Abstract
The anticoccidial effects of Galla Rhois (GR) powder, which contains a major tannin-derived component of 52.7%, were evaluated in chickens following oral infection with Eimeria tenella. One-day-old chickens were assigned to five groups (control, unsupplemented, GR 0.5% supplemented [GRS 0.5%], GRS 1.0% [GRS 1.0%] and salinomycin supplemented [SS]). The chickens were fed a standard diet supplemented or not supplemented with GR or salinomycin for 10 days prior to infection. The birds received the supplemented diets continuously until 10 days post infection. The effects of GR on a E. tenella infection were evaluated by several parameters, including body weight gain, feed intake, oocyst excretion, bloody diarrhoea, and lesion scores. Infected chickens on the GRS and SS diets had a relatively moderate body weight loss (reduction ratio < 15%) and improved feed conversion. GRS and SS chickens produced significantly fewer faecal oocysts (P<0.05) and showed milder bloody diarrhoea compared with the E. tenella-infected control group. Furthermore, the lesion scores of both the GRS 0.5% and GRS 1.0% groups were significantly lower than the scores of the unsupplemented group on day 5 post infection. The lesion scores for the GR groups were similar to the scores for the SS group. In conclusion, this study suggests that GR appears to be as efficacious as salinomycin against E. tenella infection. GR supplementation leads to a reduction in infected chickens, although infected chickens are still affected compared with the uninfected control group. GR-based diets may be beneficial in preventing or treating coccidial infections in poultry.
Introduction
Avian coccidiosis is a common parasitic infection that seriously affects chickens, and can cause great economic losses in the poultry industry (Dalloul & Lillehoj, Citation2006). This disease is caused by seven species of Eimeria that infect the intestine and are disseminated via ingestion of sporulated oocysts, resulting in reduced growth performance (Min et al., Citation2004; Dalloul & Lillehoj, Citation2006).
During the past several years, several studies have investigated the anticoccidial effects of various chemotherapeutics that have been noted for their use as dietary supplements for coccidiosis management. Many anticoccidial feed additives are well established and frequently used (Campbell, Citation2008; De Pablos et al., Citation2010). Although coccidiosis can be controlled by chemotherapeutic programmes, the constant use and misuse of anticoccidial drugs have contributed to the emergence of drug-resistant strains in the poultry industry (Chapman, Citation1997,Citation1998; Allen & Fetterer, Citation2002; Williams, Citation2002). Additionally, because the accumulation of drug residues in poultry products could pose a risk to consumers (Abbas et al., Citation2011), there are limits on the use of anticoccidial drugs. As such, new demands have emerged for alternative eradication strategies for avian coccidiosis. To control the emergence of drug-resistant strains, alternative natural drugs have been tested and are provided alongside current drugs. This additional treatment has led to an increase in the cost of poultry products. Treating poultry with plant extracts may relieve complications from coccidiosis because these are natural products that potentially contain compounds that could mitigate the development of resistance (Youn & Noh, Citation2001; Du & Hu, Citation2004; Naidoo et al., Citation2008).
Galla Rhois (GR) has long been used in traditional Asian medicine to treat diarrhoea, persistent coughing and spontaneous perspiration in man because this product has antidiarrhetic, astringent and haemostatic properties. GR is a harmless natural material (Gao et al., Citation2000) that contains a number of tannin-derived components, including methyl gallate and gallic acid (Chen et al., Citation2006; Djakpo & Yao, Citation2010). Gallotannins are a class of hydrolysable tannin polymers formed from gallic acid, which has antifungal and antiviral properties (Aziz et al., Citation1998; Pettinari et al., Citation2006; Kratz et al., Citation2008). Methyl gallate has a recognized growth-inhibiting activity against Escherichia coli that does not negatively influence the growth of lactic acid-producing bacteria (Ahn et al., Citation1998; Bae et al., Citation1998; Kang et al., Citation2008).
Although various classes of natural components have been investigated as alternate supplementations for coccidiosis in chickens, the effects of GR on Eimeria infections in vivo have not been investigated. Therefore, the aim of this study is to confirm the anticoccidial activity of GR in vivo against an experimental infection with Eimeria tenella in chickens.
Materials and Methods
Management of the experimental birds
Fertilized eggs were generated from the mating of female Rhode Island Red chickens and male Korean native chickens. The birds were hatched at the National Livestock Research Institute (Daejeon, Korea). The chickens in each group were reared coccidia-free in individual cages under routine conditions with free access to water and a commercial broiler feed formulated without anticoccidial agents. Constant artificial light for 24 h was maintained for the duration of the experiment, and the temperature was initially set at 31 to 32°C on the first day and followed by weekly reductions of 2°C until the end of the experiment.
Experimental compounds
GR powder was obtained from Samkwang Bioscience (Jeonbuk, Korea), who produced the powder from plant material and analysed its components as previously described (Ahn et al., Citation1998). Briefly, plant material (1 kg) dried in an oven at 60°C for 3 days was twice extracted with methanol at room temperature, the residue was removed by filtration (Toyo filter paper no. 2; Toyo Roshi Kaisha, Ltd., Tokyo, Japan) and the filtrate was concentrated using vacuum rotary evaporation (Iwai Co., Tokyo, Japan) followed by freezing dry to powder. The composition of the crude extracted powder was analysed using chromatography on a silica gel column (70–230 mesh; Merck, Darmstadt, Germany) and fractionation on a preparatory high-performance liquid chromatography column (Delta Prep 4000; Waters, Ontario, Canada). Tannins account for 52.7% of the total composition of GR, and methyl gallate and gallic acid comprised 16.4% and 4.3% of that, respectively. Both GR powder and sodium salinomycin (Inetfeed, Korea), a known anticoccidial drug, were supplied in standard diets for 10 days before the challenge infection with E. tenella. The chickens were fed the supplemented diets until the termination of the experiment.
Corn-based and soybean-based starter diets without any antibiotic additives were formulated as standard diets in accordance with the broiler diet used in Korea, with corn as the principal cereal and soybean meal as the protein concentrate to meet the National Research Council (NRC) nutrient requirements for broiler chickens (NRC, Citation1994). Briefly, the formulated starter diets are composed of 53.44% corn, 33.65% soybean meal, 4.16% corn gluten meal, 4.68% soybean oils, and 3.57% other ingredients. The calculated analysis values of the diets were 3100 kcal/kg metabolizable energy, 22% crude protein, 1.10% lysine, 0.05% methionine, 0.87% methionine plus cystine, 1.00% calcium and 0.50% available protein.
Experimental design for Eimeria infection and supplementation
One hundred 1-day-old chicks were divided into five groups of 20 birds each, which were randomly further subdivided into five subgroups such that the final arrangement yielded 25 cages that contained four chicks each.
Groups were given the following supplementations: Group 1 (control), unsupplemented, uninfected control group (n=20); Group 2 (untreated), unsupplemented, infected group (n=20); Group 3 (GR 0.5% supplemented [GRS 0.5%]), GR-supplemented (0.5%), infected group (n=20); Group 4 (GR 1.0% supplemented [GRS 1.0%]), GR-supplemented (1.0%), infected group (n=20); and Group 5 (Salinomycin supplemented [SS]), sodium salinomycin-supplemented (60 parts/106), infected group (n=20).
All supplementations started 10 days before infection and were continuously maintained during the experiment. The GR powder was added at the desired proportion to the standard feed meal for broiler chickens. The chickens did not receive any vaccinations during the experimental period.
For the infection, E. tenella strain 291-7 isolated from chickens in Korea (Gyeongsang National University, Jinju, Korea) and E. tenella oocysts propagated in specific pathogen-free chickens were used. Prior to infection, the virulence of the initial stock of oocysts was evaluated. The faeces of the specific pathogen-free chickens were collected, and new oocysts were purified. Next, the oocysts were incubated in a 2% potassium dichromate solution for 48 h at 30°C in a water bath to induce sporulation. After sporulation, the oocysts were stored in a refrigerator at 4°C for less than 4 weeks. Immediately prior to infection, the oocysts were cleared by flotation on 5.25% sodium hypochlorite solution and were washed three times with sterile phosphate-buffered saline. The number of sporulated oocysts was counted and diluted to a final concentration of 1×105 oocysts/ml. The chickens were orally infected with 5×104 sporulated oocysts of E. tenella by inserting a 5 cm oesophageal cannula into the crop of each chick at 10 days of age. The uninfected groups received the same volume (0.5 ml) of sterile phosphate-buffered saline. The birds were placed in wire-floored grower cages and monitored daily for signs of infection, which were defined as the excretion of oocysts in the faeces and concomitant bloody diarrhoea (Holdsworth et al., Citation2004).
Faecal materials as excreted droppings from each subgroup in each cage were collected in their entirety from day 6 to day 10 post infection (p.i.), at 2 p.m. daily. The number of oocysts was determined by two counts of duplicate, serially diluted aliquots of faecal homogenates using a McMaster counting chamber. The number of oocysts per gram of faeces (OPG) was calculated using the following formula:
Feed intake and weight gain were measured during the experimental period four times, at day 10 pre infection, and at days 0, 5 and 10 p.i. The feed conversion ratio (FCR) was calculated using the following formula:
Evaluation of supplementation effectiveness
To evaluate the efficacy of the supplementations, we recorded various indicators, such as BWG, relative weight gain (RWG) and lesion scores (Chapman, Citation1998):
BWG and RWG were analysed by weighing the chickens every 5 days during the experimental period.
To determine lesion scores, five chickens from each group were selected randomly and killed at days 5 and 10 p.i. The caecum of each chicken was observed, and the severity of the lesions was scored as one of five ranks between 0 and 4 based on the epithelial colour, fluid accumulation and overall general appearance of the intestine (serosal thickness, mucosal erosion, dilation and similar factors) according to the method of Johnson & Reid (Citation1970).
The bloody diarrhoea score for faecal materials as excreted droppings from each group was determined on a scale from 0 to 4 using previously described methods (Youn & Noh, Citation2001). Zero is considered normal, whereas 1, 2, 3 and 4 correspond to 25, 26 to 50, 51 to 75 or over 75% blood in the faeces, respectively, according to the judgement of an experienced observer.
The data of BWG, FI, FCR and bloody diarrhoea score were obtained from a total of 20 chickens in five subgroups of four chickens each until day 5 p.i. After the five chickens from each group were killed on day 5 p.i., all data were obtained from the remaining 15 chickens in five subgroups of three chickens each until day 10 p.i.
Statistical analysis
Means and analyses of variance for BWG, FI, FCR, lesion scores and oocyst excretion were statistically analysed using the general linear models procedure of the SAS software (SAS Institute, Citation1999). Group OPG counts were analysed after a natural log transformation to rectify for heterogeneity of variance and to obtain approximately normally distributed data (Snedecor & Cochran, Citation1980). Lesion scores and oocyst excretion were analysed with a Mood–Brown median test to estimate the significance of the differences between the five groups (Mood et al., Citation1974; Jean & Subhabrata, Citation2003).
The results of measured indicators are given as mean values ±standard error of deviation for the different groups at different times of periods during the experiment. Differences were considered significant at P<0.05.
Results
Evaluation of body weight gain, feed intake, relative weight gain and feed conversion ratio
The BWG, RWG, FI and FCR of the chickens in this study are presented in . Significant differences existed for BWG among the five groups from day 5 to day 10 p.i. BWGs measured immediately prior to infection showed that no significant differences existed between the groups. In the BWG and RWG analyses, both the BWG and RWG values for the GRS 0.5% and GRS 1.0% groups were higher than the values in the unsupplemented group but were lower than the values in the uninfected control group. The values were almost equivalent to the values of the unsupplemented group ().
Table 1. Effects of supplementation with GR on body weight gain, relative weight gain, feed intake and feed conversion ratios of chickens at days 5 and day 10 p.i. with E. tenella.
In the FI analysis, FI values for the GRS 1.0% group were notably elevated at days 5 and 10 p.i. compared with the unsupplemented group. Compared with the uninfected control group, FI was slightly elevated at day 5 p.i. but was reduced by day 10 p.i. (). Furthermore, FCR values for the GRS 0.5% and GRS 1.0% groups were improved compared with the unsupplemented group, although they were not improved compared with the uninfected control group.
Evaluation of oocyst output
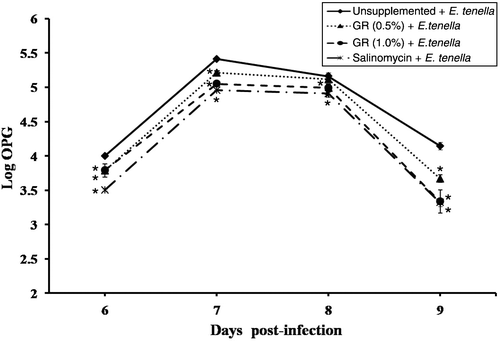
The evaluation of oocyst output in chickens infected with E. tenella from days 6 to 9 p.i. is shown in . The patterns of oocyst output in the GRS 0.5%, GRS 1.0% and SS groups are similar to that in the unsupplemented group, but the number of faecal oocysts decreased from day 6 to day 8 p.i. At day 9 p.i., the GRS 0.5%, GRS 1.0% and SS groups exhibited 11.5%, 19.5% and 19.9% reductions in faecal oocyst output, respectively, compared with the unsupplemented group ().
Figure 1. Oocyst excretion values per gram of faeces (OPG) for each group of chicks. The OPG output in each group of chickens corresponds to days 6 to 9 p.i. with E. tenella and is expressed as the logarithm of the OPG value. Error bars represent the standard deviations. *Significantly reduced oocyst excretion compared with the unsupplemented and infected groups (P < 0.05).
Evaluation of bloody diarrhoea and lesion scores
After infection, bloody diarrhoea was found in nearly all of the experimental groups from day 4 to day 7 p.i. (). In both the GRS 0.5% and GRS 1.0% groups, the severity of bloody diarrhoea was milder compared with the unsupplemented group. Compared with the SS group, the severity of the diarrhoea in the GRS 0.5% group was moderate only on days 5 and 6 p.i., and the severity in the GRS 1.0% group was moderate only on day 5 p.i. (). Furthermore, lesion scores of the GRS 1.0% (2.42±0.39) and GRS 0.5% groups (2.00±0.28) were significantly lower than scores of the unsupplemented group (3.57±0.26) on day 5 p.i., but a significant difference was not seen in any supplemented group compared with the unsupplemented group (2.14±0.34) on day 10 p.i. ().
Table 2. Bloody diarrhoea in chickens infected with E. tenella at days 2 to 10 p.i.
Table 3. Effect of supplementation with GR on lesion scores in the caecum of chickens at days 5 and 10 p.i. with E. tenella.
In addition, the lesion scores of the GRS 0.5% and GRS 1.0% groups were 32% and 44% lower than the score of the unsupplemented group, respectively. The scores of the GR groups were similar to the score of the SS group.
Discussion
Coccidiosis caused by the genus Eimeria has been controlled by various methods, including utilizing several medications, natural products and probiotics, the improvement of farm management, and the modification of the chicken immune system (Allen & Fetterer, Citation2002; Dalloul & Lillehoj, Citation2006). Thus, the present study demonstrated the partial anticoccidial effect in chickens of GR as a dietary supplement. The supplement resulted in reduced oocyst shedding, reduced bloody diarrhoea and fewer caecal lesions, as well as improvement of the growth performance and feed utilization of chickens during infection with E. tenella.
An early precedent for confirming the anticoccidial effects of diets supplemented with salinomycin comes from several experiments (McDougald et al., Citation1996; Daugschies et al., Citation1998; Conway et al., Citation1999; Chapman et al., Citation2004; Duffy et al., Citation2005) showing that salinomycin supplementation in broiler chickens during Eimeria challenge trials alleviated the negative effects of the infection on performance, feed efficiency and intestinal lesion scores. In line with earlier studies, our present study reconfirmed that salinomycin has significant anticoccidial activity and can mitigate the detrimental effects caused by infection with E. tenella. Furthermore, both GR and salinomycin were equally beneficial for improving the performance indicators that were reduced during the E. tenella infection. Thus, considering the harsh conditions in the field, further study would be required to determine the effect of GR under a more severe challenge.
Proper quantification of the oocyst inoculation is required to evaluate its pathogenic effects. The number of oocysts with which the chickens were infected should lead to a statistically significant difference between the unsupplemented/uninfected chickens and the unsupplemented/infected chickens. As such, the recommended dose of oocysts for dose determination studies of E. tenella in chickens (Conway et al., Citation1993; Holdsworth et al., Citation2004) was used in this study. Our results revealed that a moderate infection of chickens with E. tenella led to a significant difference between the control and unsupplemented groups. Furthermore, there was an improvement over the unsupplemented group in growth performance and feed efficiency in chickens supplied with GR or salinomycin.
Previous studies have been undertaken to improve our comprehension of the mode of action of plant-derived bioactive components and their effects on parasitism. These studies have focused on tannin-rich plants having a direct antiparasitic activity but also indirectly increasing host resistance (Hoste et al., Citation2006). Thus, we could consider whether the effects of GR on the infection parameters are associated with direct antiparasitic activity or indirect effects that may increase host resistance. When the chickens supplemented with 0.5% GR, 1.0% GR and salinomycin were evaluated across several infection parameters, the BWG and RWG values of the GRS groups were higher than the values of the unsupplemented group. Additionally, these values in the GRS 1.0% group were almost equal to the values in the salinomycin group. Although there were less remarkable differences in the amount of food intake by the supplemented groups compared with the unsupplemented group, the FCR values in the supplemented groups were higher than the values of the unsupplemented group due to weight elevations. Consequently, these results showed that improved weight gain and feed efficiency in GRS chickens due to the GR diet might have positive effects on host physiology and performance upon infection with E. tenella. Moreover, faecal oocyst excretion is only one parameter for the evaluation of coccidiosis (Lillehoj & Trout, Citation1996; Chapman et al., Citation2005) and several investigators have concluded that oocyst count is an unsatisfactory and unreliable parameter for judging the effectiveness of an anticoccidial (Reid et al., Citation1975). Although the total number of oocysts and the OPG counts are not synchronized, the GRS and SS groups shed 10 to 34% fewer oocysts than the unsupplemented group in OPG units. Considering these reductions in faecal oocyst output in the supplemented groups, it is possible that GR might be associated with anticoccidial effects. Infection with E. tenella typically leads to swelling and damage to the caecal wall, which alters the normal function of the chicken intestine (Jeurissen et al., Citation1996), although this effect depends on the infection rate. The GR dietary supplement effectively reduced lesion scores due to E. tenella, which is a parasite that thrives in the caecal region. This finding demonstrated that diets supplemented with GR have a protective effect against the gross pathological changes caused by E. tenella infection. Moreover, the bloody diarrhoea in the unsupplemented group was prolonged and severe, especially at days 5 and 6 p.i., which are the most important days in the lifecycle of E. tenella. This sign was relieved by GR supplementation at both the 1.0% and 0.5% levels, although the relief was not as great as that with salinomycin treatment. The use of GR as a dietary supplement may have a beneficial influence and may improve the host defence response against E. tenella. Our results confirm the possibility that GR modulates the host resistance to parasitic infection.
The present study is the first to describe the anticoccidial activity of GR. The challenge test using both 0.5% and 1.0% GR powder as a feed supplement indicated that effective control of infection with E. tenella was accomplished by the supplement, as judged by such parameters as BWG, FCR, bloody diarrhoea, lesion scores and oocyst excretion in faeces. Therefore, GR-based diets may provide benefits for preventing or treating coccidial infection in poultry. GR supplementation is thus recommended in environments where chickens face the risk of E. tenella infection. Future studies in our laboratory will focus on the mode of action of GR in order to determine how this compound may influence immune responses in avian hosts, together with the anticoccidial effects on other Eimeria species.
Acknowledgements
This research was supported by the Cooperative Research Programme for Agriculture Science & Technology Development (PJ907105032011), Rural Development Administration, Republic of Korea. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- Abbas , R.Z. , Iqbal , Z. , Blake , D. , Khan , M.N. and Saleemi , M.K. 2011 . Anticoccidial drug resistance in fowl coccidia: the state of play revisited . World's Poultry Science Journal , 67 : 337 – 350 .
- Ahn , Y.J. , Lee , C.O. , Kweon , J.H. , Ahn , J.W. and Park , J.H. 1998 . Growth-inhibitory effects of Galla Rhois-derived tannins on intestinal bacteria . Journal Applied Microbiology , 84 : 439 – 443 .
- Allen , P.C. and Fetterer , R.H. 2002 . Recent advances in biology and immunobiology of Eimeria species and in diagnosis and control of infection with these coccidian parasites of poultry . Clinical Microbiology Reviews , 15 : 58 – 65 .
- Aziz , N.H. , Farag , S.E. , Mousa , L.A. and Abo-Zaid , M.A. 1998 . Comparative antibacterial and antifungal effects of some phenolic compounds . Microbios , 93 : 43 – 54 .
- Bae , E.A. , Han , M.J. , Kim , N.J. and Kim , D.H. 1998 . Anti-Helicobacter pylori activity of herbal medicines . Biological and Pharmaceutical Bulletin , 21 : 990 – 992 .
- Campbell , W.C. 2008 . History of the discovery of sulfaquinoxaline as a coccidiostat . Journal of Parasitology , 94 : 934 – 945 .
- Chapman , H.D. 1997 . Biochemical, genetic and applied aspects of drug resistance in Eimeria parasites of the fowl . Avian Pathology , 26 : 221 – 244 .
- Chapman , H.D. 1998 . Evaluation of the efficacy of anticoccidial drugs against Eimeria species in the fowl . International Journal for Parasitology , 28 : 1141 – 1144 .
- Chapman , H.D. , Marsler , P. and LaVorgna , M.W. 2004 . The effects of salinomycin and roxarsone on the performance of broilers when included in the feed for four, five, or six weeks and infected with Eimeria species during the starter or grower phase of production . Poultry Science , 83 : 761 – 764 .
- Chapman , H.D. , Matsler , P.L. , Muthavarapu , V.K. and Chapman , M.E. 2005 . Acquisition of immunity to Eimeria maxima in newly hatched chickens given 100 oocysts . Avian Diseases , 49 : 426 – 429 .
- Chen , J.C. , Ho , T.Y. , Chang , Y.S. , Wu , S.L. and Hsiang , C.Y. 2006 . Antidiarrheal effect of Galla Chinensis on the Escherichia coli heat-labile enterotoxin and ganglioside interaction . Journal of Ethnopharmacology , 103 : 385 – 391 .
- Conway , D.P. , Dayton , A.D. and McKenzie , M.E. 1999 . Comparative testing of anticoccidials in broiler chickens: the role of coccidial lesion scores . Poultry Science , 78 : 529 – 535 .
- Conway , D.P. , Sasai , K. , Gaafar , S.M. and Smothers , C.D. 1993 . Effects of different levels of oocyst inocula of Eimeria acervulina, E. tenella, and E. maxima on plasma constituents, packed cell volume, lesion scores, and performance in chickens . Avian Diseases , 37 : 118 – 123 .
- Dalloul , R.A. and Lillehoj , H.S. 2006 . Poultry coccidiosis: recent advancements in control measures and vaccine development . Expert Review of Vaccines , 5 : 143 – 163 .
- Daugschies , A. , Gasslein , U. and Rommel , M. 1998 . Comparative efficacy of anticoccidials under the conditions of commercial broiler production and in battery trials . Veterinary Parasitology , 76 : 163 – 171 .
- De Pablos , L.M. , Dos Santos , M.F. , Montero , E. , Garcia-Granados , A. , Parra , A. and Osuna , A. 2010 . Anticoccidial activity of maslinic acid against infection with Eimeria tenella in chickens . Parasitology Research , 107 : 601 – 604 .
- Djakpo , O. and Yao , W. 2010 . Rhus chinensis and Galla chinensis – folklore to modern evidence: review . Phytotherapy Research , 24 : 1739 – 1747 .
- Du , A. and Hu , S. 2004 . Effects of a herbal complex against Eimeria tenella infection in chickens . Journal of Veterinary Medicine. B, Infectious Disease and Veterinary Public Health , 51 : 194 – 197 .
- Duffy , C.F. , Mathis , G.F. and Power , R.F. 2005 . Effects of Natustat supplementation on performance, feed efficiency and intestinal lesion scores in broiler chickens challenged with Eimeria acervulina, Eimeria maxima and Eimeria tenella . Veterinary Parasitology , 130 : 185 – 190 .
- Gao , X.M. , Xu , Z.M. and Li , Z.W. 2000 . Traditional Chinese Medicines , 263 – 266 . Beijing : People's Health Publishing House .
- Holdsworth , P.A. , Conway , D.P. , McKenzie , M.E. , Dayton , A.D. , Chapman , H.D. , Mathis , G.F. , Skinner , J.T. , Mundt , H.C. and Williams , R.B. 2004 . World Association for the Advancement of Veterinary Parasitology (WAAVP) guidelines for evaluating the efficacy of anticoccidial drugs in chickens and turkeys . Veterinary Parasitology , 121 : 189 – 212 .
- Hoste , H. , Jackson , F. , Athanasiadou , S. , Thamsborg , S.M. and Hoskin , S.O. 2006 . The effects of tannin-rich plants on parasitic nematodes in ruminants . Trends in Parasitology , 22 : 253 – 261 .
- Jean , D.G. and Subhabrata , C. 2003 . Nonparametric Statistical Inference , 4th edn , 353 – 362 . New York : Marcel Dekker .
- Jeurissen , S.H. , Janse , E.M. , Vermeulen , A.N. and Vervelde , L. 1996 . Eimeria tenella infections in chickens: aspects of host-parasite: interaction . Veterinary Immunology and Immunopathology , 54 : 231 – 238 .
- Johnson , J. and Reid , W.M. 1970 . Anticoccidial drugs: lesion scoring techniques in battery and floor-pen experiments with chickens . Experimental Parasitology , 28 : 30 – 36 .
- Kang , M.S. , Oh , J.S. , Kang , I.C. , Hong , S.J. and Choi , C.H. 2008 . Inhibitory effect of methyl gallate and gallic acid on oral bacteria . Journal of Microbiology , 46 : 744 – 750 .
- Kratz , J.M. , Andrighetti-Frohner , C.R. , Leal , P.C. , Nunes , R.J. , Yunes , R.A. , Trybala , E. , Bergstrom , T. , Barardi , C.R. and Simoes , C.M. 2008 . Evaluation of anti-HSV-2 activity of gallic acid and pentyl gallate . Biological and Pharmaceutical Bulletin , 31 : 903 – 907 .
- Lillehoj , H.S. and Trout , J.M. 1996 . Avian gut-associated lymphoid tissues and intestinal immune responses to Eimeria parasites . Clinical Microbiology Reviews , 9 : 349 – 360 .
- McDougald , L.R. , Mathis , G.F. and Conway , D.P. 1996 . Effect of semduramicin, salinomycin, and monensin on performance, shank pigmentation, and coccidial lesions in broiler chickens in floor pens . Avian Diseases , 40 : 68 – 71 .
- Min , W. , Dalloul , R.A. and Lillehoj , H.S. 2004 . Application of biotechnological tools for coccidia vaccine development . Journal of Veterinary Science , 5 : 279 – 288 .
- Mood , A.M. , Graybill , F.A. and Boes , D.C. 1974 . Introduction to the Theory of Statistics, , 3rd edn , 504 – 526 . New York : McGraw-Hill .
- NRC . 1994 . Nutrient Requirements of Poultry, , 9th edn . Washington , DC : National Academy Press .
- Naidoo , V. , McGaw , L.J. , Bisschop , S.P. , Duncan , N. and Eloff , J.N. 2008 . The value of plant extracts with antioxidant activity in attenuating coccidiosis in broiler chickens . Veterinary Parasitology , 153 : 214 – 219 .
- Pettinari , A. , Amici , M. , Cuccioloni , M. , Angeletti , M. , Fioretti , E. and Eleuteri , A.M. 2006 . Effect of polyphenolic compounds on the proteolytic activities of constitutive and immuno-proteasomes . Antioxidants and Redox Signaling , 8 : 121 – 129 .
- Reid , W.M. , Johnson , J. and Dick , J. 1975 . Anticoccidial activity of lasalocid in control of moderate and severe coccidiosis . Avian Diseases , 19 : 12 – 18 .
- SAS . 1999 . SAS OnlineDoc®, Version 8.01 . Cary , NC : SAS Institute Inc .
- Snedecor , G.W. and Cochran , W.G. 1980 . Statistical Methods , Ames , IA : Iowa State University Press .
- Williams , R.B. 2002 . Fifty years of anticoccidial vaccines for poultry (1952–2002) . Avian Diseases , 46 : 775 – 802 .
- Youn , H.J. and Noh , J.W. 2001 . Screening of the anticoccidial effects of herb extracts against Eimeria tenella . Veterinary Parasitology , 96 : 257 – 263 .