zur Vereinheitlichung der Fettbestimmung in Lebensmitteln (Suggestion to standardise the determination of fat in foodstuffs) . Fetten, Seifen, Anstrichmittel , 54 : 206 – 207 .
- Tiihonen , K. , Kettunen , H. , Bento , M.H.L. , Saarinen , M. , Lahtinen , S. , Ouwehand , A.C. , Schulze , H. and Rautonen , N. 2010 . The effect of feeding essential oils on broiler performance and gut microbiota . British Poultry Science , 51 : 381 – 392 .
- Timbermont , L. , Lanckriey , A. , Nollet , N. , Schwarzer , K. , Haesebrouck , Ducatelle , R. & Van Immerseel , F. 2010 . Control of Clostridium perfringens-induced necrotic enteritis in broilers by target-released butyric acid, fatty acids and essential oils . Avian Pathology , 39 , 117 – 121 .
- Tzenkova , R. , Kamenarska , Z. , Draganov , A. and Atanassov , A. 2010 . Composition of Artemisia annua essential oil obtained from growing wild in Bulgaria . Biotechnology & Biotechnological Equipment , 24 : 1833 – 1835 .
- Van Immerseel , F. , Boyen , F. , Gantois , I. , Timbermont , L. , Bohez , L. , Pasmans , F. , Haesebrouck , F. and Ducatelle , R. 2005 . Supplementation of coated butyric acid in the feed reduces colonization and shedding of Salmonella in poultry . Poultry Science , 84 : 1851 – 1856 .
- White , N.J. 2008 . Quinghaosu (Artemisinin): the price of success . Science , 320 : 330 – 334 .