Abstract
The present report documents an outbreak of adenoviral gizzard erosion in 22 broiler flocks in Germany. The clinical picture was characterized by uneven growth of affected broilers that resulted in considerably lower than average weight at slaughtering. Fowl adenovirus serotype 1 (FAdV-1) was isolated from gizzard lesions and histological examinations demonstrated FAdV-1-positive intranuclear inclusion bodies in gizzard epithelial cells of affected broilers by in-situ hybridization. Birds from all affected flocks originated from one broiler breeder farm. During production of affected birds, broiler breeders were between 27 and 32 weeks old. Enzyme-linked immunosorbent assay and specific virus neutralization assay of sera from parent birds demonstrated an acute FAdV-1 infection within the first 5 weeks of the production cycle. Clinically, broiler breeders exhibited a moderate fall in the hatchability of their chicks, while egg production remained normal. No further clinical signs could be observed. Genetically identical FAdV-1 strains were isolated from gizzards of embryos at the lowest point of hatchability and from affected broiler flocks raised on independent farms. For the first time, direct detection of viable FAdV-1 from gizzards of embryos and progenies of one FAdV-1-seropositive broiler breeder farm in the course of an outbreak of adenoviral gizzard erosion could be demonstrated, highlighting the importance of vertical transmission of this disease. Additionally, growth retardation and subsequent reduced average weight at the time of slaughter of broiler chickens underline the economic impact of adenoviral gizzard erosion for poultry production.
Introduction
The role of fowl adenoviruses (FAdVs) as primary pathogens has been studied with regard to natural outbreaks of inclusion body hepatitis, hepatitis/hydropericardium syndrome and gizzard erosion (Adair & Fitzgerald, Citation2008). Outbreaks of gizzard erosion associated with FAdV serotype 1 (FAdV-1) infections have been reported from commercial broiler chickens in Japan and more recently in Europe and Korea (Ono et al., Citation2001; Manarolla et al., Citation2009; Marek et al., Citation2010; Domanska-Blicharz et al., Citation2011; Lim et al., Citation2012). Gizzard erosions have been reproduced experimentally by oral and ocular infection with FAdV-1 field isolates in specific-pathogen-free (SPF) White Leghorn chickens as well as in commercial broilers (Okuda et al., Citation2001a, Citationb). Horizontal transmission of FAdV-1 through direct contact as well as aerosol infection and the resulting gizzard erosions have been documented in an experimental setting (Ono et al., Citation2007).
Vertical transmission has been described as an important route for the spread of FAdVs in general, essential for an early infection of chicken progenies. In the early 1950s, chicken embryo lethal orphan virus (CELO, FAdV-1) was isolated from chicken embryos inoculated with an unrelated pathological specimen and recognized as an endogenous virus of the egg, transmitted from parent bird to the embryonated egg (Yates & Fry, Citation1957). Subsequently, several different sources reported the accidental isolation of FAdVs also from uninoculated fertile eggs and reactivation of the viruses in cell cultures prepared from vertically infected embryos (Du Bose & Grumbels, Citation1959; Cook, Citation1968; McFerran et al., Citation1975). These findings resulted in the establishment of SPF flocks for industrial and scientific purposes. Moreover, vertical transmission seems to be an important factor in the epidemiology of inclusion body hepatitis and hepatitis/hydropericardium syndrome (Saifuddin & Wilks, Citation1991; Toro et al., Citation2001; Mazaheri et al., Citation2003; Grgic et al., Citation2006). Similarly, it can also be hypothesized that trans-ovarial transmission from breeder to broiler flocks may play an important role in the epidemiology of adenoviral gizzard erosion. Although a number of outbreaks of adenoviral gizzard erosion have been described and vertical transmission has been suspected in some cases, no direct evidence has been reported.
The present study investigated the role of vertical transmission, from broiler breeder to progenies, in the course of an outbreak of adenoviral gizzard erosion. For this purpose clinical data were analysed and histological, virological and serological investigations were performed.
Materials and Methods
Case history
Affected broilers were reported from 22 different flocks in Germany. One-day-old birds were placed on the farms between week 17 and week 20 of 2011. All birds belonged to the same breed (Ross 308) and were raised indoors on deep litter. Flock size ranged from 7000 to 62,000 broilers. The first signs of clinical problems were noticed in week 19. Roughly the same picture presented itself in all affected flocks. The chicks started growing apart from 9 days old onwards. We estimated that about 5 to 10% of the birds per flock were affected. No additional clinical signs were seen. Mortality was recorded to be in the normal range. No pathogenic agents were detected by routine bacteriological investigations. Polymerase chain reactions (PCRs) were performed occasionally to detect infectious bursal disease virus, chicken anaemia virus and reovirus were negative.
All affected broiler flocks were recognized to originate from the same broiler breeder farm. This farm comprised three flocks (A, B, C), 25,000 birds each. During production of the affected broilers, broiler breeders were between 27 and 32 weeks old. Furthermore, the broiler breeders on this farm experienced a moderate drop in the number of hatched chicks between weeks 29 and 32 of life, while egg production remained normal. No further clinical signs could be observed from birds on the broiler breeder farm.
Dataset of production parameters
Production parameters included the average weight at 7, 14, 21 and 28 days of life for three broiler flocks affected with gizzard erosions (“positive” flocks traced back to a single broiler breeder farm) and three broiler flocks without any clinical signs of gizzard erosions harvested during the same time period (“negative” flocks originated from different breeder flocks). Flocks in both categories were selected randomly. In addition, the total mortality, total selection, age at harvest, average weight at harvest and plant condemnation rate of broiler carcasses at slaughter for the 22 “positive” and 48 “negative” broiler flocks were analysed. The relationship between the origin of the broiler flocks and average weight at harvest was evaluated through an analysis of variance model adjusting for age at harvest. The arithmetic means of average weight at 7, 14, 21 and 28 days of life, total selection, total mortality and condemnation rates of carcasses at slaughter of “positive” and “negative” flocks were analysed by Student's t test. Data were analysed using commercial statistical software (PASW v 17.0; SPSS Inc., Chicago, Illinois, USA). Differences with P<0.05 were considered significant.
Post-mortem examination and sampling
Routine necropsy of 5 to 20 culled chicks per affected broiler flock was performed and gross pathological lesions were documented. Affected gizzards from a few selected birds were taken for histological and virological investigations. In order to demonstrate vertical transmission of FAdV-1, dead-in-shell chicks of the suspected broiler breeder farm were retrieved from the eggs at the lowest point of hatchability. Embryos were examined and gizzard samples were collected. Organ samples were pooled and kept frozen at − 20°C until virus isolation and PCR were performed.
Histology
After post-mortem examinations, gizzard samples of affected broilers were fixed in 3.5% neutral buffered formalin. The samples were then embedded in paraffin. Tissue slices 3 µm in thickness were prepared using a Microm HM 360 microtome (Microm Laborgeräte GmbH, Walldorf, Germany). They were mounted on glass slides and routine staining of the tissue slices was performed using haematoxylin and eosin. In order to confirm FAdV-1 DNA in the gizzard sections, in-situ hybridization was carried out following a protocol described by Liebhart et al. (Citation2006) using a digoxigenin-labelled specific DNA probe based on the FAdV-1 long fibre gene (5′-CGGGGTCGCAGCAGCTGCAGCTCGCGAGCGGAGAACTCG-3′).
Virus isolation, PCR and sequence analysis
Virus isolation was carried out on primary chicken embryo liver cells, prepared from 14-day-old SPF chicken embryos (VALO; Lohmann Tierzucht GmbH, Cuxhaven, Germany) following a method described by Schat & Purchase (Citation1998). Gizzard samples were homogenized in phosphate-buffered saline containing 100,000 IU/ml penicillin and 1 mg/ml streptomycin and were filter sterilized (0.2 µm syringe filter; VWR, Vienna, Austria). This solution was inoculated on nearly confluent chicken embryo liver cells and each sample was passaged up to three times or until a cytopathic effect was observed. A sample was considered negative when no cytopathic effect was noted after three passages.
Using the DNeasy Blood and Tissue kit (Qiagen, Vienna, Austria) according to the manufacturer's instructions, DNA was extracted from gizzard sample homogenates and tissue culture supernatant. Detection of FAdV DNA was done by amplifying the loop 1 region of the hexon gene, following a protocol described by Meulemans et al. (Citation2001). The resulting amplification products were analysed by 1.5% agarose gel electrophoresis and stained with ethidium bromide. Fragments of the correct length were excised from the gel, purified using the QIAquick gel extraction kit (Qiagen) and used as templates for direct fluorescence-based sequencing by LGC Genomics GmbH (Berlin, Germany) and Eurofins MWG Operon (Ebersberg, Germany). Assembly and analyses were performed with Accelrys Gene, version 2.5 (Accelrys Inc., San Diego, California, USA) and Lasergene software (DNASTAR Inc., Madison, Wisconsin, USA). Additionally, the nucleotide sequences of the long and short fibres from broiler and dead-in-shell chick isolates were investigated and compared with each other, as well as with “pathogenic” FAdV-1 isolates described by Marek et al. (Citation2010).
Serology
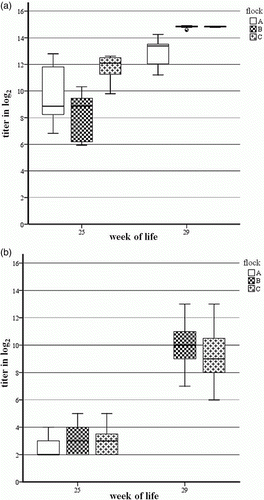
Blood samples from broiler breeder Flocks A, B and C (24 samples per flock) from the suspected broiler breeder farm were taken in weeks 25 and 29 of life in the course of routine monitoring procedures. Blood samples were tested by commercial enzyme-linked immunosorbent assay (ELISA) and by serum neutralization test (VNT). Investigations with “FAdV Group 1 ELISA” (BioChek Ltd, London, UK) were carried out and evaluated according to the manufacturer's instructions. For the VNT, sera were inactivated for 30 min at 56°C in a thermomixer (Eppendorf, Vienna, Austria). The VNT was performed according to a constant virus diluted serum method using 100 µl of 100 median tissue culture infective dose/100 µl FAdV-1 virus isolated from progeny broilers with gizzard erosions. The plates were incubated for 5 days at 37°C under 5% CO2 conditions and were investigated for a cytopathic effect. An antibody titre above 3 log2 was regarded as positive. Unfortunately, blood samples from Flock A at week 29 of life were not available for VNT.
Results
Production parameters
The dataset of average weight at 7, 14, 21 and 28 days of life revealed a significantly decreased weight gain in “positive” broiler flocks from 14 days of life onwards (). Additional production parameters are outlined in . Statistics showed that “positive” broiler flocks were documented to have a significantly higher total mortality rate (P<0.02). “Positive” broiler flocks registered significantly higher selection rates (P<0.005), up to 7.4%, during the rearing period. The arithmetic mean of average weight at harvest was 165.9 g lower in “positive” flocks than in “negative” flocks. The association between the occurrence of gizzard erosions within a flock and average weight when adjusted for age at harvest was significant (P<0.005). The plant condemnation rate was not different from “negative” flocks slaughtered at the same time (P=0.9).
Table 1. Average body weight (g) at 7, 14, 21 and 28 days of life for broilers from three flocks originating from the FAdV-1-seropositive broiler breeder farm (“positive farms”) and from three broiler flocks without clinical signs of gizzard erosions hatched from other broiler breeder flocks (“negative farms”).
Table 2. Production parameters (total mortality, selection rate, age at harvest, average weight, plant condemnation rate) of 22 broiler flocks originating from the FAdV-1-seropositive broiler breeder farm (“positive farms”) and from 48 broiler flocks hatched from other broiler breeder flocks (“negative farms”).
Necropsy
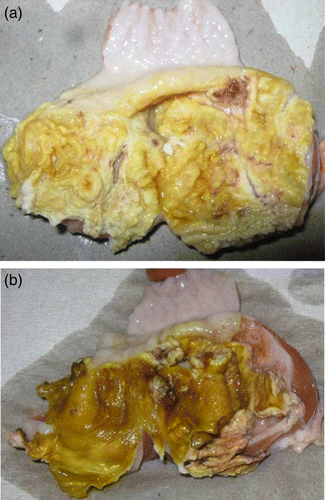
Post-mortem examinations from 9-day-old and older affected broiler chicks showed single or multiple areas of gizzard erosions, characterized by brown or black coloured areas of variable sizes in the koilin layer (a,b). The gizzard mucosa underneath the degenerative koilin layer was inflamed and frequently ulcerative. In a few cases, gizzards were dilated with bloody fluids. Generally, no significant lesions were present in any other organ. The pathological findings were seen until the chickens were slaughtered.
Figure 1. Gizzards of (1a) 19-day-old and (1b) 14-day-old broilers with erosions in the koilin layer and inflammation of the mucosa.
Examination of dead-in-shell chicks revealed that the embryos died between 13 and 18 days of age. Some embryos showed signs of dwarfing.
Histology
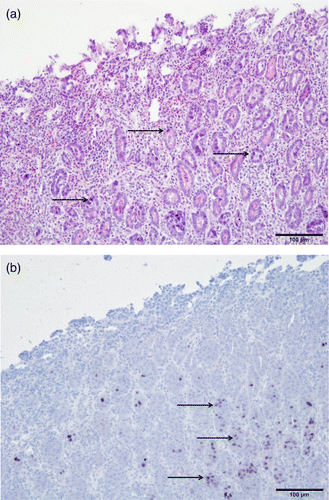
Extensive erosion and loss of the koilin layer was characteristic for the investigated gizzards. Multiple basophilic intranuclear inclusion bodies were observed in the nuclei of degenerating and necrotizing glandular epithelial cells (a). An inflammatory cell infiltration consisting of lymphocytes and heterophils was detected in the mucosa, as well as the submucosa and muscle layer. The intranuclear inclusion bodies of the affected epithelial cells were found positive for FAdV-1 DNA, as demonstrated by in-situ hybridization (b).
Figure 2. Histopathological lesions of the gizzard from an affected broiler. 2a: Erosion of gizzard epithelial cells and infiltration of inflammatory cells in the mucosal membrane. Basophilic intranuclear inclusion bodies can be observed in gizzard epithelial cells (arrows). Haematoxylin and eosin staining. Bar = 100 µm. 2b: In-situ hybridization of gizzard sample. Positive reaction of inclusion bodies with FAdV-1 DNA (arrows). Bar = 100 µm.
Polymerase chain reaction and virus isolation
FAdV was detected by PCR and virus isolation from gizzard of broilers with gizzard erosions. It was possible to isolate FAdV from a pool of gizzards from dead-in-shell chicks at the lowest point of hatchability from Flock C of the broiler breeder farm, although not from Flocks A and B. Sequence analysis of hexon gene PCR products showed isolates to be FAdV-A, serotype 1. These FAdV-1 isolates from broilers and from dead-in-shell chicks had 100% sequence identity to each other within the hexon gene, and the long and the short fibre genes. Furthermore, comparing nucleic acid sequences of long and short fibre genes from FAdV-1 isolates from this case study with “pathogenic” FAdV-1 documented by Marek et al. (Citation2010) showed them to be 100% identical.
Serology
The ELISA detected eight, two and 22 of 24 serum samples positive in week 25 of life from Flocks A, B and C, respectively. In comparison, neutralizing antibodies against FAdV-1 were found in four, seven and six of the 24 serum samples tested, with low titres. High antibody titres could be demonstrated by VNT and ELISA in all serum samples investigated at 29 weeks of age. Comparing the arithmetic mean of titres, the differences between the early and late serum samples were much more pronounced in the homologous virus neutralization assay (+6.74 log2) than in the ELISA (+4.31 log2; ).
Discussion
The present study describes an outbreak of gizzard erosion in 22 broiler flocks. The results of histological and virological investigations indicate that an FAdV-1 infection was the reason for the gizzard erosions. Multiple basophilic intranuclear inclusion bodies were observed within nuclei of degenerating and necrotizing glandular epithelial cells of the gizzard as reported in previous studies of natural outbreaks (Abe et al., Citation2001; Domanska-Blicharz et al., Citation2011). Detection of FAdV-1 DNA in intranuclear inclusion bodies by in-situ hybridization was used for further diagnostic specification.
Clinical observations from the present study indicate that outbreaks of adenoviral gizzard erosion may be found and diagnosed in connection with uneven growth of broiler flocks in the field. Only Domanska-Blicharz et al. (Citation2011) have so far reported uneven growth, depression and dull feathers in connection with an outbreak of gizzard erosions in a Polish broiler flock but detailed clinical data were not reported. Production parameters in this case study show growth retardation of affected broilers already within the first 7 to 14 days of the rearing period. By performing experimental studies, a decreased weight gain was noticed in broiler chickens infected by oral and ocular routes with FAdV-1 approximately 10 days post infection (Okuda et al., Citation2001a). In contrast, experimental infection with FAdV-1 in SPF layers caused neither weight loss nor any other clinical signs (Okuda et al., Citation2001b). As a result of uneven growth, selection rates in flocks with gizzard erosions were significantly higher than in flocks without. The selection process during the rearing period aims to minimize flock variability by monitoring and managing flock uniformity, although it depends heavily on the experience and diligence of flock management personal. Furthermore, significantly lower average weights were documented in broiler flocks with gizzard erosions when compared with flocks slaughtered in the same time period, indicating considerable economic losses. Interestingly, most previous reported cases of adenoviral gizzard erosion were derived from examinations of gizzards directly at the slaughter line from flocks that were reported to have no apparent clinical signs whatsoever and whose production parameters were unremarkable, if investigated at all (Ono et al., Citation2001, Citation2003; Manarolla et al., Citation2009). There are, however, a few documented cases of elevated mortality, generally without any accompanying clinical signs, in the course of adenoviral gizzard erosion (Tanimura et al., Citation1993; Abe et al., Citation2001). Even though mortality during the rearing process in the present study seemed within the norm, we were able to document a statistically higher total mortality rate in flocks with gizzard erosions when compared with flocks reared and slaughtered during the same time period. The FAdV-1 infection seems to have no further negative impact on marketing suitability of broilers, since the plant-condemnation rate did not differ between “positive” and “negative” flocks. Considering that there are a number of broiler diseases which might be more obvious in case of uneven growth precise diagnosis is of high importance to detect unnoticed cases of adenoviral gizzard erosion.
All affected broiler flocks originated from the same broiler breeder farm. Parent birds can serve as a viral reservoir from which transmission through the embryonated eggs may lead to introduction of FAdV-1 into progeny flocks and consequently to induction of adenoviral gizzard erosion. In the present case, results of a commercial “FAdV group 1 ELISA” demonstrated a rise of FAdV antibodies in the first 5 weeks of the broiler breeder production cycle, although nearly all samples from Flock C were seropositive from the beginning. Because the applied ELISA detects FAdV antibodies independent of the serotype, it remains questionable whether the detected antibodies were solely due to the FAdV-1 infection. Antibodies to several different FAdV serotypes have been documented some time ago in broiler breeders applying a serotype specific serum neutralization assay (Monreal, Citation1984). Results of a VNT, using the FAdV-1 field strain, isolated from the gizzard of affected progeny flocks as an antigen, showed a definite increase of serum neutralizing antibodies in the course of 5 weeks and demonstrated an acute infection with the homologous FAdV-1. In contrast to a previous study (Dawson et al., Citation1979), the FAdV-1 infection in this case resulted in a moderate depression of the hatching rate. In another previous study, Cook (Citation1968) described embryo mortality over the period of 2 to 3 weeks in the course of a natural infection with CELO. In accordance with experimental studies with CELO (Yates & Fry, Citation1957), some embryos examined in the present case showed growth retardation.
It was possible to isolate FAdV-1 from a pool of gizzards taken from dead-in-shell chicks at the lowest point of hatchability from one of the three flocks of the broiler breeder farm. These results are in agreement with experimental studies indicating that vertical transmission occurs even in the presence of high levels of virus neutralizing antibodies (Mazaheri et al., Citation2003). Furthermore, the same research suggests that vertical transmission may occur only intermittently. The FAdV-1 strains isolated from gizzards of embryos at the lowest point of hatchability were genetically identical with strains isolated from affected broiler flocks raised at independent farms. Moreover, these isolates demonstrated 100% sequence identity within the hexon, long and short fibre genes to “pathogenic” isolates, typed from gizzard erosion outbreaks (Marek et al., Citation2010). Toro et al. (Citation2001) reported the vertical transmission of FAdV-4 from infected breeders to progeny and the development of disease signs in progeny up to 4 weeks after experimental infection.
In the present case, no problems of growth retardation or occurrence of gizzard erosions in progeny flocks beyond week 32 of the breeders were noticed, indicating that vertical induction of adenoviral gizzard erosion was a time-limited incidence. Unfortunately, we were unable to investigate embryos for the presence of FAdV-1 at a later date because no samples were available.
The present study confirms that vertical transmission of FAdV-1 and subsequent manifestation of adenoviral gizzard erosion occurs within broiler production. Furthermore, considerable economic losses in the course of FAdV-1-induced gizzard erosions were documented in the present outbreak, backing arguments for precise monitoring and diagnostics.
References
- Abe , T. , Nakamura , K. , Tojo , T. and Yuasa , N. 2001 . Gizzard erosion in broiler chicks by group I avian adenovirus . Avian Diseases , 45 : 234 – 239 . doi: 10.2307/1593034
- Adair , B.M. and Fitzgerald , S.D. 2008 . “ Group 1 adenovirus infections ” . In Diseases of Poultry , 12th ed , Edited by: Saif , Y.M. , Fadly , A.M. and Glisson , J.R. 260 – 286 . Ames , IA : Iowa State University Press .
- Cook , J.K.A. 1968 . Isolation of a CELO virus from fertile chicken eggs . The Veterinary Record , 82 : 294
- Dawson , G.J. , Yates , V.J. , Chang , P.W. , Orsi , L.N. and Pronovost , A.D. 1979 . Egg transmission of avian adenovirus-associated virus and CELO virus during a naturally occurring infection . The American Journal of Veterinary Research , 40 : 1624 – 1627 .
- Domanska-Blicharz , K. , Tomczyk , G. , Smietanka , K. , Kozaczynski , W. and Minta , Z. 2011 . Molecular characterization of fowl adenoviruses isolated from chickens with gizzard erosions . Poultry Science , 90 : 983 – 989 . doi: 10.3382/ps.2010-01214
- Du Bose , R.T. and Grumbels , L.C. 1959 . The relationship between quail bronchitis virus and chicken embryo lethal orphan virus . Avian Diseases , 3 : 321 – 344 . doi: 10.2307/1587679
- Grgic , H. , Philippe , C. , Ojkic , D. and Nagy , E. 2006 . Study of vertical transmission of fowl adenoviruses . Canadian Journal of Veterinary Research , 70 : 230 – 233 .
- Liebhart , D. , Weissenböck , H. and Hess , M. 2006 . In-situ hybridization for the detection and identification of Histomonas meleagridis in tissues . Journal of Comparative Pathology , 135 : 237 – 242 . doi: 10.1016/j.jcpa.2006.08.002
- Lim , T.H. , Kim , B.Y. , Kim , M.S. , Jang , J.H. , Lee , D.H. , Kwon , Y.K. , Lee , J.B. , Park , S.Y. , Choi , L.S. and Song , C.S. 2012 . Outbreak of gizzard erosion associated with fowl adenovirus infection in Korea . Poultry Science , 91 : 1113 – 1117 . doi: 10.3382/ps.2011-02050
- Manarolla , G. , Pisoni , G. , Moroni , P. , Gallazzi , D. , Sironi , G. and Rampin , T. 2009 . Short communications adenoviral gizzard erosions in Italian chicken flocks . The Veterinary Record , 164 : 754 – 756 . doi: 10.1136/vr.164.24.754
- Marek , A. , Schulz , E. , Hess , C. and Hess , M. 2010 . Comparison of the fibers of fowl adenovirus a serotype 1 isolates from chickens with gizzard erosions in Europe and apathogenic reference strains . Journal of Veterinary Diagnostic Investigation , 22 : 937 – 941 . doi: 10.1177/104063871002200613
- Mazaheri , A. , Prusas , C. , Voß , M. and Hess , M. 2003 . Vertical transmission of fowl Adenovirus serotype 4 investigated in specified pathogen-free birds after experimental infection . Archiv für Geflügelkunde , 67 : 6 – 10 .
- McFerran , J.B. , Adair , B. and Connor , T.J. 1975 . Adenoviral antigens (CELO, QBV, GAL) . The American Journal of Veterinary Research , 36 : 527 – 529 .
- Meulemans , G. , Boschmans , M. , Van den Berg , T.P. and Decaesstecker , M. 2001 . Polymerase chain reaction combined with restriction enzyme analysis for detection and differentiation of fowl adenoviruses . Avian Pathology , 30 : 655 – 660 . doi: 10.1080/03079450120092143
- Monreal , G. 1984 . Nachweis von neutralisierenden Antikörpern gegen 11 Serotypen der aviären Adenoviren . Archiv für Geflügelkunde , 48 : 245 – 250 .
- Okuda , Y. , Ono , M. , Yazawa , S. , Imai , Y. , Shibata , I. and Sato , S. 2001a . Pathogenicity of serotype 1 fowl adenovirus in commercial broiler chickens . Avian Diseases , 45 : 819 – 827 . doi: 10.2307/1592862
- Okuda , Y. , Ono , M. , Yazawa , S. , Shibata , I. and Sato , S. 2001b . Experimental infection of specific-pathogen-free chickens with serotype-1 fowl adenovirus isolated from a broiler chicken with gizzard erosions . Avian Diseases , 45 : 19 – 25 . doi: 10.2307/1593007
- Ono , M. , Okuda , Y. , Shibata , I. , Sato , S. and Okada , K. 2007 . Reproduction of adenoviral gizzard erosion by the horizontal transmission of fowl adenovirus serotype 1 . Journal of Veterinary Medical Science , 69 : 1005 – 1008 . doi: 10.1292/jvms.69.1005
- Ono , M. , Okuda , Y. , Yazawa , S. , Shibata , I. , Sato , S. and Okada , K. 2003 . Outbreaks of adenoviral gizzard erosion in slaughtered broiler chickens in Japan . The Veterinary Record , 153 : 775 – 779 .
- Ono , M. , Okuda , Y. , Yazawa , S. , Shibata , I. , Tanimura , N. Kimura , K. 2001 . Epizootic outbreaks of gizzard erosion associated with adenovirus infection in chickens . Avian Diseases , 45 : 268 – 275 . doi: 10.2307/1593040
- Saifuddin , M.D. and Wilks , C.R. 1991 . Vertical transmission of avian adenovirus associated with inclusion body hepatitis . New Zealand Veterinary Journal , 39 : 50 – 52 . doi: 10.1080/00480169.1991.35659
- Schat , K.A. and Purchase , H.G. 1998 . “ Cell-culture methods ” . In A Laboratory Manual for the Isolation and Identification of Avian Pathogens , 4th ed , Edited by: Swayne , D.E. , Glisson , J.R. , Jackwood , M.W. , Pearson , J.E. and Reed , W.M. 223 – 234 . Kennett Square : University of Pennsylvania .
- Tanimura , N. , Nakamura , K. , Imai , K. , Maeda , M. , Gobo , T. Nitta , S. 1993 . Necrotizing pancreatitis and gizzard erosion associated with adenovirus infection in chickens . Avian Diseases , 37 : 606 – 611 . doi: 10.2307/1591697
- Toro , H. , González , O. , Escobar , C. , Cerda , L. , Morales , M.A. and González , C. 2001 . Vertical induction of the inclusion body hepatitis/hydropericardium syndrome with fowl adenovirus and chicken anemia virus . Avian Diseases , 45 : 215 – 222 . doi: 10.2307/1593031
- Yates , V.J. and Fry , D.E. 1957 . Observations on a chicken embryo lethal orphan (CELO) virus . The American Journal of Veterinary Research , 18 : 657 – 660 .