Abstract
Aspergillosis caused by Aspergillus fumigatus seems to be more prevalent in some avian species than in others. We compared the development of aspergillosis in 8-month-old Gyr-Saker hybrid falcons and 8-month-old pigeons after a single intratracheal inoculation of different dosages of A. fumigatus conidia (107, 105 and 103). Clinical signs, including vomiting, discoloration of the urates, loss of appetite and dyspnoea, were observed in four out of five falcons and in four out of five pigeons inoculated with 107 A. fumigatus conidia. Necropsy revealed the presence of granulomas in the air sacs and/or lungs in four out of five falcons and in four out of five pigeons in the high dosage group. A. fumigatus was isolated from these granulomas in three falcons and in three pigeons. The presence of fungal hyphae was detected with Periodic acid Shiff reagent staining in three out of five falcons and in three out of five pigeons in the high dosage group. Avian respiratory macrophages were clearly present in and around the fungal granulomas. In the other dosage groups, no granulomas, positive A. fumigatus cultures or fungal hyphae were present, except for one falcon in the middle dosage group in which a sterile granuloma without fungal hyphae was noticed. In conclusion, the study shows that adult falcons and pigeons are susceptible to aspergillosis after inoculation of a single dose of conidia intratracheally.
Introduction
Respiratory disease due to Aspergillus species is a major cause of morbidity and mortality in captive and free-ranging birds (Tell, Citation2005; Beernaert et al., Citation2008; Olias et al., Citation2010). In the genus Aspergillus, especially Aspergillus fumigatus and to a lesser extent Aspergillus flavus, Aspergillus niger, Aspergillus terreus and Aspergillus nidulans are causative agents of aspergillosis (Jones & Orosz, Citation2000). A. fumigatus is a ubiquitous saprophytic fungus that sporulates abundantly and releases a huge number of conidia into the air. Inhaled conidia can reach the lungs and air sacs due to their small size (Fedde, Citation1998; Beernaert et al., Citation2010).
Although aspergillosis most probably occurs in all avian species, it is seen more often in captive waterfowl, wading birds, penguins, raptors, pheasants and passerines (Bauck, Citation1994; Kearns, Citation2003). At present, it is still not clear why these birds appear to be more susceptible to aspergillosis. In the literature, the factors necessary to induce clinical disease after exposure to A. fumigatus conidia are not known. Even though a multifactorial aetiology seems to be common, the intrinsic susceptibility to aspergillosis may be host dependent.
The aim of the present study was to compare the intrinsic susceptibility of otherwise healthy adult hybrid falcons and pigeons to aspergillosis, after a single intratracheal inoculation with different numbers of A. fumigatus conidia.
Materials and Methods
A. fumigatus strain and inoculum preparation
The A. fumigatus strain K125 (accession number of rRNA and ITS genes: HE 864321) used in this study was isolated from a lung granuloma of a Gyr-Saker (Falco rusticolus×Falco cherrug) hybrid falcon, which died from severe aspergillosis. The sample was stored at −80°C via Microbank™ (Pro-Lab Diagnostics, Novolab, Belgium) until use. Five-day-old cultures of this strain on Sabouraud dextrose agar (SAB) (CM0041; Oxoid Ltd, Basingstoke, UK) were washed with 5 ml of 0.01% Tween 20 in Hank's balanced salt solution (HBSS) to harvest A. fumigatus conidia. The conidia were washed three times in 0.01% Tween 20 in HBSS (3200×g, 10 min at 4°C) and the suspension was adjusted to a concentration of 103, 105 or 107 A. fumigatus conidia/0.5 ml in HBSS by haemacytometer count. Numbers of viable conidia were determined by plating serial 10-fold serial dilutions in 0.01% Tween 20 in HBSS on SAB plates. The number of colony-forming units/ml was calculated after incubation at 37°C for 20 h. The final conidial suspensions had a viable count of 2.5×107, 1.89×105 and 2.25×103 colony-forming units/ml.
Experimental birds
Eighteen adult male Gyr-Saker (F. rusticolus×F. cherrug) hybrid falcons were obtained from one breeder. The birds’ health was evaluated by a general examination, endoscopy, and bacteriological, virological and parasitological examination. All air sacs on both sides from each falcon were visualized and were free of signs of aspergillosis. To detect anti-Aspergillus antibodies, the Aspergillus haemagglutination test (Hemkit® Aspergillus IHA; Ravo Diagnostika, Freiburg Germany) was used. The Aspergillus haemagglutination test was negative for all falcons. Excreta were collected for 5 days from each falcon and mixed. Bacteriological analysis was performed using direct plating on brilliant green agar and enrichment on buffered peptone water/brilliant green tetrathionate broth. This test was negative for the presence of Salmonella spp. Polymerase chain reaction testing for the detection of herpesvirus DNA in blood was negative. Parasitological analysis was performed using a saturated salt solution in water and microscopic examination. No endoparasite ova could be detected. All birds were considered healthy before the trial, especially free of signs of aspergillosis. The falcons were perched according to standard falconry techniques with a 12-h photoperiod during the trial (Parry-Jones, Citation2008). One-day-old chicks were provided to each bird each day.
Twenty adult racing pigeons (Columba livia domestica) were divided into four groups. The birds’ health was evaluated by general examination, endoscopy, and bacteriological, virological and parasitological examination as described for the falcons. All pigeons were considered healthy before the trial. During the experiment, each bird was housed individually with a 12-h photoperiod. The birds received a commercial pigeon diet ad libitum and had free access to fresh drinking water.
All experiments were performed with the permission of the Ethical Committee of the Faculty of Veterinary Medicine, Merelbeke, Ghent University, Belgium (EC 2010/111; EC 2011/138).
Experimental design
Falcons
Three groups of five falcons were inoculated intratracheally with 103, 105 or 107 A. fumigatus conidia in 0.5 ml HBSS, respectively, and one group of three falcons was sham-inoculated intratracheally with 0.5 ml HBSS. The inoculation was performed under general anaesthesia with Isoflurane (Isoflo®; Medini, Oostkamp, Belgium) and a paediatric endotracheal tube (Ø 2.5×4.1-L. 165 mm) (Vygon, Ecouen, France) was used for the intratracheal inoculation. The birds were weighed daily and observed at least twice daily. At 28 days post inoculation (p.i.) all falcons were euthanized by an intravenous injection of 1 ml T61 (Intervet, Mechelen, Belgium) in the vena basilica under general anaesthesia.
Pigeons
Three groups of five pigeons were inoculated intratracheally with 0.5 ml of 103, 105 or 107 A. fumigatus conidia in HBSS, respectively, and one group of five pigeons was sham-inoculated intratracheally with 0.5 ml HBSS. The inoculation was performed under general anaesthesia with Isoflurane and an intravenous cannula (18 G×1¾ inch) (Vasovet, Tuttlingen, Germany) was used for the intratracheal inoculation. The birds were weighed daily and observed at least twice daily. At 28 days p.i. all pigeons were euthanized by an intravenous injection of 1 ml T61 in the vena basilica under general anaesthesia.
Clinical follow-up
The presence of ruffled feathers, dyspnoea, sneezing and stridor were scored daily. During the trial, birds with severe dyspnoea (open beak breathing) or extreme weight loss were considered irreversibly fatally ill and suffering, and therefore were euthanized. This was noted as “mortality”.
Environmental sampling
To measure the environmental load of A. fumigatus conidia, air samples from the experimental units were collected using the MAS-100 Eco impaction air-sampler (Merck, Whitehouse Station, New Jersey, USA). A sampling volume of 100 l was chosen. Twice a week, samples were collected in triplicate on SAB agar plates and incubated at 37°C under aerobic conditions to quantify A. fumigatus.
Gross, histopathological and immunohistochemical examination
At necropsy, macroscopic lesions were noted. Samples of the lungs, air sacs, liver, spleen, kidney and granulomas were fixed in phosphate-buffered formaldehyde solution, sectioned and stained with haematoxylin and eosin or Periodic acid Shiff reagent for visualization of fungal elements.
To visualize respiratory macrophages in the lungs, air sacs and granulomas, concanavalin A staining was performed (Greenfield et al., Citation1988). Briefly, antigen retrieval in the deparaffinized sections, breaking the protein cross-links formed by formalin fixation and uncovering hidden antigenic sites, of the lungs, air sacs and granulomas, was performed using a pressure cooker. The sections were heated for 15 min at 850 W and 15 min at 300 W in the microwave oven. Subsequently, they were cooled down for 20 min and thereafter treated with 3% hydrogen peroxide in methanol for 5 min at room temperature to block endogenous peroxidase activity. After rinsing with phosphate-buffered saline (PBS), the sections were incubated with peroxidase-labelled concanavalin A (L6397; Sigma-Aldrich, St Louis, Missouri, USA) at 20 µg/ml for 60 min in a humid chamber at room temperature. After rinsing with PBS, the reaction product was developed with a hydrogen peroxide and diaminobenzidine solution (prepared following manufacturer's instructions) for 5 min. Finally, the sections were counterstained with haematoxylin and mounted for examination.
Mycological examination and microsatellite length polymorphism
To isolate A. fumigatus from the birds, samples of the trachea, lungs, air sacs, heart, pericardium, liver, kidney, brain, pectoral muscle, and abdominal fluid were inoculated on SAB plates and incubated for 72 h at 37°C at aerobic conditions. After identification of the isolated fungi, microsatellite length polymorphism was conducted on each colony to confirm that mycoses during this study originated from the inoculated strain; this was performed as previously described (Van Waeyenberghe et al., Citation2011).
Galleria mellonella virulence assay
To assess the virulence of isolate K125 and K24, 10 sixth-instar larvae of G. mellonella were injected with 1×106 A. fumigatus conidia of K125 and K24 in 10 µl PBS, respectively, into the haemocoel through the last left proleg using a Myjector U-100 Insulin syringe (Verdifarm, Beringen, Belgium). After infection, the larvae were incubated in plastic containers, and the number of dead larvae was scored daily. Larvae were considered dead when they displayed no movement in response to touch. All tests were performed in triplicate.
Results
Clinical signs
Falcons
Clinical signs were seen only in the high and middle dosage groups. In the high dosage group, vomiting was observed on day 2 p.i. in four out of five birds. One bird also vomited on 10 days p.i. A greenish coloration of the urates was seen in three out of five birds. A loss of appetite was noted in four out of five birds during the first days p.i. One bird also showed a reduced appetite from day 10 to day 14, one bird from day 13 to day 28 and one bird from day 20 to day 28.
In the middle dose group, one bird vomited on days 17 and 18 p.i. and showed greenish coloration of its urates from day 19 to day 23 p.i. From day 18 to day 22 p.i., this bird also exhibited a loss of appetite.
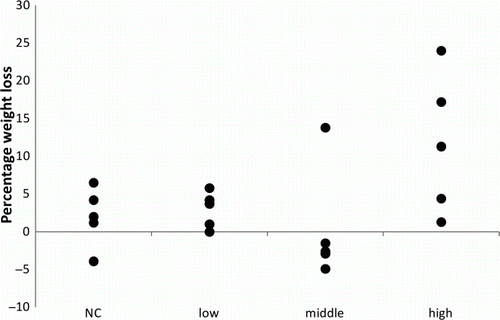
A summary of the clinical signs is presented in . An overview of the weight loss in the different groups is presented in . No mortality was observed in any group.
Figure 1. Total weight loss as a percentage of the initial weight of the falcons, inoculated with a high dosage (107), a middle dosage (105) or a low dosage (103) of A. fumigatus spores and a negative control (NC) group.
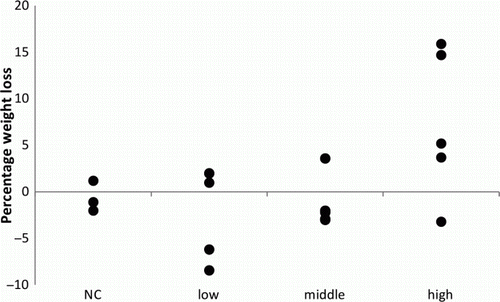
Table 1. Clinical signs in falcons and pigeons inoculated with 107 (high dosage), 105 (middle dosage) or 103 (low dosage) A. fumigatus conidia in the trachea.
Pigeons
In the high dosage group, dyspnoea and a reduced appetite were observed after 2 days p.i. in four out of five birds. On day 4 p.i., one of these four birds died in its cage. The respiratory signs of the other three birds remained present for the following 14 days. On day 13 p.i., one of the remaining four birds was found dead with blood in the oral cavity. From 15 days p.i. onward, the dyspnoea of the other birds improved and the appetite returned. In the three other groups, none of these signs were observed. A summary of the clinical signs is presented in . An overview of the weight loss in the different groups is presented in .
Pathological, mycological, histopathological and immunohistochemical findings
In the high dosage group of inoculated falcons, necropsy revealed the presence of granulomas in the air sacs in four out of five birds. In two of these birds, granulomatous lesions were also observed in the lung. Lesions were found at the left side as well as the right side of the respiratory system in two out of four birds. A. fumigatus was isolated from the lesions of the air sacs in three birds. In the middle dosage group, one bird had a granulomatous lesion in the air sacs, although A. fumigatus could not be isolated. No lesions were observed in the low dosage group and the negative control group.
In the high dosage group of pigeons, necropsy revealed the presence of granulomatous lesions in the lungs in four out of five birds. In two of these birds, granulomatous lesions were also seen in the air sacs and kidney. The lesions were all bilateral in nature. A. fumigatus was isolated from the lesions in three birds. No macroscopic lesions were observed in the other groups.
The histopathological findings of the observed lesions in falcons and pigeons showed a severe heterophilic and granulomatous inflammation, with large accumulations of necrotic heterophils, surrounded by a continuous rim of epithelioid, multinucleate giant cells and macrophages. Periodic acid Shiff staining revealed the presence of fungal elements in three of the six air sac granulomas in the falcons and in three out of four granulomas in pigeons. The spleens of the falcons and pigeons were evaluated for the presence of circovirus inclusions. No circovirus inclusions were noted in the spleen. A summary of the pathological, mycological and histopathological lesions is presented in . No lesions or fungal elements were detected in the other organs. In granulomas of the air sacs and lungs, concanavalin A staining revealed a large number of macrophages and giant cells surrounding the necrotic foci ().
Figure 3. Fungal granuloma in the air sac of a falcon, stained with peroxidase-labelled concanavalin A (20 µg/ml). Darkly stained macrophages (arrow) are distributed at the edge of the granuloma.
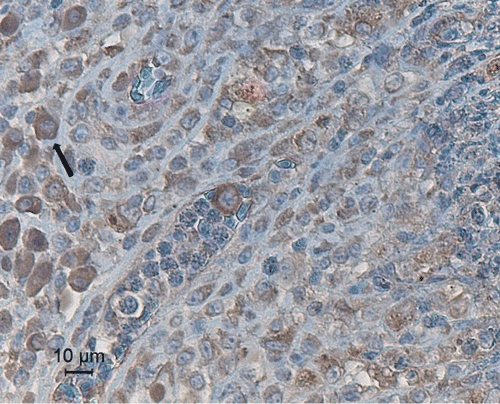
Table 2. Pathological, mycological and histopathological findings in falcons and pigeons inoculated with 107 (high dosage), 105 (middle dosage) or 103 (low dosage) A. fumigatus conidia in the trachea.
Environmental sampling
In eight measurements, an average 140±86 A. fumigatus conidia/m3 air were detected in the experimental unit of the falcons and an average 71±31 A. fumigatus conidia/m3 air were detected in the experimental unit of the pigeons.
Microsatellite length polymorphism
The genotypes of the A. fumigatus, isolated from the lesions of the falcons, were identical to the genotype of the inoculated strain in two out of three falcons. In one falcon, besides the genotype of the inoculated strain, a second genotype was obtained from three out of four lesions.
The genotypes of the A. fumigatus isolated from the lesions of the pigeons were all identical to the genotype of the inoculated strain.
G. mellonella virulence assay
No differences in survival rate of the larvae were observed between the two A. fumigatus isolates. After 72 h, all larvae inoculated with the different A. fumigatus conidia were found dead.
Discussion
In the present study, the occurrence of aspergillosis after single exposure to different dosages of A. fumigatus conidia was examined in two avian species. A single-dose exposure of 107 A. fumigatus conidia was capable of causing disease in 8-month-old Gyr-Saker hybrid falcons and pigeons. Although several authors claim that birds of prey, especially gyrfalcon (Falco rusticollis) and its hybrids, golden eagle (Aquila chrysaetos), osprey (Pandion haliaetus), goshawk (Accipiter gentilis), roughlegged hawk (Buteo lagopus) and red-tailed hawk (Buteo jamaicensis), are highly susceptible to aspergillosis (Redig, Citation1993; Joseph, Citation2000; Tell, Citation2005; Silvanose, 2012, personal communication), the expected difference in species susceptibility between 8-month-old hybrid falcons and 8-month-old pigeons was not observed. On the other hand, age-related susceptibility to aspergillosis is reported for falcons (Joseph, Citation2000), pigeons (Beernaert et al., Citation2008), turkeys (Femenia et al., Citation2007) and white storks (Olias et al., Citation2010). Therefore, infection trials with young hybrid falcons and pigeons should be performed to determine the influence of age in the development of aspergillosis within the model used.
In our study, adult pigeons developed aspergillosis after intratracheal inoculation of 107 A. fumigatus conidia. In a study of Beernaert et al. (Citation2008), adult pigeons did not develop aspergillosis after intratracheal inoculation of as many as 108 conidia of a different A. fumigatus strain. This may suggest that virulence of the A. fumigatus strain involved is more important than species susceptibility in the development of aspergillosis. Such differences in virulence of A. fumigatus strains were already demonstrated in turkeys (Peden & Rhoades, Citation1992) and in mouse models of invasive pulmonary aspergillosis (Mondon et al., Citation1996; Aufauvre-Brown et al., Citation1998). In the non-vertebrate host model of G. mellonella, no differences in virulence between the two strains were observed. Furthermore, Olias et al. (Citation2011) demonstrated that under field conditions strain pathogenicity does not play a major role.
Although animal movements may contribute to the generation of conidial aerosols (Dyar et al., Citation1984; Arné et al., Citation2011), the Aspergillus conidial concentrations did not exceed the general indoor concentrations of 175 conidia/m3 (Ault & Schott, Citation1994). In poultry houses, concentrations up to 2.1×103 conidia/m3 were recorded in spring (Ault & Schott, Citation1994). However, Vanhee et al. (Citation2009, Citation2010) reported much lower concentrations indoors, in poultry houses and pigeon houses, as observed in our study. The falcons in the present study inhaled approximately 50 A. fumigatus conidia from ambient air each day. This concentration did not harm healthy adult hybrid falcons as the birds of the control group did not show any signs of disease or pathological lesions. Microsatellite length polymorphism demonstrated that the inoculated strain was responsible for the disease in the experimental birds. The detection of additional strains is not uncommon as birds may be infected by several strains (Olias et al., Citation2011).
After 10 and 17 days, two falcons (one of the high dosage group and one of the middle dosage group) vomited and showed a loss of appetite but recovered completely. Interestingly, these two birds had sterile granulomas in the air sacs. One pigeon also had a sterile granuloma in the lung after clinical recovery. Clearance of the fungal infection in these birds might explain the sterile granulomas. In turkeys and chickens, respectively, clearance of fungal infections from the lung and air sacs was also demonstrated after 7 to 10 days p.i. and up to 3 weeks p.i., respectively (Taylor & Burroughs, Citation1973; Femenia et al., Citation2007). This finding proves that aspergillosis in clinically healthy birds can present as a self-limiting disease, also in supposedly susceptible species. However, apart from the health condition of the host, this seems to be also dependent on the infection dose.
Host defence mechanisms against A. fumigatus include innate as well as adaptive immunity. Respiratory macrophages, belonging to the innate immune system, prevent germination and establishment of early infection (Van Waeyenberghe et al., Citation2012). Nevertheless, inhalation of an overwhelming amount of conidia results in germination of conidia inside avian respiratory macrophages and colonization of the respiratory tract (Van Waeyenberghe et al., Citation2012). In cases of infection, many respiratory macrophages are present in and around the fungal granulomas as demonstrated in this study. Demarcation of the fungal burden with these macrophages could support clearance of the disease as observed in several birds.
In conclusion, clinically healthy falcons seem equally as susceptible as pigeons to aspergillosis after single-dose exposure to A. fumigatus conidia and the development of aspergillosis is dose-dependent under experimental conditions. According to the available literature, this study demonstrates for the first time that a single-dose exposure to A. fumigatus conidia is sufficient to cause a clinical disease in falcons. This should be considered in future, as under clinical conditions aspergillosis is not always a multifactorial disease and can also be induced by an overwhelming amount of conidia.
Acknowledgements
This work was supported by the Institute for the Promotion of Innovation through Science and Technology in Flanders (IWT Vlaanderen), Brussels, Belgium.
References
- Arné , P. , Thierry , S. , Wang , D. , Deville , M. , Le Loc'h , G. , Desoutter , A. , Féménia , F. , Nieguitsila , A. , Huang , W. , Chermette , R. & Guillot , J. 2011 . Aspergillus fumigatus in poultry . International Journal of Microbiology , doi: 10.1155/2011/746356 .
- Aufauvre-Brown , A. , Brown , J.S. and Holden , D.W. 1998 . Comparison of virulence between clinical and environmental isolates of Aspergillus fumigatus . European Journal of Clinical Microbiology and Infectious Diseases , 17 : 778 – 780 . doi: 10.1007/s100960050184
- Ault , S.K. & Schott , M. 1994 . Aspergillus, Aspergillosis and Composting Operations in California . Research Services Section, Grants and Research Branch, Markets, Research and Technology Division, California Integrated Waste Management Board, California Environmental Protection Agency .
- Bauck , L. 1994 . Mycoses . In B.W. Ritchie , G.J. Harrison & L.R. Harrison . Avian Medicine: Principles and Application (pp. 997 – 1006 ). Lake Worth , FL : Wingers Publishing .
- Beernaert , L.A. , Pasmans , F. , Haesebrouck , F. and Martel , A. 2008 . Modelling Aspergillus fumigatus infections in racing pigeons (Columba livia domestica) . Avian Pathology , 37 : 545 – 549 . doi: 10.1080/03079450802382280
- Beernaert , L.A. , Pasmans , F. , Van Waeyenberghe , L. , Haesebrouck , F. and Martel , A. 2010 . Aspergillus infections in birds: a review . Avian Pathology , 39 : 325 – 331 . doi: 10.1080/03079457.2010.506210
- Dyar , P.M. , Fletcher , O.J. and Page , R.K. 1984 . Aspergillosis in turkeys associated with use of contaminated litter . Avian Diseases , 28 : 250 – 255 . doi: 10.2307/1590149
- Fedde , M.R. 1998 . Relationship of structure and function of the avian respiratory system to disease susceptibility . Poultry Science , 77 : 1130 – 1138 .
- Femenia , F. , Fontaine , J. , Lair-Fulleringer , S. , Berkova , N. , Huet , D. , Towanou , N. , Rakotovao , F. , Granet , O.–I. , Le Loc'h , G. , Arné , P. and Guillot , J. 2007 . Clinical, mycological and pathological findings in turkeys experimentally infected by Aspergillus fumigatus . Avian Pathology , 36 : 213 – 219 . doi: 10.1080/03079450701332337
- Greenfield , C.L. , Sanders , F.S. and Dietert , R.R. 1988 . Detection of avian macrophages with concanavalin A . Avian Pathology , 17 : 803 – 820 . doi: 10.1080/03079458808436503
- Jones , M.P. and Orosz , S.E. 2000 . The diagnosis of aspergillosis in birds . Seminars in Avian and Exotic Pet Medicine , 9 : 52 – 58 . doi: 10.1053/AX.2000.4619
- Joseph , V. 2000 . Aspergillosis in raptors . Seminars in Avian and Exotic Pet Medicine , 9 : 66 – 74 . doi: 10.1053/AX.2000.4617
- Kearns , K.S. 2003 . Avian aspergillosis . In K.S. Kearns & B. Loudis . Recent Advances in Avian Infectious Diseases . Document number A1902.0903 . Ithaca : International Veterinary Information Service .
- Mondon , P. , De Champs , C. , Donadille , A. , Ambroise Thomas , P. and Grillot , R. 1996 . Variation in virulence of Aspergillus fumigatus strains in a murine model of invasive pulmonary aspergillosis . Journal of Medical Microbiology , 45 : 186 – 191 . doi: 10.1099/00222615-45-3-186
- Olias , P. , Gruber , A.D. , Hafez , H.M. , Lierz , M. , Slesiona , S. , Brock , M. and Jacobsen , I.D. 2011 . Molecular epidemiology and virulence assessment of Aspergillus fumigatus isolates from white stork chicks and their environment . Veterinary Microbiology , 148 : 348 – 355 . doi: 10.1016/j.vetmic.2010.08.029
- Olias , P. , Gruber , A.D. , Winfried , B. , Hafez , H.M. and Lierz , M. 2010 . Fungal pneumonia as a major cause of mortality in White Stork (Ciconia ciconia) chicks . Avian Diseases , 54 : 94 – 98 . doi: 10.1637/9088-092509-Reg.1
- Parry-Jones , J. 2008 . Raptor husbandry and falconry techniques . In J. Chitty & M. Lierz . BSAVA Manual of Raptors, Pigeons and Passerines (pp. 7 – 13 ). Wiley-Blackwell , New Jersey , USA .
- Peden , W.M. and Rhoades , K.R. 1992 . Pathogenicity differences of multiple isolates of Aspergillus fumigatus in turkeys . Avian Diseases , 36 : 537 – 542 . doi: 10.2307/1591746
- Redig , P.T. 1993 . Avian aspergillosis . In M.E. Fowler . Zoo and Wild Animal Medicine: Current Therapy 3 (pp. 178 – 181 ). W.B. Saunders Inc , Philadephia , USA .
- Taylor , J.J. and Burroughs , E.J. 1973 . Experimental avian aspergillosis . Mycopathologia et Mycologia Applicata , 51 : 131 – 141 . doi: 10.1007/BF02141104
- Tell , L.A. 2005 . Aspergillosis in mammals and birds: impact on veterinary medicine . Medical Mycology , 43 : S71 – S73 . doi: 10.1080/13693780400020089
- Van Waeyenberghe , L. , Pasmans , F. , Beernaert , L.A. , Haesebrouck , F. , Vercammen , F. , Verstappen , F. , Dorrestein , G.M. , Klaassen , C.H.W. and Martel , A. 2011 . Microsatellite typing of avian clinical and environmental isolates of Aspergillus fumigatus . Avian Pathology , 40 : 73 – 77 . doi: 10.1080/03079457.2010.540229
- Van Waeyenberghe , L. , Pasmans , F. , D'herde , K. , Ducatelle , R. , Favoreel , H. , Li , SJ. , Haesebrouck , F. and Martel , A. 2012 . Germination of Aspergillus fumigatus inside avian respiratory macrophages is associated with cytotoxicity . Veterinary Research , 43 : 32 doi: 10.1186/1297-9716-43-32
- Vanhee , L.M.E. , Nelis , H.J. and Coenye , T. 2009 . Quantification and identification of airborne Aspergillus fumigatus using SPC . Environmental Science and Technology , 43 : 3233 – 3239 . doi: 10.1021/es803435a
- Vanhee , L.M.E. , Nelis , H.J. and Coenye , T. 2010 . Rapid quantification of itraconazole-resistant Aspergillus fumigatus in air . Journal of Microbiological Methods , 81 : 197 – 199 . doi: 10.1016/j.mimet.2010.02.012