Abstract
The use of attenuated vaccines or the occurrence of low virulent T-lymphotropic or B-lymphotropic viruses in flocks may alter the immune responses of young chicks in spite of the absence of clinical signs. Infections with a low virulent T-lymphotropic chicken infectious anaemia virus (lvCIAV) followed by infection with an intermediate B-lymphotropic infectious bursal disease virus (iIBDV) were conducted in specific pathogen free chicks. Thirty-six 1-day-old chicks were infected with the lvCIAV strain (CAV-VAC®) and a similar number of chicks were inoculated with phosphate-buffered saline. At 14 days after lvCIAV infection, one group of 18 lvCIAV-infected chicks and one group of 18 uninfected chicks were infected with an iIBDV strain. At 4, 7 and 14 days post infection with iIBDV, six chicks from each group were euthanized and lymphoid organs were collected. Detection of lvCIAV and iIBDV genomes was conducted by polymerase chain reaction and reverse transcriptase-polymerase chain reaction, respectively. Double-labelled lymphoid subsets from the thymus, spleen and bursa were studied by cytofluorometric analysis. The results reveal that previous infection with lvCIAV increases the occurrence of the lvCIAV and iIBDV genome in thymus and/or bursa without the occurrence of clinical signs in dually lvCIAV/iIBDV-infected chicks. However, the decreases of B cells in spleen and bursa and increases of T-cell subsets in bursa observed in chicks infected with iIBDV did not occur in chicks previously infected with lvCIAV. Taken together, these results suggest that previous infection of young chicks with lvCIAV decreases lymphoid disorders induced by iIBDV while subsequent iIBDV infection increases the lvCIAV genome in lymphoid organs.
Introduction
The control of poultry infectious diseases is an ongoing challenge because of environmental changes, changes in practice and pathogen evolution. The use of vaccinal or attenuated strains of lymphotropic viruses, such as infectious bursal disease virus (IBDV) or chicken infectious anaemia virus (CIAV), may modulate the innate and adaptative immune responses of the host leading to increased virulence of important pathogens (Kaiser, Citation2010).
Infectious bursal disease has been one of the most important avian viral diseases since its first appearance about 50 years ago and continues to pose a considerable threat to the poultry industry worldwide. The virus is ubiquitous, highly contagious, spreads by direct contact between infected and susceptible chickens and is resistant to environmental exposure (Lukert & Saif, Citation2003). When chicks are infected at an early age, clinical and subclinical infections occurring in flocks stem from humoral immunosuppression. The outcome of an IBDV infection largely depends on the viral strain and the amount of the infecting virus, the age and the breed of the bird, the route of inoculation, and the presence or absence of neutralizing antibodies (Müller et al., Citation2003). IBDV isolates have been classified into pathotypes according to their virulence: mild intermediate (i) strain (subclinical infection), and virulent or very virulent (vv) strains causing clinical signs and mortality. The IBDV replication leads to the destruction of IgM-bearing lymphoid cells in the bursa of Fabricius (Ivanyi & Morris, Citation1976; Hirai & Calnek, Citation1979; Hirai et al., Citation1981) and causes an acute immunosuppressive disease in the chicken. The IBDV can alter to a lesser extent other lymphoid organs such as the thymus, caecal tonsils and spleen (Ivanyi & Morris, Citation1976; Hirai et al., Citation1981; Rodenberg et al., Citation1994; Elankumaran et al., Citation2002). Thymic lesions correlate with extensive apoptosis of thymocytes in the absence of IBDV replication (Sharma et al., Citation1993; Tanimura & Sharma, Citation1998). It was demonstrated that functional T cells are essential to control the IBDV replication in the acute phase of the infection whilst also being involved in tissue damage and delayed recovery (Rautenschlein et al., Citation2002).
The immunological disorders induced by IBDV may differ according to virulence of the strain. Tanimura & Sharma (Citation1998) performed in vivo studies on the bursa of Fabricius in specific pathogen free (SPF) chickens following inoculation with different strains of IBDV, including a classical virulent strain and an attenuated vaccinal strain. The appearance of large numbers of apoptotic lymphocytes in IBDV antigen-positive and antigen-negative bursal follicles was seen in SPF chicks infected with the classical IBDV strain. A dramatic infiltration of CD4+ and CD8+ T cells occurred at 7 days post infection (p.i.) in the bursa of virulent IBDV-infected 3-week-old SPF chickens (Sharma et al., Citation2000). The intrabursal T cells promote bursal tissue damage (apoptosis) and delay tissue recovery without IBDV replication (Tanimura & Sharma, Citation1998; Sharma et al., Citation2000). However, apoptotic cells in the bursa slowly increased in the attenuated vaccine IBDV-infected birds. Lower numbers of T cells infiltrating the bursal follicle and an increase of viral replication were observed in cyclosporin A-treated chickens, suggesting that T-cell immunodeficiency may promote viral persistence in the bursa (Rautenschlein et al., Citation2002). In addition, passive administration of neutralizing anti-IBDV antibodies is protective for T-cell-intact chickens, but not for thymectomized and cyclosporin A-treated chickens, indicating that T-cell involvement is critical for the protection of chickens against IBDV infection (Tanimura & Sharma, Citation1998; Sharma et al., Citation2000).
CIAV, a circovirus, can infect chickens and decrease thymic and splenic T cells, compromising the ability of birds to mount a successful T-cell-dependent immune response against invading pathogens (Adair et al., Citation1991). In a sequential study, CIAV was detected as soon as 4 days p.i. in the spleen and thymus of CIAV-infected 1-day-old chickens (Smyth et al., Citation1993). The virus can replicate in dividing cells such as T-cell precursors in the thymus or dividing T cells in response to antigenic stimulation, and in haemocytoblasts in the bone marrow (reviewed in Adair, Citation2000; Schat & Skinner, Citation2008). B cells and their precursors are not susceptible to CIAV infection and no substantial depletion of B-cell numbers comparable with the dramatic depletion in numbers of T cells is observed following CIAV infection. When chickens are infected at 3 or 6 weeks of age they rapidly develop a resistance to the experimentally-induced disease, although they remain susceptible to viral replication since CIAV-infected cells were detected in the thymus and rarely in other tissues (Smyth et al., Citation2006). Furthermore, the virus can persist in chickens long after seroconversion in reproductive tissues (Cardona et al., Citation2000). We have recently observed the persistence of a vaccinal strain of CIAV up to 28 days p.i. leading to lymphoid cell disorders in the thymus and spleen in a few SPF chicks (Vaziry et al., Citation2011). Splenic CD4+ cells and small thymic CD4+CD8+ cells as well as their CD4 expression level decreased after 14 days in lvCIAV-infected birds, suggesting a T-cell immunosuppression. The anti-CIAV antibody response remained low and disappeared after 18 days.
Previous co-infection experiments of virulent CIAV with other immunosuppressive viruses, such as IBDV, reticuloendotheliosis virus and Marek's disease virus, showed synergistic effects on the pathogenesis, clinical signs and immunosuppression (reviewed in Schat & van Santen, Citation2008). Chickens inoculated simultaneously with virulent CIAV and IBDV experienced a prolonged acute phase prior to recovery or mortality (Cloud et al., Citation1992a). Imai et al. (Citation1999) observed that vvIBDV infection inhibited production of virus-neutralizing antibody to CIAV up to 40 days of age and the CIAV was isolated at higher titres from plasma and blood cells as a result of the IBDV-induced humoral immunosuppression. Virulent CIAV infection of 1-day-old chicks, followed by vaccination 2 weeks later with Newcastle disease virus or infectious laryngotracheitis virus resulted in compromised protection when challenged with the relevant virus. (Cloud et al., Citation1992b).
The immune consequences of subclinical infections induced by attenuated or low virulent (lv) CIAV and IBDV viruses have not been studied despite the use of attenuated (vaccinal) strains and the broad occurrence of subclinical infections with these viruses in the field. In this work, we verify whether B-cell and T-cell disorders, the presence of viral genomes, and humoral anti-viral responses induced by iIBDV infection in young chicks are modulated by a previous lvCIAV infection.
Materials and Methods
Chickens and experimental design
Embryonated SPF eggs (including freedom from CIAV and IBDV) were obtained from the Veterinary Laboratories Agency (Nepean, Ontario, Canada) and were incubated, hatched and reared in the Faculty of Veterinary Medicine facilities (St-Hyacinthe, Quebec, Canada). All procedures were approved by the Université de Montréal animal care committee. Seventy-two 1-day-old SPF chicks were divided into four groups of 18 birds each and housed separately in chicken isolators under sterile conditions in a room under negative pressure. At hatching, birds from two groups (36 chicks) received an intraperitoneal injection of 5 µl of the lvCIAV strain (CAV-VAC®; Intervet, Millsboro, Delaware, USA) while the other two groups (36 chicks) were inoculated with the same volume of phosphate-buffered saline (PBS). The viral titre in the commercial CAV-VAC® vaccine is not available but the recommended volume for vaccination has been used. At 14 days of age, one group of uninfected chicks (iIBDV-infected group) and one group of lvCIAV-infected chicks (dually lvCIAV/iIBDV-infected group) were infected intraperitoneally with 100 TCID50/µl (5 µl) of the intermediate IBDV-QT1, a Quebec isolate of infectious bursal virus (Reddy & Silim, 1991a). This strain was isolated from a turkey showing arthritis and respiratory signs and shows partial antigenic difference with the IBDV belonging to the serotype 1, known to be pathogenic for chickens. This variant may be considered an intermediate IBDV strain for fowl since no clinical signs were observed in SPF chicks. The second group of uninfected chicks (control group) and lvCIAV-infected chicks received PBS. The use of the intraperitoneal, rather than the oral or ocular, route for the administration of viruses was chosen to increase the likelihood of occurrence of clinical signs (van Santen et al., Citation2004), to favour infection of macrophages (Mueller et al., Citation1979) and to permit a comparable distribution of viruses in lymphoid organs between chicks. At 18, 21 and 28 days p.i. with lvCIAV, or at 4, 7 and 14 days p.i. with iIBDV, six chicks from each group were weighed, blood-sampled by cardiac puncture and euthanized by CO2 anoxia.
Sampling and cell extraction
The blood samples were collected directly into heparinized microhaematocrit tubes for packed cell volume (PCV) determinations and also for white blood cell counts (WBC) and differential analysis. The thymus, spleen, bone marrow and bursa were collected under sterile conditions and subjected to lymphocyte extraction procedure. Samples of sera and caecal tonsils were also collected and kept frozen until testing. Isolation of lymphocytes from the spleen and the thymus was conducted by mincing each tissue into fragments in RPMI 1640 media supplemented with 20% foetal bovine serum and antibiotic–antimycotic solution (GIBCO Laboratories, Grand Island, New York, USA), and the resulting tissue suspensions were passed through a 70 µm cell strainer (Falcon Scientific Co., Montreal, Quebec, Canada). Lymphocytes from the spleen and thymus cell suspensions were further enriched by centrifugation at 1000×g for 20 min on a Lymphoprep gradient (Cedarlane, Hornby, Ontario, Canada). Bursa tissues were homogenized by glass beads. The recovered lymphocyte layer from spleen and thymus and the original cell suspension from bursa samples were washed in fresh media by centrifugation at 500×g for 10 min. The cell suspensions of thymus, spleen, and bursa were enumerated using a haemacytometer with trypan blue (Fischer Scientific, Montréal, Quebec, Canada), adjusted to 106 viable cells per 1 ml and used for different assays.
Haematology
Peripheral leukocyte analyses such as PCV, WBC and differential percentages of heterophils, monocytes, lymphocytes, eosinophils, and basophils were performed by May–Grunwald's staining and light microscopic examination.
Immunolabelling of lymphocyte subsets
The phenotype of lymphocyte subpopulations such as CD4+CD8−, CD4−CD8+, CD4+CD8+, CD3−CD8+, CD3−IgM+, and CD3+TCRγδ+ cells was determined by double immunolabellings (CD4 and CD8, CD3 and CD8, CD3 and IgM, or CD3 and TCRγδ markers) using fluorescein isothiocyanate (FITC)-conjugated anti-CD4, anti-IgM, anti-CD3 or anti-TCR-γδ, and phycoerythrine (PE)-conjugated anti-CD8a or anti-CD3 monoclonal antibodies (Southern Biotech, Birmingham, Alabama). For each double staining, 1×106 cells from thymic, splenic and bursal lymphocyte suspensions were incubated with 1 µg anti-chicken monoclonal antibodies labelled with FITC or PE for 30 min at 4oC. Cells were then washed gently three times in RPMI-1640 and fixed overnight at 4oC in PBS, pH 7.2, containing 1% formaldehyde (Fischer Scientific). Cytofluorometric analysis of positive FITC-stained and PE-stained cells was performed on a FACScan cytofluorometer (Becton Dickinson, Mountain View, California, USA) using CellQuest software (Becton Dickinson, San Jose, California, USA). Analysis was done on 10,000 events and discrete viable lymphoid cell populations were gated according to forward scatter versus 90o angle scatter parameters. Percentages of different lymphoid cell subpopulations in thymus, spleen and bursa were determined by multiparametric analysis.
Chicken infectious anaemia and infectious bursal disease viral genome detection
Total DNA or RNA was extracted from thymus, spleen, bursa, and caecal tonsils using Trizol® LS Reagent (Life Technologies, Grand Island, New York, USA) according to the manufacturer's procedure. After the phase separation step, the aqueous upper phase was collected for RNA precipitation while DNA was isolated from the interphase and the phenol phase.
Viral VP3 DNA of CIAV detection by polymerase chain reaction
To detect the viral genome in the samples, fragments of 374 base pairs (bp) located between nucleotides 472 and 846, and of 203 bp located between nucleotides 588 and 791, were targeted to be amplified in conventional and nested polymerase chain reaction (PCR), respectively. A set of primers was designed and used as follows:
Forward (5′-CTCTCCAAGAAGATACTCCAC-3′),
Reverse (5′-GCTCGTCTTGCCATCTTA-3′), and
Forward nested (5′-ATCACTCTATCGCTGTGTGG-3′)
Reverse nested (5′-GGAGTAGTGGTAATCAAGC-3′).
The PCR programme consisted of an initial denaturation at 94°C for 5 min, and 35 cycles of 94°C for 35 sec, 58°C for 55 sec, and 72°C for 1 min followed by a final extension at 72°C for 5 min. PCR products were separated by 100 V electrophoresis in a 1.4% agarose gel in TAE buffer (40 mM Tris and 2 mM ethylenediamine tetraacetic acid, pH 8.0) containing 0.5 mg/ml ethidium bromide for 60 min, and were visualized under an ultraviolet light transilluminator.
Viral VP2 RNA of IBDV detection by reverse transcriptase-PCR
To detect the infectious bursal disease viral genome in the lymphoid organs samples, a 604 bp product, in the VP2 gene, was amplified by a two step reverse transcriptase (RT)-PCR. In the first step, the cDNA was produced in 25 µl reaction volume using SuperScript® II Reverse Transcriptase (Life Technologies) and used as templates in the IBDV PCR with the following primers:
Forward (5′-TGTAAAACGACGGCCAGTGCATGCGGTATGTGAGGCTTGGTGAC-3′),
Reverse (5′-CAGGAAACAGCTATGACCGAATTCGATCC TGTTGCCACTCTTTC-3′).
The PCR programme consisted of a primary denaturation at 95°C for 5 min, and 35 cycles of 95°C for 30 sec, 64°C for 45 sec, 70°C for 1 min and a final extension at 70°C for 10 min. PCR products were separated by 100 V electrophoresis in a 1.2% agarose gel in TAE buffer containing 0.5 mg/ml ethidium bromide for 60 min and visualized under an ultraviolet light transilluminator.
Proliferation assay of lymphocytes
Metabolic activity of lymphocytes from lvCIAV-infected and/or iIBDV-infected groups of chicks were evaluated by the tetrazolium salt reduction test (Promega, Madison, Wisconsin, USA) and compared with the unvaccinated uninfected control group. Thymic and splenic lymphocytes were seeded at 105 per well (in 100 µl volume) in flat-bottomed microtitre plates in RPMI 1640 containing 20% foetal bovine serum, 2-mercaptoethanol (55 µM) and antibiotics (GIBCO Laboratories). Splenic and thymic cells were incubated with optimal concentration of ConA (100 µg/ml, 1000 µg/ml, respectively) for 72 h at 37oC and 5% CO2. 3-(4,5-Dimethylthiazol-2-y1)-5-(3-carboxymethoxyphenyl)-2-(4-sulphophenyl)-2H tetrazolium (MTS) and phenazine methosulphate (PMS) were added and the optical density (OD) was recorded at 490 nm using a plate reader 4 h later (Molecular Devices, Sunnyvale, California, USA). The level of stimulation was calculated using the formula: (OD stimulated – OD of unstimulated control / OD of unstimulated control).
Specific anti-CIAV and anti-IBDV antibodies
Specific anti-CIAV antibodies were quantified in serum by enzyme-linked immunosorbent assay (ELISA) using the IDEXX FlockChek CIAV test kit according to the manufacturer's procedure (IDEXX Laboratories, Inc., Westbrook, Maine, USA). Titration of anti-IBDV antibodies in chick sera was carried out using commercial IBD-Plus kits provided by Synbiotics Corporation (San Diego, California, USA) according to the manufacturer's protocol. Regular titres and sample-to-positive values calculated by Synbiotics software were used for statistical analysis.
Statistical analysis
Percentages of blood cells, blastic transformation, lymphocyte subsets in lymphoid organs, presence of nucleic acids for CIAV and IBDV, and numbers of seropositive chicks for each virus from lvCIAV-infected and/or iIBDV-infected birds groups were analysed with a one-way analysis of variance (ANOVA) test using statistical software (PASW version 18; IBM SPSS Inc., Chicago, IL, USA). Following the significant main effect, a post-hoc Tukey analysis was performed on the four groups. Results from lvCIAV-infected and/or iIBDV-infected groups were compared with control birds, lvCIAV-infected or iIBDV-infected groups according to experiments. Values of P ≤ 0.05 were considered significant.
Results
Haematologic evaluation of lvCIAV-infected SPF chicks infected with a low virulent strain of IBDV
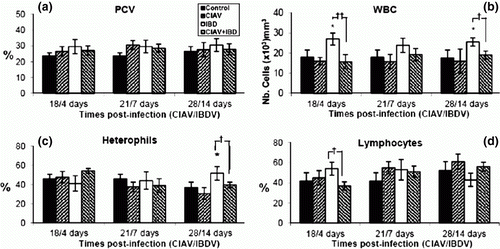
Seventy-two SPF chicks were separated into four groups, two of which were vaccinated with lvCIAV at 1 day of age. At 14 days of age, one lvCIAV-infected group and one uninfected group were then infected with iIBDV. The other two groups received PBS. Six chicks of each group were sacrificed at 4, 7 and 14 days p.i. with iIBDV (corresponding to 18, 21 and 28 days p.i. with lvCIAV). No clinical or anaemia signs, reduction of weight gain, or thymic atrophy as revealed by gross examination were observed in any chick. No significant effect on PCV was observed in any of the groups at any time p.i. (a). The WBC increased in iIBDV-infected chicks at 4 and 14 days p.i. (P ≤ 0.05). The WBC decreased in the iIBDV-infected birds when they were previously infected with lvCIAV (P ≤ 0.01 and 0.05) (b when compared with the iIBDV-infected group). Percentages of heterophils increased at 14 days p.i. in iIBDV-infected birds (P ≤ 0.05). Such an increase was not observed in dually lvCIAV/iIBDV-infected birds when compared with iIBDV-infected birds (P ≤ 0.05) (c). Percentages of lymphocytes decreased in dually lvCIAV/iIBDV-infected birds when compared with the iIBDV-infected group at 4 days p.i (18 days p.i. with lvCIAV) (P ≤ 0.05) (d). In addition, lymphoid cells isolated from thymus decreased in iIBDV-infected chicks (123×106±85 cells) when compared with control chicks (296×106±83 cells) (P ≤0.05) but significantly increased in dually lvCIAV/iIBDV-infected (428×106±142 cells) when compared with iIBDV-infected chicks (P ≤ 0.05). Similarly, lymphoid cells isolated from the bursa also decreased in iIBDV-infected chicks (14.8×106±5.8 cells) when compared with the control group (83.8×106±31.4 cells) (P ≤ 0.01) and increased in dually lvCIAV/iIBDV-infected chicks (106×106±34 cells) when compared with iIBDV-infected chicks (P ≤ 0.001). Numbers of lymphoid cells isolated from spleen and bone marrow did not differ significantly between the four groups of chicks.
Figure 1. Haematological examination of blood from control, lvCIAV-infected, iIBDV-infected and dually lvCIAV/iIBDV-infected groups of SPF chicks. Groups of 18 SPF chicks were infected with lvCIAV at 1 day of age and/or infected with the iIBDV strain 14 days later. Two other groups of 18 1-day-old SPF chicks received PBS and were infected, or not, with iIBDV 14 days later. Haematocrit (PCV) (1a), WBC (1b), percentages of blood heterophils (1c) and lymphocytes (1d) were determined at 18/4, 21/7 and 28/14 days p.i. Values are means of six chicks per group and error bars represent standard deviations. P values were calculated with the ANOVA (post-hoc, Tukey) tests either compared with the control group (*P ≤ 0.05) or with the iIBDV-infected group (†P ≤ 0.05, ††P ≤ 0.01).
Presence of lvCIAV and iIBDV genomes in lymphoid organs
We have previously reported that the lv strain of CIAV (CAV-VAC strain) may persist in the thymus and spleen for up to 28 days in SPF chicks impairing thymopoiesis and CD4+ cell subsets (Vaziry et al., Citation2011). To verify whether a subsequent infection with a iIBDV strain favours the presence of the lvCIAV strain in lymphoid organs from lvCIAV-infected 1-day-old chicks, VP3 DNA of lvCIAV or VP2 RNA of IBDV were revealed by PCR and RT-PCR, respectively, in the thymus, spleen, bursa and caecal tonsils of bird groups infected with lvCIAV and/or iIBDV.
Results shown in indicate that the lvCIAV genome was only detected in one or eight out of 17 chicks in the thymus and spleen, respectively, between 18 and 28 days p.i. (P ≤ 0.01). VP3 DNA of CIAV was detected in the thymus of a higher number of dually lvCIAV/iIBDV-infected chicks (5/16) between 18 and 28 days p.i. when compared with lvCIAV-infected or iIBDV-infected groups, respectively (P ≤ 0.05 and 0.01). Bursa and caecal tonsils remained negative for lvCIAV DNA in successive PCR and nested PCR tests in the lvCIAV-infected group. VP3 DNA of CIAV was not detected in any of the tested organs from the iIBDV-infected group. The VP3 DNA of CIAV, however, was detected in the bursa of three out of the six birds from dually lvCIAV/iIBDV-infected birds at 28/14 days p.i. (P ≤ 0.05 when compared with lvCIAV-infected birds).
Table 1. Detection of VP3 DNA of lvCIAV and VP2 of iIBDV in thymus, spleen, bursa and caecal tonsils of lvCIAV-vaccinated and/or iIBDV-infected SPF chicks.
IBDV VP2 RNA was detected in the bursa of four out of 12 birds after 7 days p.i. in iIBDV-infected chicks, but a higher number of chicks expressed IBDV RNA when dually infected with vCIAV and iIBDV (seven out of 11) (P ≤ 0.05). No IBDV RNA was detected in the thymus or spleen of these birds. The RT-PCR carried out on the organs of 18-day-old, 21-day-old and 28-day-old chicks demonstrated that the lvCIAV-infected groups were free of IBDV genome. No CIAV or IBDV genes were detected in any organ from the control group (results not shown). These results indicate that CIAV genome was more frequently detected in the thymus and bursa of lvCIAV-infected chicks when they were infected with the iIBDV strain 14 days later, while the IBDV genome was detected in the bursa of a significantly higher number of chicks when they were previously infected with lvCIAV.
Blastic transformation of thymic and splenic lymphoid cells
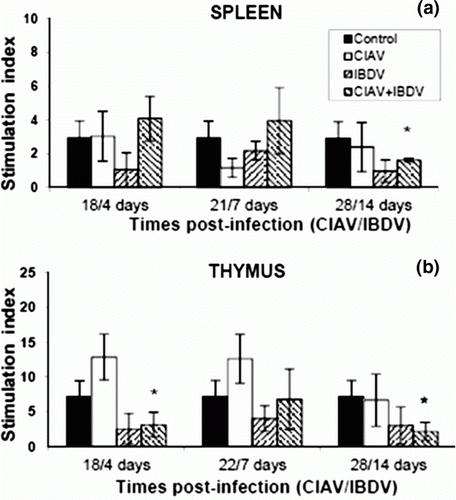
Virulent CIAV and IBDV viruses are known to induce disorders in lymphoid cell blastic transformation (Cloud et al., Citation1992b; Rauw et al., Citation2007). Lymphoid cells from thymus and spleen isolated from lvCIAV-infected and/or iIBDV-infected groups of chicks were cultured in the presence of ConA, and the metabolic activity (measured by MTS/PMS, reflecting cell proliferation) was recorded at 72 h post stimulation. As shown in Figures 2a,b, stimulation levels of splenic cells decreased in dually lvCIAV/iIBDV-infected chicks only at 28/14 days p.i. (P ≤ 0.05) while they decreased significantly at 18/4 and 28/14 days p.i. in the thymus (P ≤ 0.05).
Figure 2. Blastic transformation of splenic (2a) and thymic (2b) lymphocytes of control, lvCIAV-infected, iIBDV-infected and dually lvCIAV/iIBDV-infected groups of SPF chicks. Two groups of 18-day-old SPF chicks were infected with lvCIAV and/or with the iIBDV strain 14 days later. Two other groups of 18 1-day-old SPF chicks received PBS and were infected, or not, with iIBDV 14 days later. Splenic and thymic lymphoid cells were stimulated with ConA (100 µg/ml and 1000 µg/ml, respectively) for 72 h. Metabolic activity of these lymphocytes was evaluated by the tetrazolium salt reduction test at 18/4, 21/7 and 28/14 days p.i. Values are means of six chicks per group and error bars represent standard deviations. P values were calculated with the ANOVA (post-hoc, Tukey) tests compared with the control group (*P ≤ 0.05).
Analysis of lymphoid cell subpopulations
To verify whether the increased occurrence of lvCIAV or iIBDV genome in thymus and/or bursa may be associated with immunodisorders in lymphoid cell populations, as previously reported following CIAV infection with a low virulent vaccinal strain in 1-day old chicks (Vaziry et al., Citation2011), percentages of different lymphoid subsets were analysed in thymus, spleen and bursa from the four groups of chicks. The lymphoid cells were isolated, double-immunolabelled with fluorescent antibodies against CD4, CD8, CD3, TCR-γδ and IgM, and analysed by cytofluorometry. The lymphoid cells were divided into the following subpopulations: single-positive CD4 T cells (CD4+CD8−), single-positive CD8 T cells (CD4−CD8+), double-positive T cells (CD4+CD8+), B cells (IgM+), T cells expressing the TCR γδ (TCRγδ+) and NK-like cells (CD3−CD8+). The times tested were 18, 21 and 28 days p.i. with lvCIAV corresponding, respectively, to 4, 7 and 14 days p.i. with iIBDV (18/4, 21/7 and 28/14 days p.i.)
Thymic cell subpopulations
As shown in a, infection with lvCIAV in 1-day-old chicks and/or with iIBDV at 14 days of age did not significantly alter the CD4+CD8− cell subset at any of the times tested. However, the percentage of CD4+CD8− cells decreased in dually lvCIAV/iIBDV-infected chicks when compared with cells from the iIBDV-infected group at 21/7 and 28/14 days p.i. (P ≤ 0.05).
Figure 3. Percentages of lymphocyte subpopulations in the thymus of control, lvCIAV-infected, iIBDV-infected and dually lvCIAV/iIBDV-infected groups of SPF chicks. Groups of 18 SPF chicks were infected with lvCIAV at 1 day of age and/or infected with the iIBDV strain 14 days later. Two other groups of 18 1-day-old SPF chicks received PBS and were infected, or not, with iIBDV 14 days later. Thymic cells were double-labelled with anti-CD4, anti-CD8, anti-CD3, anti-IgM and anti-TCR-γδ antibodies conjugated to FITC or PE and analysed by cytofluorometry. The mean percentages of thymic CD4+CD8− (3a), CD4−CD8+ (3b), CD4+CD8+(3c), and TCR-γδ+ (3d) subpopulations were determined at 18/4, 21/7 and 28/14 days p.i. Values are means of six chicks per group and error bars represent standard deviations. P values were calculated with the ANOVA (post-hoc, Tukey) tests compared with either the control group (*P≤ 0.05, **P≤ 0.01) or the iIBDV-infected group (†P≤ 0.05, ††P≤ 0.01).
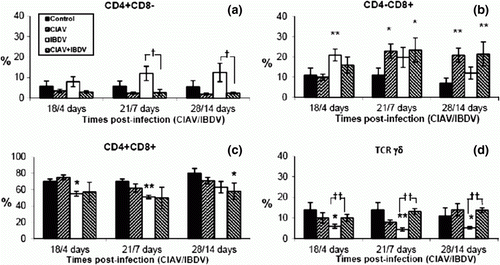
The percentage of thymic CD4−CD8+ cells increased in lvCIAV-infected chicks at 21 and 28 days p.i. (P ≤ 0.05 to 0.01) (b). These cells significantly increased transiently at 4 days p.i. with iIBDV in iIBDV-infected group (P≤ 0.01). The CD4−CD8+ percentages in dually lvCIAV/iIBDV-infected chicks increased similarly to that observed in lvCIAV-infected chicks at 21 and 28 days p.i. with lvCIAV (P ≤ 0.05 to 0.01).
However, the double-positive thymocytes (CD4+CD8+) decreased slightly in iIBDV-infected chicks at 4 and 14 days p.i. (P ≤ 0.05), but also in dually lvCIAV/iIBDV-infected chicks at 28/14 days p.i. (P ≤ 0.05) (c).
The percentages of thymic TCRγδ+ decreased in iIBDV-infected birds (P ≤0.05 to 0.01), but increased in dually lvCIAV/iIBDV-infected when compared with iIBDV-infected group at the three times tested (P ≤ 0.01).
Splenic lymphocyte subpopulations
The phenotypic analysis of lymphoid subsets in the spleen from lvCIAV-infected and/or iIBDV-infected young chicks revealed that percentages of CD4+CD8− cells decreased in lvCIAV-infected chicks (P ≤ 0.05 to 0.01) (Figure 4a). A low decrease of these cells was also observed in iIBDV-infected chicks at 14 days p.i. (P ≤0.05). No changes were noted in dually lvCIAV/iIBDV-infected chicks when compared with the control group, but significant decreases were observed when compared with the iIBDV-infected chicks at 4 and 14 days p.i. (P ≤ 0.05 to 0.01).
Concomitantly, CD4−CD8+ cell percentages slightly increased in the lvCIAV-infected group at 21 and 28 days p.i. (P ≤ 0.05 to 0.01) (b). CD4−CD8+ cells also increased in iIBDV-infected chicks at 14 days p.i. (P ≤ 0.05). These cells increased in dually lvCIAV/iIBDV-infected chicks at 21/7 days p.i. (P ≤ 0.05) but also increased at 18/4 and 21/7 days p.i. when compared with cells from the iIBDV-infected group (P ≤ 0.01). The percentage of CD4−CD8− cells decreased in dually lvCIAV/IIBDV-infected group at 28/14 days p.i. when compared with the iIBDV-infected group (P ≤ 0.05).
Figure 4. Percentages of lymphocyte subpopulations in the spleen of control, lvCIAV-infected, iIBDV-infected and dually lvCIAV/iIBDV-infected groups of SPF chicks. Groups of 18 SPF chicks were infected with lvCIAV at 1 day of age and/or infected with a iIBDV strain 14 days later. Two other groups of 18 1-day-old SPF chicks received PBS and were infected, or not, with iIBDV 14 days later. Spleen cells were double-labelled with anti-CD4, anti-CD8, anti-CD3, anti-IgM and anti-TCR-γδ antibodies conjugated to FITC or PE and analysed by cytofluorometry. The mean percentage of splenic CD4+CD8− (4a), CD4+CD8− (4b), CD4+CD8+(4c), TCR-γδ+ (4d), IgM+ (4e), and CD3−CD8+ NK cells (4f) subpopulations was determined at 18/4, 21/7 and 28/14 days p.i. Values are means of six chicks per group and error bars represent standard deviations. P values were calculated with the ANOVA (post-hoc, Tukey) tests compared with either the control group (*P≤ 0.05, **P≤ 0.01, ***P≤ 0.001) or the iIBDV-infected group (†P≤ 0.05, ††P≤ 0.01).
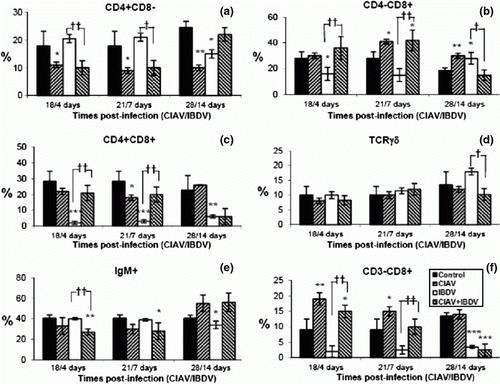
The percentages of CD4+CD8+ cells rapidly decreased in iIBDV-infected chicks (P≤0.01 to 0.001) (c) but were rather increased when chicks were dually lvCIAV/iIBDV-infected at 18/4 and 21/7 days p.i. in comparison with chicks only infected with iIBDV (P ≤ 0.01).
B lymphocytes (IgM+-bearing cells) slightly decreased in iIBDV-infected chicks, reaching a significant level only after 14 days p.i. (P ≤ 0.05) (e). The decreases of B cells, however, occurred sooner in dually lvCIAV/iIBDV-infected chicks when compared with both the control group (P ≤ 0.05 to 0.01) and the iIBDV-infected group (P ≤ 0.01). Percentages of B cells significantly increased in dually lvCIAV/iIBDV-infected chicks at 28/14 days p.i. when compared with the iIBDV-infected group (P < 0.01).
Percentages of NK cells (CD3−CD8+), however, increased in lvCIAV-infected groups at 18 days p.i. but gradually decreased thereafter (P ≤ 0.01 and 0.05) (f). The NK cells decreased in the iIBDV-infected group but only reached significant levels at 14 days p.i. due to a high variability between chicks and low percentages of these cells (P ≤ 0.001). However, NK cells increased in dually lvCIAV/iIBDV-infected chicks at 18/4 and 21/7 days p.i. when compared with cells from the iIBDV-infected group (P≤0.01) but rather decreased at 28/14 days (P≤0.001), as also seen in the iIBDV-infected group.
The splenic TCRγδ cells were not significantly altered by infection with either lvCIAV or iIBDV except for a low decrease in dually infected chicks at 28/14 days p.i. when compared with the iIBDV-infected group (P ≤ 0.05) (d).
Bursal lymphocyte subpopulations
In the bursa of Fabricius, percentages of CD4+CD8− decreased both in lvCIAV-infected groups and iIBDV-infected groups (a) (P ≤ 0.05 to 0.01). The CD4+CD8− cells also decreased in dually lvCIAV/iIBDV-infected chicks (P ≤ 0.01) but to a lesser extent than the iIBDV-infected groups (P ≤ 0.01).
Figure 5. Percentages of lymphocyte subpopulations in the bursa of control, lvCIAV-infected, iIBDV-infected and dually lvCIAV/iIBDV-infected groups of SPF chicks. Groups of 18 SPF chicks were infected with lvCIAV at 1 day of age and/or infected with a iIBDV strain 14 days later. Two other groups of 18 1-day-old SPF chicks received PBS and were infected, or not, with iIBDV 14 days later. Bursal cells were double-labelled with anti-CD4, anti-CD8, anti-CD3 and anti-IgM antibodies conjugated to FITC or PE and analysed by cytofluorometry. The percentages of bursal CD4+CD8− (5a), CD4−CD8+ (5b), CD4+CD8+(5c), and IgM+ (5d) subpopulations were determined at 18/4, 21/7 and 28/14 days p.i. Values are means of six chicks per group and error bars represent standard deviations. P values were calculated with the ANOVA (post-hoc, Tukey) tests compared with either the control group (*P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001) or the iIBDV-infected group (†P≤ 0.05, ††P≤ 0.01, †††P≤ 0.001).
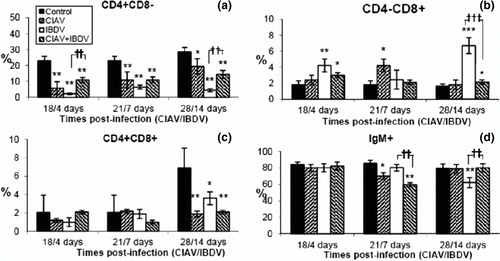
Percentages of bursal CD4−CD8+ cells increased transiently in the lvCIAV-infected chicks at 21 days p.i. (P ≤ 0.05) (b). These cells also increased in iIBDV-infected birds at 4 and 14 days p.i. and at 18/4 and 28/14 days p.i. in the dually lvCIAV/iIBDV-infected group (P ≤ 0.05 to 0.001). However, increase of CD4−CD8+ cells at 28/14 days p.i. was markedly higher in chicks only infected with iIBDV when compared with chicks infected with both viruses (P ≤ 0.001).
The lvCIAV, iIBDV and dual lvCIAV/iIBDV infections reduced the percentages of CD4+CD8+ cells only at 28/14 days p.i. (c) (P ≤ 0.05 and 0.01). However, low percentages of these cells in the bursa and high variability between chicks do not support a strong biological significance of the CD4+CD8+ disturbances.
Percentages of IgM+ cells decreased transiently at 21 days of age in lvCIAV-infected chicks (P < 0.05) (d). iIBDV infection in chicks only reduced B-cell percentages significantly at 14 days p.i. (P < 0.01). B cells decreased transiently in dually lvCIAV/iIBDV-infected chicks at 21/7 days p.i. but increased at 28/14 days p.i. when compared with both control and/or iIBDV-infected groups (P < 0.01).
Production of anti-CIAV and anti-IBDV antibodies
The humoral responses of SPF chicks to the lvCIAV and/or iIBDV infections were monitored by ELISA tests. Infection with lvCIAV did not produce a notable humoral response in most of the infected chicks when administered at hatching () since only two seropositive chicks (out of 36 birds) were observed in both lvCIAV-infected groups (one at 18 days p.i.) and dually lvCIAV/iIBDV-infected groups (one at 21 days p.i.). The antibody titres were also very low. The anti-IBDV antibodies were only detected in one chick from the iIBDV-infected group at 14 days p.i. and in three out of 12 chicks of the dually lvCIAV/iIBDV-infected group at 21/7 (2/6) and 28/14 (1/6) days p.i. Anti-IBDV antibodies were also detected in one chick from the lvCIAV-infected group but the titre was very low. No anti-CIAV or IBDV antibodies were observed in the control group (results not shown).
Table 2. Anti-CIAV or anti-IBDV antibody levels in sera of lvCIAV-vaccinated and/or iIBDV-infected SPF chicks at various times p.i.
Discussion
The present work studied the immune and viral consequences of the low virulent T-lymphotropic CIAV strain on a subsequent subclinical infection with an intermediate B-lymphotropic IBDV strain. The results reveal that previous infection with lvCIAV of young chicks later infected with iIBDV increases the number of chicks with lvCIAV and iIBDV genomes in thymus and/or bursa without occurrence of clinical signs. However, decreases of B cells in spleen and bursa and increases of T-cell subsets in bursa observed in chicks infected with iIBDV did not occur when chicks had been previously infected with lvCIAV. Taken together, these results suggest that previous infection with lvCIAV in young chicks decreases lymphoid disorders induced by iIBDV while subsequent iIBDV infection increases lvCIAV genome in thymus and bursa.
This is the first report of interaction between a low virulent strain of T-lymphotropic CIAV and a low virulent strain (intermediate) of a B-lymphotropic IBDV in young chicks leading to subclinical infections, increased viral genomes in thymus and/or bursa and decreased T-cell disorders induced by iIBDV. In the present study, neither clinical signs nor anaemia or decrease of weight gain were observed in the birds infected with lvCIAV and/or iIBDV strains. Increases of WBC were only noted in the iIBDV-infected group at 4 and 14 days p.i., reflecting a low but not significant increase of lymphocytes at 4 days p.i. and significant increase of heterophils at 14 days p.i. However, such increases were abrogated when birds were previously infected with lvCIAV, suggesting that lvCIAV may alter the cellular effects induced by the iIBDV strain used in this study.
We have recently demonstrated that the genome of this lvCIAV strain can persist in the thymus and spleen of some of the young SPF chicks up to 28 days p.i. without any apparent clinical signs (Vaziry et al., Citation2011). In the present work, we have observed similar proportions of birds with CIAV genome in the thymus and spleen. It was previously demonstrated that chickens rapidly develop resistance to clinical disease within 2 weeks of age when they are infected with a pathogenic CIAV before 7 days old (Jeurissen et al., Citation1992). The presence of infected cells in the thymic cortex of birds infected with CIAV as late as 6 weeks of age has been reported (Noteborn & Koch, Citation1995), however, confirming that CIAV can persist in thymus up to 14 days p.i.
We report here that prior infection with lvCIAV, 14 days before subsequent infection with the iIBDV strain, increases the occurrence of chicks expressing the CIAV genome in the thymus and bursa. Simultaneously, more of these chicks expressed the VP2 RNA of iIBDV in their bursa, suggesting that the presence of the lvCIAV genome may favour iIBDV genome expression up to 14 days p.i. The QT-1 IBDV isolate used in this work is a variant isolated from turkeys showing arthritis and respiratory signs (Reddy & Silim, Citation1991a). Turkeys are susceptible to infection with IBDV but resistant to clinical disease, and most turkey isolates belong to the non-pathogenic serotype 2 (McFerran et al., Citation1980). However, serological studies revealed that the QT-1 IBDV isolate belongs partially to serotype 1, known to be pathogenic for chickens, and it expresses another neutralization site, suggesting that this isolate can be a variant from serotype 1 (Reddy & Silim, Citation1991b). Preliminary experiments indicated that SPF chicks showed no clinical signs when infected with this isolate, revealing its low virulent nature. This low virulence is also supported by the fact that viral genome was detected in bursa or caecal tonsils of less than 50% of birds infected with iIBDV up to 14 days p.i. To avoid the possibility that the QT-1 strain cannot reach lymphoid tissue following inoculation by the oral route (the most common mode of IBDV infection), the virus has been injected intraperitoneally in order to favour the uptake of virus by phagocytic resident macrophages in the gut and its distribution in the bloodstream (Müller et al., 2010).
The lower recovery of viral genome in bursa and spleen than is usually expected in a virulent IBDV infection may reflect the low tropism of this variant for B cells or an efficient antiviral T-cell response. Sharma et al. (Citation1989) have observed that variant serotype 1 isolates can cause similar histological lesions in bursa to serotype 1 isolates but differed by the level of inflammatory responses. T cells may modulate the pathogenesis of IBDV by limiting the viral replication in bursa in the first 5 days p.i. and by subsequently destroying B cells (Rautenschlein et al., Citation2002). It was demonstrated that T-cell immunosuppression induced by cyclosporin treatment increased the levels of viral antigen in bursal follicles (Kim et al., Citation2000). We can thus hypothesize that iIBDV infection with the QT-1 isolate may be efficiently controlled by T cells in iIBDV-infected chicks.
A prolonged acute phase prior to recovery or mortality following simultaneous infections with pathogenic CIAV and IBDV has already been reported (Cloud et al., Citation1992a). The effects of CIAV infection following exposure of chicks varied according to the IBDV strain (Rosenberger & Cloud, Citation1989). Imai et al. (Citation1999) have studied the effect of vvIBDV infection on CIAV infectivity and persistency. They dually infected a group of chickens at 35-day-old and 40-day-old with vvIBDV and CIAV, respectively. They found that vvIBDV infection inhibited the production of virus-neutralizing antibody to CIAV, and increased CIAV titres in plasma and blood cells. Virulent IBDV infection increased the susceptibility of birds to infection with CIAV resulting in an increased mortality rate in CIAV inoculates. Birds that survived virulent IBDV infection became more susceptible to other infectious agents with reduced ability to respond to the vaccines (Allan et al., Citation1972; Müller et al., Citation2003). These effects may result from immunosuppression caused by IBDV (Sharma et al., Citation2000). We have used low virulent strains of CIAV and IBDV and expected a lower presence of viral genomes in lymphoid organs or blood lymphocytes, such as observed in this work.
However, considering the ubiquitous nature of these two viruses and the occurrence of lvCIAV (vaccinal strain) or iIBDV strains in the field, our findings about the immune disorders during subclinical infection and/or following viral persistency induced by lvCIAV infection (Vaziry et al., Citation2011) are not to be ignored. The fact that iIBDV infection in young birds increased the presence of lvCIAV without clinical signs may reflect the inability of the chick's immune responses to clear the two low pathogenic viruses from lymphoid organs. It was demonstrated that SPF chicks are susceptible to CIAV infection within the first 2 weeks of age but can remain susceptible up to at least 21 days when they are previously infected with IBDV at 1 day of age (Cloud et al., Citation1992a), suggesting that the protective role of B cells has been altered following IBDV infection.
We have observed a decrease in the number of lymphoid cells isolated from the bursa and a gradual decrease of B-cell percentages in bursa and spleen of iIBDV-infected chicks. The decreases observed are certainly lower than the B-cell depletion induced by virulent IBDV strains as reported by Kim et al. (Citation2000). The replication of virulent IBDV leads to the destruction of lymphoid cells not only in the bursa of Fabricius but also, to a lesser extent, in other lymphoid organs, such as caecal tonsils and the spleen (reviewed in Berg, Citation2000). It was also reported that IBDV caused rapid atrophy and recruitment of T cells in the bursa, and that the virulence level reflects the apoptotic process and inflammation (Liu & Vakharia, Citation2006).
The serotype 1 isolates of IBDV are known to induce bursal necrosis accompanied by an inflammatory response in contrast to that seen with variant serotype 1 isolates (Sharma et al., Citation1989). We have observed increases of CD4−CD8+ cell percentages in the thymus and bursa of iIBDV-infected chicks, accompanied by decreases in CD4+CD8− only in bursa, suggesting occurrence of a cytotoxic T-cell response in bursa rather than Th-dependent humoral response. Tanimura & Sharma (Citation1998) have previously demonstrated that infection with vvIBDV resulted in extensive viral replication in the bursa and the caecal tonsils with an associated accumulation of T cells. The presence of viral antigen in the bursa and the germinal centres of the caecal tonsils is accompanied by CD3+ cell recruitment in vvIBDV-infected chickens. Increases of intrabursal T cells can limit viral replication in the early phase of the infection but, at the same time, can promote bursal damage and delay tissue recovery (Rautenschlein et al., Citation2002). A recent study revealed critical differences in bursal lesions, infiltration of T cells, expression of pro-inflammatory cytokines and Toll-like receptors 3 and 7 according to the virulence of IBDV strains (Rauf et al., Citation2011).
We have observed that lvCIAV infection 14 days before the iIBDV infection of young chicks did not induce the decrease of B cells nor the increase of CD4−CD8+ cells, but a lower decrease of CD4+CD8− cells in bursa, while no decrease of splenic CD4−CD8+ cells was noted within the first 7 days p.i. Our results suggest that previous lvCIAV infection in 1-day-old chicks may reduce the recruitment of cytotoxic CD4−CD8+ cells in bursa following iIBDV infection, decreasing the bursal B-cell depletion as shown by the number of lymphoid cells isolated from the bursa in dually lvCIAV/iIBDV-infected chicks. It was already demonstrated that, in the absence of T-cell function, the incidence of apoptotic bursal cells and the expression of cytokines such as interleukin-2 and interferon-γ were significantly reduced in comparison with T-cell-intact birds, indicating that T-cell immunodeficiency may partially protect against B-cell apoptosis and inflammatory response (Rautenschlein et al., Citation2002). In contrast, overproduction of interferon-γ by T lymphocytes occurred in chickens infected with IBDV and played a key role in the pathogenesis and immunosuppression induced by this virus (Rauw et al., 2007).
The increase of iIBDV genome presence following lvCIAV infection may also result from T-cell disorders induced by lvCIAV. We have previously reported that lvCIAV infection in 1-day-old SPF chicks decreased thymic CD4+CD8− and small CD4+CD8+ cells, splenic and bursal CD4+CD8− cells as well as expression levels of CD4 on these cells up to 28 days p.i. (Vaziry et al., Citation2011). Markowski-Grimsrud & Schat (Citation2003) also demonstrated that CIAV infection decreased cytotoxic T cells. The increase of lvCIAV genome in the thymus and bursa and its presence in spleen of dually lvCIAV/iIBDV-infected chicks suggest that T-cell subsets recruited in the bursa from thymus or spleen are lvCIAV-infected. They are probably not able to act efficiently as cytotoxic cells against iIBDV-infected B cells, thus favouring an increase of the iIBDV genome in the bursa, as observed in dually lvCIAV/iIBDV-infected chicks. Previous lvCIAV infection of young chicks thus prevents iIBDV-induced cytotoxic T-cell recruitment in the bursa, decreasing the loss of B cells but increasing the presence of iIBDV genome.
On the contrary, increases of lvCIAV genome in thymus and bursa of the dually lvCIAV/iIBDV-infected chicks seem to disagree with the increase of bursal B cells in chicks, suggesting that primary humoral response would be improved. Humoral responses play a major role in the viral clearance of CIAV infection as the age-related resistance to CIAV can be antibody-mediated since persistent infection occurred in older chickens following bursectomy (Hu et al., Citation1993). We have observed a decrease of CD4+CD8− cells in the spleen and bursa from lvCIAV-infected birds and low seroconversions, as previously reported (Vaziry et al., Citation2011). Similar results have been noted in dually lvCIAV/iIBDV-infected chicks. The CD4+CD8− cells are essential for B-cell activation and to mount an efficient antiviral humoral response. Decreases of CD4+CD8− cells cannot support a humoral response even in the presence of higher numbers of B cells. An increase of the lvCIAV genome in the thymus and bursa may thus be favoured by impairment of the collaboration between CD4+ and B cells in the humoral response process and low seroconversion.
In addition, low seroconversion to iIBDV in the IBDV-infected group may result from the decrease of the numbers of bursal lymphoid cells. It is well known that the cytolytic effect of IBDV on B cells leads to impaired primary humoral response (review in Sharma et al., Citation2000). It was also reported that vaccinal IBDV strains induced suppression of the primary antibody response to antigens during the first 6 weeks of virus exposure (Giambrone et al., Citation1977; Rodenberg et al., Citation1994; Kim et al., Citation1999). We have observed no significant seroconversion to CIAV or IBDV in dually lvCIAV/iIBDV-infected chicks when compared with iIBDV-infected or lvCIAV-infected groups. The low seroconversion levels noted was not due to ELISA tests used. Indeed, the ELISA test chosen in this work was more sensitive for iIBDV antibody detection than the neutralization test since the QT-1 strain used partially differs in neutralization sites from the reference virulent IBDV strains (Reddy & Silim, 1991a, b). Moreover, vvIBDV-exposed chickens produced suboptimal levels of antibodies against many antigens or infectious agents (Cho, Citation1970; Faragher et al., Citation1974; Rosenberger & Gelb, Citation1978). We observed one lvCIAV-infected chick with a low titre of anti-IBDV antibodies. The groups of chicks were maintained in separate isolators during the experiments. We cannot exclude an accidental contamination since the antibody titre to IBDV is the lowest observed in all seropositive chicks. However, no IBDV genome was observed in lymphoid organs in any of the chicks from the control group or the lvCIAV-infected group.
Our results also indicate that iIBDV infection decreased percentages of splenic NK cells (CD3−CD8+) and thymic TCRγδ cells. Such decreases were not observed in lvCIAV-infected or dually lvCIAV/iIBDV-infected chicks. It was previously reported that NK cells were not affected by infection with virulent IBDV (Sharma & Lee, Citation1983). Decreases of NK or thymic TCR-γδ cell percentages following iIBDV infection may well reflect increases of other cell subsets, such as CD4−CD8+ cells.
The immunological consequences of iIBDV infection 2 weeks after lvCIAV infection may also differ from simultaneous infections with pathogenic viral strains, such as carried out in previous studies. Cloud et al. (Citation1992b) demonstrated that lymphocytes harvested from birds inoculated simultaneously with pathogenic CIAV and IBDV had significantly lower responses to mitogens and suppression of cell-mediated responsiveness of peripheral blood and splenic lymphocytes than in IBDV-infected chicks. In our work, thymic or splenic cell responses to ConA were significantly diminished in dually lvCIAV/iIBDV-infected chicks. However, it was demonstrated by Poonia & Charan (Citation2004) that the peripheral lymphocyte response to phytohaemagglutinin A did not depend on the virulence level of IBDV strains. The weak decrease of responses to ConA may thus reflect the presence of viral CIAV genome in the thymus.
Taken together, our results indicate that the lvCIAV infection impairs the T-cell recruitment and subsequent B-cell depletion in the bursa induced by the subsequent iIBDV infection. The ineffectiveness of the immune responses in the elimination of these viruses may account for the subclinical infection and viral presence in lymphoid organs. These observations are critical in the establishment of control procedures of subclinical infections in avian flocks.
Further work is in progress to evaluate the immune consequences of CIAV vaccination on lv infectious bursal disease infections and viral persistency in commercial flocks.
Acknowledgements
The authors want to thank Mrs M. Costa for her help in statistical analysis and M. Burnette for revision of the manuscript.
References
- Adair , B.M. 2000 . Immunopathogenesis of chicken anemia virus infection . Developmental and Comparative Immunology , 24 : 247 – 255 . doi: 10.1016/S0145-305X(99)00076-2
- Adair , B.M. , McNeilly , F. , McConnell , C.D.G. , Todd , D. , Nelson , R.T. and McNulty , M.S. 1991 . Effects of chicken anemia agent on lymphokine production and lymphocyte transformation in experimentally infected chickens . Avian Diseases , 35 : 783 – 792 . doi: 10.2307/1591611
- Allan , W.H. , Faragher , J.T. and Cullen , G.A. 1972 . Immunosuppression by the infectious bursal agent in chickens immunised against Newcastle disease . The Veterinary Record , 90 : 511 – 512 . doi: 10.1136/vr.90.18.511
- Cardona , C.J. , Oswald , W.B. and Schat , K.A. 2000 . Distribution of chicken anaemia virus in the reproductive tissues of specific-pathogen-free chickens . Journal of General Virology , 81 : 2067 – 2075 .
- Cho , B.R. 1970 . Experimental dual infections of chickens with infectious bursal and Marek's disease agents. I. Preliminary observation on the effect of infectious bursal agent on Marek's disease . Avian Diseases , 14 : 665 – 675 . doi: 10.2307/1588638
- Cloud , S.S. , Lillehoj , H.S. and Rosenberger , J.K. 1992a . Immune dysfunction following infection with chicken anemia agent and infectious bursal disease virus. I. Kinetic alterations of avian lymphocyte subpopulations . Veterinary Immunology and Immunopathology , 34 : 337 – 352 . doi: 10.1016/0165-2427(92)90174-O
- Cloud , S.S. , Rosenberger , J.K. and Lillehoj , H.S. 1992b . Immune dysfunction following infection with chicken anemia agent and infectious bursal disease virus. II. Alterations of in vitro lymphoproliferation and in vivo immune responses . Veterinary Immunology and Immunopathology , 34 : 353 – 366 . doi: 10.1016/0165-2427(92)90175-P
- Elankumaran , S. , Heckert , R.A. and Moura , L. 2002 . Pathogenesis and tissue distribution of a variant strain of infectious bursal disease virus in commercial broiler chickens . Avian Diseases , 46 : 169 – 176 . doi: 10.1637/0005-2086(2002)046[0169:PATDOA]2.0.CO;2
- Faragher , J.T. , Allan , W.H. and Wyeth , P.J. 1974 . Immunosuppressive effect of infectious bursal agent on vaccination against Newcastle disease . The Veterinary Record , 95 : 385 – 388 . doi: 10.1136/vr.95.17.385
- Giambrone , J.J. , Donahoe , J.P. , Dawe , D.L. and Eidson , C.S. 1977 . Specific suppression of the bursa-dependent immune system of chicks with infectious bursal disease virus . The American Journal of Veterinary Research , 38 : 581 – 583 .
- Hirai , K. and Calnek , B.W. 1979 . In vitro replication of infectious bursal disease virus in established lymphoid cell lines and chicken B lymphocytes . Infection and Immunity , 25 : 964 – 970 .
- Hirai , K. , Funakoshi , T. , Nakai , T. and Sahimakura , S. 1981 . Sequential changes in the number or immunoglobulin-bearing B lymphocytes in infectious bursal disease virus-infected chickens . Avian Diseases , 25 : 484 – 496 . doi: 10.2307/1589940
- Hu , L.B. , Lucio , B. and Schat , K.A. 1993 . Abrogation of age-related resistance to chicken infectious anemia by embryonal bursectomy . Avian Diseases , 37 : 157 – 169 . doi: 10.2307/1591469
- Imai , K. , Mase , M. , Tsukamoto , K. , Hihara , H. and Yuasa , N. 1999 . Persistent infection with chicken anaemia virus and some effects of highly virulent infectious bursal disease virus infection on its persistency . Research in Veterinary Science , 67 : 233 – 238 . doi: 10.1053/rvsc.1999.0313
- Ivanyi , J. and Morris , R. 1976 . Immunodeficiency in the chicken. IV. An immunological study of infectious bursal disease . Clinical Experimental Immunology , 23 : 154 – 165 .
- Jeurissen , S.H.M. , Janse , M.E. , Van Rooselaar , D.J. , Koch , G. and De Boer , G.F. 1992 . Susceptibility of thymocytes for infection by chicken anaemia virus is related to pre- and post-hatching development . Developmental and Comparative Immunology , 2 : 123 – 129 . doi: 10.1155/1992/52484
- Kaiser , P. 2010 . Advances in avian immunology—prospects for disease control: a review . Avian Pathology , 39 : 309 – 324 . doi: 10.1080/03079457.2010.508777
- Kim , I.J. , Gagic , M. and Sharma , J.M. 1999 . Recovery of antibody-producing ability and lymphocyte repopulation of bursal follicles in chickens exposed to infectious bursal disease virus . Avian Diseases , 43 : 401 – 413 . doi: 10.2307/1592637
- Kim , I.J. , You , S.K. , Kim , H.G. , Yeh , H.Y. and Sharma , J.M. 2000 . Characteristics of intrabursal T lymphocytes induce by infectious bursal disease virus . Journal of Virology , 74 : 8884 – 8892 . doi: 10.1128/JVI.74.19.8884-8892.2000
- Liu , M. and Vakharia , V.N. 2006 . Nonstructural protein of infectious bursal disease virus inhibits apoptosis at the early stage of virus infection . Journal of Virology , 80 : 3369 – 3377 . doi: 10.1128/JVI.80.7.3369-3377.2006
- Lukert , P.D. & Saif , Y.M. 2003 . Infectious bursal disease . In B.W. Calnek , H.J. Barnes , C.W. Beard , L.R. McDougald , & Y.M. Saif , Diseases of Poultry 160 – 180 . Ames : Iowa State University Press .
- Markowski-Grimsrud , C.J. and Schat , K.A. 2003 . Infection with chicken anaemia virus impairs the generation of pathogen-specific cytotoxic T lymphocytes . Immunology , 109 : 283 – 294 . doi: 10.1046/j.1365-2567.2003.01643.x
- McFerran , J.B. , McNulty , M.S. , McKillop , E.R. , Connor , T.J. , McCracken , R.M. , Collins , D.S. and Allan , G.M. 1980 . Isolation and serological studies with infectious bursal disease virus from fowl, turkeys and ducks: demonstration of a second serotype . Avian Pathology , 9 : 395 – 404 . doi: 10.1080/03079458008418423
- Müller , H. , Islam , M.R. and Raue , R. 2003 . Research on infectious bursal disease—the past, the present and the future . Veterinary Microbiology , 97 : 153 – 165 . doi: 10.1016/j.vetmic.2003.08.005
- Müller , R. , Kaufer , I. , Reinacher , M. and Weiss , E. 1979 . Immunofluorescent studies of early virus propagation after oral infection with infectious bursal disease virus (IBDV) . Zentralblatt Für Veterinärmedizin, Reihe B , 26 : 345 – 352 . doi: 10.1111/j.1439-0450.1979.tb00823.x
- Noteborn , M.H.M. and Koch , G. 1995 . Chicken anaemia virus infection: molecular basis of pathogenicity . Avian Pathology , 24 : 11 – 13 . doi: 10.1080/03079459508419046
- Poonia , B. and Charan , S. 2004 . Infiltration by CD4+ and CD8+ lymphocytes in bursa of chickens infected with infectious bursal disease virus (IBDV): strain-specific differences . Indian Journal Experimental Biology , 42 : 823 – 829 .
- Rauf , A. , Khatri , M. , Murgia , M.V. , Jung , K. and Saif , Y.M. 2011 . Differential modulation of cytokine, chemokine and Toll like receptor expression in chickens infected with classical and variant infectious bursal disease virus . Veterinary Research , 42 : 85 – 96 . doi: 10.1186/1297-9716-42-85
- Rautenschlein , S. , Yeh , H.Y. , Ngenda , M.K. and Sharma , J.M. 2002 . Role of intrabursal T cells in infectious bursal disease virus (IBDV) infection: T cells promote viral clearance but delay follicular recovery . Archives of Virology , 147 : 285 – 304 . doi: 10.1007/s705-002-8320-2
- Rauw , F. , Lambrecht , F. and van den Berg , T. 2007 . Pivotal role of ChIFNg in the pathogenesis and immunosuppression of infectious bursal diseas . Avian Pathology , 36 : 367 – 374 . doi: 10.1080/03079450701589159
- Reddy , S.K. and Silim , A. 1991a . Isolation of infectious bursal disease virus from turkeys with arthritic and respiratory symptoms in commercial farms in Quebec . Avian Diseases , 35 : 3 – 7 . doi: 10.2307/1591287
- Reddy , S.K. and Silim , A. 1991b . Comparison of neutralizing antigens of recent isolates from infectious bursal disease virus . Archives of Virology , 117 : 287 – 296 . doi: 10.1007/BF01310772
- Rodenberg , J. , Sharma , J.M. , Belzer , S.W. , Nordgren , R.M. and Naqi , S. 1994 . Flow cytometric analysis of B cell and T cell populations in specific-pathogen-free chickens infected with infectious bursal virus . Avian Diseases , 38 : 16 – 21 . doi: 10.2307/1591831
- Rosenberger , J.K. and Cloud , S.S. 1989 . The effects of age, route of exposure, and coinfection with infectious bursal disease virus on the pathogenicity and transmissibility of chicken anemia agent (CAA) . Avian Diseases , 33 : 753 – 759 . doi: 10.2307/1591156
- Rosenberger , J.K. and Gelb , J. Jr. 1978 . Response to several avian respiratory viruses as affected by infectious bursal disease virus . Avian Diseases , 22 : 95 – 105 . doi: 10.2307/1589512
- Schat , K.A. & Skinner , M.A. 2008 . Avian immunosuppressive diseases and immune evasion . In F. Davison , B. Kaspers , & K.A. Schat , Avian Immunology 299 – 322 . London , Elsevier .
- Schat , K.A. & van Santen , V.L. 2008 . Chicken infectious anemia virus . In Y.M. Saif , A.M. Fadley , J.R. Glisson , L.R. McDougald , L.K. Nolan , & D.E. Swayne. , Diseases of Poultry 211 – 235 . New York Wiley-Blackwell Publishing .
- Sharma , J.M. , Dohms , J.E. and Metz , A.L. 1989 . Comparative pathogenesis of serotype 1 and variant serotype 1 isolates of infectious bursal disease virus and their effect on humoral and cellular immune competence of specific-pathogen-free chickens . Avian Diseases , 33 : 112 – 124 . doi: 10.2307/1591076
- Sharma , J.M. , Dohms , J. , Walser , M. and Snyder , D.B. 1993 . Presence of lesions without virus replication in the thymus of chickens exposed to infectious bursal disease virus . Avian Diseases , 37 : 741 – 748 . doi: 10.2307/1592023
- Sharma , J.M. , Kim , I.J. , Rautenschlein , S. and Yeh , H.Y. 2000 . Infectious bursal disease virus of chickens: pathogenensis and immunosuppression . Developmental and Comparative Immunology , 24 : 223 – 235 . doi: 10.1016/S0145-305X(99)00074-9
- Sharma , J.M. and Lee , L.F. 1983 . Effects of infectious bursal disease virus on natural killer cell activity and mitogenic response of chicken lymphoid cells: role of adherent cells in cellular immune suppression . Infection & Immunity , 42 : 747 – 754 .
- Smyth , J.A. , Moffett , D.A. , Connor , T.J. and McNulty , M.S. 2006 . Chicken anaemia virus inoculated by the oral route causes lymphocyte depletion in the thymus in 3-week-old and 6-week-old chickens . Avian Pathology , 35 : 254 – 259 . doi: 10.1080/03079450600717349
- Smyth , J.A. , Moffett , D.A. , McNulty , M.S. , Todd , D. and Mackie , D.P. 1993 . A sequential histopathologic and immunocytochemical study of chicken anemia virus infection at one day of age . Avian Diseases , 37 : 324 – 338 . doi: 10.2307/1591656
- Tanimura , N. and Sharma , J.M. 1998 . In situ apoptosis in chickens infected with infectious bursal disease virus . Journal of Comparative Pathology , 118 : 15 – 27 . doi: 10.1016/S0021-9975(98)80024-8
- van den Berg , T.P. 2000 . Acute infectious bursal disease in poultry: a review . Avian Pathology , 29 : 175 – 194 . doi: 10.1080/03079450050045431
- Van Santen , V.L. , Joiner , K.S. , Murray , C. , Petrenko , N. , Hoerr , F.J. and Toro , H. 2004 . Pathogenesis of chicken anemia virus: comparison of the oral and the intramuscular routes of infection . Avian Diseases , 48 : 494 – 504 . doi: 10.1637/7155-010904R
- Vaziry , A. , Silim , A , Bleau , C. , Frenette , D. and Lamontagne , L. 2011 . Chicken infectious anaemia vaccinal strain persists in the spleen and thymus of young chicks and induces thymic lymphoid cell disorders . Avian Pathology , 40 : 377 – 385 . doi: 10.1080/03079457.2011.586330