Abstract
Nine hundred and fifty-five pathology cases collected in Ontario between 1992 and 2011 from wild free-ranging Canada geese, trumpeter swans and mute swans were retrospectively evaluated for the pathology associated with avian bornavirus (ABV) infection. Cases were selected based on the presence of upper gastrointestinal impaction, central nervous system histopathology or clinical history suggestive of ABV infection. The proportion of birds meeting at least one of these criteria was significantly higher at the Toronto Zoo (30/132) than elsewhere in Ontario (21/823). Central, peripheral and autonomic nervous tissues were examined for the presence of lymphocytes and plasma cells on histopathology. The presence of virus was assessed by immunohistochemistry and reverse transcriptase-polymerase chain reaction (RT-PCR) on frozen brains and on formalin-fixed paraffin-embedded tissues. Among selected cases, 86.3% (44/51) were considered positive on histopathology, 56.8% (29/51) were positive by immunohistochemistry, and RT-PCR was positive on 88.2% (15/17) of the frozen brains and 78.4% (40/51) of the formalin-fixed paraffin-embedded samples. Histopathological lesions included gliosis and lymphoplasmacytic perivascular cuffing in brain (97.7%), spinal cord (50%), peripheral nerves (55.5%) and myenteric ganglia or nerves (62.8%), resembling lesions described in parrots affected with proventricular dilatation disease. Partial amino acid sequences of the nucleocapsid gene from seven geese were 100% identical amongst themselves and 98.1 to 100% identical to the waterfowl sequences recently described in the USA. Although ABV has been identified in apparently healthy geese, our study confirmed that ABV can also be associated with significant disease in wild waterfowl species.
Introduction
In 2009, we identified a novel genotype of avian bornavirus (ABV) in association with non-suppurative inflammation in the central, peripheral and autonomic nervous systems of several Canada geese (Branta canadensis) and trumpeter swans (Cygnus buccinator) in southern Ontario as part of our surveillance programme for diseases in free-ranging wildlife (Smith et al., Citation2010; Delnatte et al., Citation2011). ABV was first identified in 2008 and proposed as the aetiology of proventricular dilatation disease (PDD), a significant pathological syndrome that has been reported worldwide in more than 80 species of psittacine birds (Honkavuori et al., Citation2008; Kistler et al., Citation2008). Subsequent research, including inoculation studies in birds (Gancz et al., Citation2009; Gray et al., Citation2010; Mirhosseini et al., Citation2011; Payne et al., Citation2011b; Piepenbring et al., Citation2012) and investigations of outbreaks (Kistler et al., Citation2010; Gancz et al., Citation2010; Wunschmann et al., Citation2011; Heffels-Redmann et al., Citation2012), provided strong supporting evidence for a causal relationship. Psittacine birds affected with PDD exhibit various non-specific gastrointestinal and neurological signs with a high case fatality rate once clinical signs develop. Characteristic gross pathological findings of PDD include poor body condition, proventricular and ventricular dilatation, and duodenal distension (Shivaprasad et al., Citation1995; Berhane et al., Citation2001; Gancz et al., Citation2010). Lymphoplasmacytic infiltrates within myenteric ganglia and nerves of the proventriculus and ventriculus are considered the histological hallmarks of PDD (Ritchie et al., Citation1998) and are considered by some authors to be pathognomonic for the disease (Berhane et al., Citation2001; Schmidt et al., Citation2003). Similar infiltrates are frequently present in the brain, spinal cord, peripheral nerves (Shivaprasad et al., Citation1995; Berhane, Citation2004), adrenal glands, heart (Vice, Citation1992), eye (Steinmetz et al., Citation2008; Korbel & Rinder, Citation2011) and autonomic nerves and ganglia adjacent to various tissues (Doneley et al., Citation2007). In infected parrots, ABV exhibits a high tropism for neuroectodermal cells including neurons, astroglia and ependymal cells of the central nervous system, neurons of the peripheral nervous system, and adrenal cells, but is also present in many other tissues, as demonstrated by immunohistochemistry (IHC), western blot analysis and reverse transcription-polymerase chain reaction (RT-PCR) (Honkavuori et al., Citation2008; Gancz et al., Citation2009; Kistler et al., Citation2010; Lierz et al., Citation2009; Ouyang et al., Citation2009; Rinder et al., Citation2009; Weissenböck et al., Citation2009a; Weissenböck et al., Citation2009b; Lierz et al., Citation2010; Raghav et al., Citation2010; Wunschmann et al., Citation2011).
Although PDD had been considered a disease of captive psittacine birds, consistent histopathological lesions have been identified occasionally in a variety of other avian species, including passerines, falconiformes, piciformes and anseriformes (Daoust et al., Citation1991; Gregory et al., Citation2000; Perpiñán et al., Citation2007; Weissenböck et al., Citation2009b; Delnatte et al., Citation2011). In 1991, Daoust et al. described non-suppurative encephalitis and ganglioneuritis in two emaciated free-ranging Canada geese from eastern Canada that showed proventricular dilatation (Daoust et al., Citation1991). The similarity to PDD was highlighted in that publication, particularly as common causes of proventricular dilatation in waterfowl, such as chronic lead poisoning and mechanical obstruction, had been excluded. Attempts to identify a causative agent, including viral culture in several cell lines and inoculation of young mice and chicken embryos, were not successful.
Since our initial report of ABV in waterfowl, others have identified the presence of the virus in apparently healthy wild Canada geese and mute swans in the USA using oropharyngeal/cloacal swabs and brain samples. No clinical examination or necropsies were performed on these birds (Payne et al., Citation2011a; Guo et al., Citation2012). Partial nucleotide sequences of ABV identified in Canadian and American waterfowl are very similar, sharing 95.8 to 100% nucleotide identity as determined using pairwise comparison, with sequences clustering separately from those previously described in psittacines and other avian species, and from Borna disease virus (BDV) (Delnatte et al., Citation2011; Payne et al., Citation2011a). It thus appears that the ABV identified in waterfowl is a separate genotype within the family Bornaviridae, and that the virus may be present in birds that do and do not appear to suffer from clinical disease.
The objectives of this study were to describe the results of a detailed retrospective investigation evaluating the presence of ABV in wild Canada geese and swans in southern Ontario and to assess the correlation among clinical signs, histopathological lesions and presence of ABV in tissues.
Materials and Methods
Case selection
Nine hundred and fifty-five necropsy reports from the Toronto Zoo (n=132) and the Canadian Cooperative Wildlife Health Centre—Ontario Region (CCWHC-Ont; n=823) databases from 1992 to 2011 were reviewed to identify Canada geese or swans with upper gastrointestinal impaction (oesophagus, proventriculus or ventriculus); non-suppurative encephalitis, myelitis, neuritis or ganglioneuritis; or having a clinical history suggestive of ABV infection (e.g. weakness, neurological signs). Cases were excluded if a specific diagnosis accounted for the clinical signs and pathological lesions; for example, soybean impaction, lead poisoning or West Nile virus infection. Six additional cases (Canada geese) with a histological diagnosis of non-suppurative encephalitis were submitted from the CCWHC—Atlantic Region (CCWHC-Atl), including the two birds reported in Daoust's paper in 1991 (Daoust et al., Citation1991). Nine negative control birds were selected from the CCWHC-Ont database based on the absence of these inclusion criteria.
The following necropsy information was reviewed as available: species, geographic location where found, age class, sex, clinical history, gross necropsy findings including body condition, histopathology, and the results of any additional diagnostic tests such as toxicologic and microbiologic testing.
Histopathology
Slides from all selected cases were reviewed by one pathologist (D.A.S.), blinded by case, for the presence of histological lesions. Tissue samples had been formalin-fixed paraffin-embedded (FFPE), sectioned at 4 µm and stained with haematoxylin and eosin. Nerves and ganglia in all sections of all tissues (central, peripheral and autonomic nervous system) were specifically examined for the presence of lymphocytes and plasma cells. A case was considered positive for ABV-consistent histopathology if non-suppurative inflammation was present in any nervous tissue.
Immunohistochemistry
IHC was performed on one or more sections of FFPE brain from each case as available. When brain was not available, spinal cord or peripheral nerves were assessed. Briefly, IHC was performed on 4 µm tissue sections mounted on charged slides and using an automated staining instrument (Dako Autostainer; Dako Canada Inc., Mississauga, Ontario, Canada) with rabbit polyclonal antiserum raised against the ABV nucleocapsid (ABV-N) protein (provided by the deRisi Laboratory, UC-SF, San Francisco, California, USA). Detailed description of the cloning, recombinant expression, purification, and generation of polyclonal antisera against the ABV nucleocapsid (ABV2-N) protein has been published previously (Gancz et al., Citation2009; Raghav et al., Citation2010). Following manual deparaffinization and rehydration, tissue sections were treated with 3% hydrogen peroxide to quench endogenous peroxidase activity. Antigen retrieval was accomplished by incubation with Proteinase K (Dako) for 6 min. Sections were then incubated with rabbit anti-ABV-N antiserum (1:2000) for 30 min, followed by 30-min incubation with a goat antimouse/antirabbit polymer visualization system (UltraVision ONE; Lab Vision Corp., Fremont, California, USA). All incubations were completed at room temperature. Nova Red was used as the chromogen (Vector Laboratories, Burlington, Ontario, Canada). ABV-positive control brain sections included with each run were from a psittacine previously tested by both IHC and RT-PCR. For negative reagent controls, duplicate sections of each control and test tissue were subjected to the same immunohistochemical procedure with substitution of pre-immune rabbit serum at a similar protein concentration to the anti-ABV-N antisera. All slides were interpreted by one author (J.D.), who was blinded to case information. A case was considered IHC-positive when strong, diffuse intranuclear staining was present in neurons or glial cells.
Nucleic acid isolation and RT-PCR
FFPE tissues and thawed frozen or fresh tissue or cloacal swabs collected at necropsy (when available) were evaluated for the presence of ABV by RT-PCR. The paraffin blocks were the same as those used for IHC, and thus included one or more sections of brain in most cases, or spinal cord or peripheral nerves when brain was not available.
RNA extraction was performed on FFPE sections of brain using the RNeasy® FFPE Kit (Qiagen Inc., Mississauga, Ontario, Canada) according to the manufacturer's instructions. Total nucleic acids were extracted from thawed frozen or fresh brain samples and cloacal swabs using the MagMAX-96 Viral RNA Isolation Kit in a MagMAX Express-96 Magnetic Particle Processor (Applied Biosystems Inc., Foster City, California, USA) from 50 µl aliquots of 5 to 10% tissue suspensions or, for cloacal swabs, from 50 µl aliquots of virus transport media (Starplex Scientific Inc., Etobicoke, Ontario, Canada).
Thawed frozen or fresh brain samples and cloacal swabs collected at necropsy were evaluated for the presence of ABV by conventional gel-based RT-PCR detection of the ABV-N gene and by real-time RT-PCR (rtRT-PCR) detection of the ABV matrix (ABV-M) gene. FFPE tissues were evaluated for the presence of ABV by rtRT-PCR detection of the ABV-M gene.
For the conventional gel-based RT-PCR, the primers used were directed against ABV-N and have previously been used for ABV identification in psittacine birds (Kistler et al., Citation2008). The RT-PCR was carried out in 25 µl reactions using the One-Step RT-PCR Kit (Qiagen Inc.).
The rtRT-PCR assay was a duplex test with two sets of primers and TaqMan probes: ABV_M_120201, targeting psittacine ABV matrix gene sequences; and ABVG_M_111029, targeting geese ABV matrix gene sequences (). The amplification was carried out in 25 µl reactions in a LightCycler 480 Real-Time PCR System (Roche, Laval, Quebec, Canada) using the AgPath-ID One-Step RT-PCR Kit (Applied Biosystems Inc.) under cycling parameters described in . A rtRT-PCR crossing-point value (Cp) less than 37.00 was considered positive, between 37.00 and 39.99 inconclusive, and equal to or greater than 40.00 negative. A case was classified as ABV-PCR-positive if one or more frozen, fresh or FFPE samples tested were positive on at least one RT-PCR (either conventional or rtRT-PCR), and negative if all samples tested were found to be negative.
Table 1. Real-time RT-PCR primer and probe sequences used to identify ABV in frozen and fixed paraffin-embedded tissues from free-ranging waterfowl in Ontario.
Table 2. Real-time RT-PCR cycling parameters used to identify ABV in frozen and fixed paraffin-embedded tissues from free-ranging waterfowl in Ontario.
Sequencing and sequence analysis
Partial nucleotide sequences of ABV-N genes were determined at the Laboratory Services, University of Guelph, Ontario, Canada. Sequences were assembled with the SeqMan module of the Lasergene 10.0 software package (DNASTAR, Inc., Madison, Wisconsin, USA). The sequences reported in the present study have been deposited in GenBank under accession numbers JX258867, JX258868, JX258869, JX258870, JX258871, JX258872 and JX258873. Multiple alignments were carried out with the MegAlign module of the Lasergene 10.0 software package using the default settings of its ClustalW algorithm. Sequence comparisons were carried out using a 313-base pair (bp) fragment from nucleotides 692 to 1004, relative to the complete genomic sequence of ABV isolate bil, GenBank accession number EU781967. Phylogenetic trees were also generated with the MegAlign module of Lasergene software using its default settings.
Statistical evaluation
Statistical analyses were performed using GraphPad QuickCalcs software (http://www.graphpad.com/quickcalcs/). Fisher's exact test was used for comparisons among species (overall and within each location) and between locations (overall and for each species) for the percentage of birds meeting the inclusion criteria; results of histopathology, IHC, RT-PCR on frozen brain and rtRT-PCR on FFPE; and distribution of lesions (histopathology and IHC). When the difference was significant (P<0.05), the odds ratio was calculated and reported with a 95% confidence interval. For rtRT-PCR, the mean Cp values were compared between type of tissues (frozen brain vs. FFPE) using paired t tests, and between location and species using unpaired t tests. Two-tailed P<0.05 was considered significant.
Test agreement was estimated using overall percentage agreement, positive percentage agreement and negative percentage agreement (Feinstein & Cicchetti, Citation1990) for all combinations of histopathology, IHC, RT-PCR on fresh/frozen tissues and RT-PCR on FFPE. Test agreement was carried out for each species and for all species combined.
Results
Cases meeting inclusion criteria
Forty-one of 555 Canada geese (7.4%), 8/321 trumpeter swans (2.5%) and 2/79 mute swans (2.5%) met at least one of the inclusion criteria and were included in the study (). The proportion of Canada geese selected was significantly higher than the proportion of swans (P=0.0007; odds ratio = 3.11; 95% confidence interval [1.54; 6.29]), and was significantly higher at the Toronto Zoo (27/88) than elsewhere in Ontario (14/467) (P<0.0001; odds ratio = 14.32; 95% confidence interval [7.12; 28.8]). There was no difference between the two sites for trumpeter swans (P=0.07) or mute swans (P=1.00).
Table 3. Number of Canada geese (Branta canadensis), trumpeter swans (Cygnus buccinator) and mute swans (Cygnus olor) meeting initial case inclusion criteria in a retrospective survey of necropsy databases from Toronto Zoo and CCWHC-Ont from 1992 to 2011.
Summaries of clinical history and gross necropsy findings in all selected geese and swans are presented in and . Clinical signs from the necropsy histories that met the clinical inclusion criteria included: somnolence, weakness and lethargy; and suspected blindness, lameness, ataxia, hypermetria, inability to stand or to fly, torticollis, head tremors, stargazing and opisthotonos. Of the selected cases, 30/41 (73.2%) Canada geese, 5/8 (62.5%) trumpeter swans and 1/2 (50.0%) mute swans were in poor body condition.
Table 4. Summary of clinical history, gross necropsy findings, histopathology, IHC and RT-PCR results in 41 wild Canada geese (Branta canadensis) evaluated for the presence of ABV.
Table 5. Summary of clinical history, gross necropsy findings, histopathology, IHC and RT-PCR results in eight wild trumpeter swans (Cygnus buccinator) and two wild mute swans (Cygnus olor) evaluated for the presence of ABV.
Six additional Canada geese from the CCWHC-Atl database with nervous system histopathology were also included in this study: 3/6 showed upper gastrointestinal impaction, 5/6 were in poor body condition and 3/3 that were not found dead showed neurological signs prior to death.
Diagnostic findings
A summary of the histopathology findings and IHC and RT-PCR results for all selected geese and swans are presented in and . Among selected cases, the proportion of cases identified as positive for each diagnostic test (histopathology, IHC, RT-PCR on frozen brain and RT-PCR on FFPE) did not differ significantly among species or between locations.
Histopathology
Of the 41 selected Canada geese, 35 (85.4%) were considered positive on histopathology, including 23 of the 27 cases from the Toronto Zoo and 12 out of 14 cases found elsewhere in Ontario. All selected trumpeter swans and one of the two selected mute swans were considered positive on histopathology. The tissue distribution of non-suppurative inflammatory lesions is shown in and , and some characteristic histological lesions are illustrated in .
Figure 1. Histological lesions in waterfowl infected with ABV. Haematoxylin and eosin staining. 1a: Trumpeter swan, cerebrum. Marked and extensive perivascular lymphoplasmacytic cuffing. 1b: Canada goose, cerebrum. Large perivascular lymphoplasmacytic cuff. 1c: Canada goose, cerebrum. Well-defined glial nodule. 1d: Canada goose, vagus nerve. Diffuse and perivascular lymphoplasmacytic infiltration. 1e: Canada goose, adrenal. Interstitial infiltration by lymphocytes and plasma cells primarily around chromaffin cell islets. 1f: Canada goose, proventriculus. Lymphoplasmacytic infiltration within a myenteric ganglion.
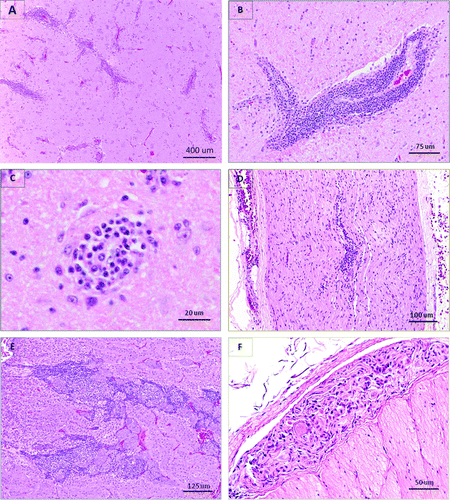
Table 6. Tissue distribution of non-suppurative inflammatory lesions, immunohistochemical staining and RT-PCR results in 35 wild Canada geese (Branta canadensis) evaluated for the presence of ABV.
Table 7. Tissue distribution of non-suppurative inflammatory lesions, immunohistochemical staining and RT-PCR results in eight wild trumpeter swans (Cygnus buccinator) and one wild mute swan (Cygnus olor) evaluated for the presence of ABV.
All selected cases except one had histological lesions in the brain, which included perivascular cuffing by lymphocytes, plasma cells and, less frequently, macrophages; and focal and diffuse gliosis. Less common additional neuropathology included non-suppurative inflammation of the cerebral meninges; Wallerian degeneration with swollen axons, dilated axonal sheaths and gitter cells; and rare malacia and cerebral oedema. The most commonly affected parts of the brain were the cerebrum (30/33 geese and 8/9 swans; overall 90.5%) and the brainstem (26/30 Canada geese and 8/9 swans; overall 87.2%) followed by the optic lobes (14/21 Canada geese and 2/2 swans; overall 69.6%) and the cerebellum (22/34 Canada geese and 7/8 swans; overall 69.0%). Lesions were noticed in both grey and white matter, in cerebellar peduncles, in Purkinje layers, in choroid plexi and in the pituitary (pars nervosa). Lymphoplasmacytic perivascular infiltrates and Wallerian degeneration were also observed in peripheral nerves (11/21 Canada geese and 4/6 swans; overall 55.6%), including the brachial plexi, sciatic nerves and vagus nerves, and in spinal cord (3/6 Canada geese and 2/4 swans; overall 50.0%).
Among the histopathology-positive cases, 23/34 geese and 4/9 swans had lymphocytes or plasma cells within myenteric nerves or ganglia; with the intensity of the infiltrate varying greatly from nerve to nerve along the gastrointestinal tract of an individual bird. Lesions were present in the oesophagus (15/27 geese and 0/4 swans; overall 48.4%), proventriculus (16/30 geese and 0/6 swans; overall 44.4%), ventriculus (8/24 geese and 0/5 swans; overall 27.6%), intestines (17/34 geese and 4/9 swans; overall 48.8%) and cloaca (4/15 geese and 0/2 swans; overall 23.5%). Among histopathology-positive cases, the proportions of Canada geese showing histological lesions in the proventriculus and ventriculus were significantly higher when compared with combined values for trumpeter and mute swans (P=0.024 and P=0.0146, respectively). The distribution of histological lesions in other tissues did not differ significantly among species. All histopathology-positive cases with a history of upper gastrointestinal impaction had characteristic lesions in the oesophagus, proventriculus and/or ventriculus. Four Canada geese and one mute swan were included in this study based on the presence of upper gastrointestinal dilation but did not show any histological lesions in any nervous tissues, and thus were considered histopathology-negative cases.
Most cases with ganglioneuritis of the gastrointestinal tract also showed perivascular, perineural or periganglional non-suppurative infiltrates in the serosa, and/or tracking through the muscularis. However, as outlined in Materials and Methods, this alone was insufficient for the classification of a case as “histopathology-positive”.
Lymphocytes and/or plasma cells were present within autonomic nerves or ganglia adjacent to the adrenal (14/25 geese and 3/5 swans; overall 56.7%), thyroid (n=7), heart (n=4), kidney (n=3), testes (n=1), ovary (n=1), lung (n=1) and pancreas (n=1). Non-suppurative interstitial adrenalitis and nephritis were noted, respectively, in 43.3% (11/25 geese and 2/5 swans) and 54.5% (21/35 geese and 3/9 swans) of histopathology-positive cases.
Other recurrent histological findings that were considered incidental included hepatic and splenic haemosiderosis and amyloidosis, pulmonary anthracosis, renal tubular mineralization, skeletal muscle degeneration, perivascular hepatitis and the presence of parasites in various tissues (renal and intestinal coccidia, vascular schistosomes, and gastrointestinal nematodes and cestodes).
Immunohistochemistry
Out of the 41 selected Canada geese, 23 were positive by IHC including 18/27 cases from the Toronto Zoo and 5/14 cases from the CCWHC-Ont. Six of eight trumpeter swans were positive by IHC, including 2/3 cases from the Toronto Zoo and 4/5 cases from the CCWHC-Ont. Neither mute swan was positive on IHC. Immunohistochemical staining was present consistently in the nucleus and occasionally in the cytoplasm of both neurons and glial cells (). Staining intensity varied from mild to intense among cases. Staining that was considered non-specific cross-reactivity with cellular proteins was sometimes observed in circulating leukocytes.
Figure 2. (Colour online) Canada goose, cerebrum. Immunohistochemical staining for ABV antigen using rabbit polyclonal antiserum raised against ABV nucleocapsid protein. Note the intense intranuclear staining in neurons and glial cells, and the less frequent intracytoplasmic staining in neurons.
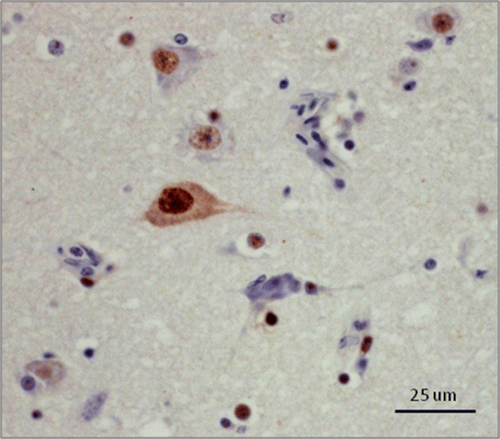
The tissue distribution of IHC staining is presented in and . The ABV antigen was most commonly detected in the optic lobes (11/16 Canada geese and 2/2 swans; overall 72.2%) and in the brainstem (16/23 Canada geese and 3/6 swans; overall 65.5%) followed by the cerebrum (19/32 Canada geese and 4/7 swans; overall 59.0%) and the cerebellum (15/27 Canada geese and 2/5 swans; overall 53.1%). The tissue distribution of immunohistochemical staining did not differ significantly among species.
RT-PCR results
Fresh/frozen brains were available for only 16 Canada geese and one mute swan. Fifteen of the 16 Canada geese (93.8%) tested positive by at least one RT-PCR method (gel-based N-gene RT-PCR or M-gene rtRT-PCR), and one was negative by gel-based RT-PCR but no brain was available for subsequent rtRT-PCR. The mute swan tested negative by both methods. For the rtRT-PCR, the Cp values of the positive birds ranged from 12.78 to 33.15 with a mean and standard deviation of 21.75 (± 5.85). The mean Cp for frozen brain samples was significantly lower in Canada geese that did not originate from the Toronto Zoo (P=0.032).
Four cloacal swabs were tested by RT-PCR; three were taken from Canada geese for which frozen brain was positive by RT-PCR and one was collected from a mute swan for which the brain was negative. Only one of three goose swabs was positive, with a Cp of 16.95, and the swan swab was negative.
Out of the 51 selected birds, 40 were positive by rtRT-PCR on FFPE tissues, including 32/41 Canada geese, 7/8 trumpeter swans and 1/2 mute swans. The result for one Canada goose was inconclusive (Cp = 37.4). The Cp values of the positive birds ranged from 11.81 to 36.41 with a mean and standard deviation of 24.22 (± 6.47). The mean Cp for FFPE samples did not differ significantly between locations or among species, and did not differ significantly from the mean value obtained from frozen brain in birds that were tested by both methods.
Agreement among tests
A summary of the overall, positive and negative percentage agreements among diagnostic tests for all species combined and for overall percentage agreement for each species alone is presented in . Test agreement was not examined by location. For the 51 birds selected in this study, the overall percentage agreements among tests (histopathology, IHC, rtRT-PCR on frozen brain and rtRT-PCR on FFPE tissue) ranged from 70.6 to 92.2%, with the highest overall percentage agreement being between histopathology and rtRT-PCR on FFPE samples (all species considered together). Positive percentage agreement ranged from 65.9 to 96.6%, and was above 90% for rtRT-PCR (FFPE) and histopathology, rtRT-PCR (FFPE) and rtRT-PCR (frozen brain), IHC and rtRT-PCR (FFPE), and IHC and rtRT-PCR (frozen brain). The negative percentage agreement ranged from 0 to 100%, with 100% agreement between histopathology and IHC, and histopathology and rtRT-PCR (FFPE).
Table 8. Summary of overall, positive and negative percentage agreements among diagnostic tests in Canada geese (Branta canadensis), trumpeter swans (Cygnus buccinator) and mute swans (Cygnus olor) evaluated for the presence of ABV.
Cases submitted from the CCWHC-Atl
All six Canada geese cases received from the CCWHC-Atl had histological lesions consistent with ABV based on their initial histopathology report and reassessment of tissues that were available at the time of this review. Blocks of paraffin-embedded brain were not available from the two cases included in Daoust et al. (Citation1991); testing was thus carried out on sections of spinal cord showing inflammatory lesions from one bird (Bird A) and peripheral nerve without lesions in the second (Bird B). The results of testing for these two birds are as follows: Bird A, rtRT-PCR-positive, IHC-negative; Bird B, rtRT-PCR inconclusive, IHC-negative. Blocks of paraffin-embedded brain were available from three additional cases, all three were positive by rtRT-PCR and two were also positive by IHC. A block of paraffin-embedded spinal cord was available from one additional case, and was positive by rtRT-PCR and negative by IHC.
Birds negative for inclusion criteria
At the commencement of the study, six Canada geese, two trumpeter swans and one mute swan were selected from the CCWHC-Ont database as negative controls, based on the absence of the previously defined inclusion criteria. All birds were negative on histopathology and IHC. Frozen brain was available from four birds (one Canada goose, two trumpeter swans, one mute swan) and all four samples were negative for ABV on conventional RT-PCR. A sample from only one of these cases was available for re-testing with rtRT-PCR, and was again negative. FFPE tissues were negative for ABV by rtRT-PCR in five Canada geese, and were positive in one Canada goose (Cp = 36.09), two trumpeter swans (Cp = 27.33 and 32.20) and one mute swan (Cp = 36.25).
Sequence analysis
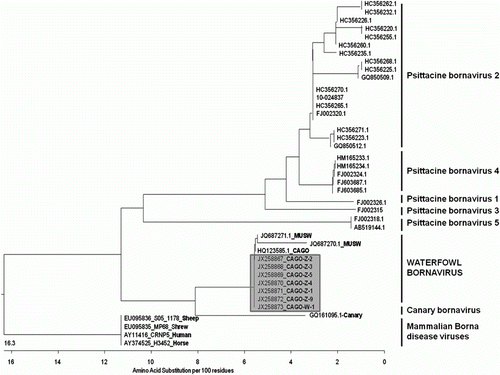
Seven RT-PCR products from positive Canada geese were sequenced and their nucleotide sequences were 100% identical amongst themselves. Using pairwise sequence comparison based on amino acid sequence, these N-gene sequences were compared with the available ABV sequences in GenBank and a phylogenetic tree based on the amino acid sequences was generated (). The N-gene sequences from Ontario geese were 100% identical to a Canada goose sequence from the USA (GenBank accession number HQ123585.1) and 100% and 98.1% identical to two mute swan sequences from the USA (GenBank accession number JQ687271.1 and JQ687270.1, respectively). Our Ontario geese N-gene sequences were 84.6% (GenBank accession number FJ002315.1, ABV-genotype 3) to 87.5% (GenBank accession numbers FJ002320.1, HC356270.1 and HC356265.1, all ABV-genotype 2) identical to sequences from parrots; 93.1% identical to the sequence from a canary (GenBank accession number GQ161095.1); and 85.6% identical to various mammalian bornavirus sequences available in the GenBank (GenBank accession numbers EU095836-S05-1178-sheep, EU095835-MP68-shrew, AY11416-CRNP5-human, AY374525-H3452-horse).
Figure 3. Phylogenetic tree illustrating the genetic relationships between the waterfowl bornavirus and other bornaviruses, based on a partial amino acid sequence of the nucleocapsid protein gene. Representatives of the five known ABV genotypes, the canary genotype and several representatives of BDV are included. Sequences are identified with GenBank accession number followed by species (if non-psittacine). The seven Canada goose sequences originating in this study are highlighted in grey. Scale bar indicates the genetic distance among clusters. CAGO, Canada goose; MUSW, mute swan.
Discussion
The objective of this study was to retrospectively identify cases of ABV infection in wild waterfowl species and to characterize associated pathology in cases with the presence of clinical signs or pathological lesions that would suggest ABV infection in psittacines. ABV was detected by RT-PCR in 78.4% (40/51) of the selected cases, indicating the effectiveness of our criteria. Birds infected with ABV but not showing the specified inclusion criteria would not have been chosen, thus our method was not appropriate to estimate the prevalence of infected birds within the databases. Other authors have looked for infection in asymptomatic waterfowl species and reported prevalences from 5 to 50% depending on the species, location and type of sample (Payne et al., Citation2011a; Guo et al., Citation2012). The percentage of ABV-infected birds selected from our pathology databases was thus significantly higher than the background level observed in apparently healthy flocks.
Pathological findings in ABV-infected birds were very similar to those typically found in parrots affected with PDD (Shivaprasad et al., Citation1995; Berhane et al., Citation2001; Gancz et al., Citation2010). Although both neurological signs and gastrointestinal impaction were seen associated with ABV infection, the former was more consistently identified within this group of cases. Non-suppurative perivascular cuffing in the central nervous system was dramatic and widespread in the majority of birds; however, lesions in peripheral nerves and in the autonomic system could be very subtle and had often been missed on initial histopathologic assessment. Many cases showed a marked degree of autolysis, making evaluation of the gastrointestinal tract difficult, and inconsistent sample collection also affected assessment of lesion distribution. Lesions in the nerves and ganglia of the proventriculus and ventriculus were, however, present in a significantly greater proportion of Canada geese as compared with trumpeter and mute swans, perhaps indicating a difference in pathology among species.
Overall, the results of the diagnostic tests used in this study (histology, IHC, RT-PCR on tissues, RT-PCR on FFPE) appeared well correlated. Actual assessment and comparison of specificity and sensitivity is difficult due to the absence of a recognized gold standard for ABV infection, and the difficulty in identifying a true control group of uninfected birds. Test agreement was thus estimated using overall, positive and negative percentage agreements (Feinstein & Cicchetti, Citation1990). In this study, histopathological lesions, with an emphasis on careful examination of a broad selection of nerves and ganglia, correlated strongly with the presence of virus in brain. Using histopathology alone would probably result in false positives, particularly if only brain is examined, as non-suppurative encephalitis is common to several neurotropic viral diseases. Lesions in the autonomic nerves and ganglia and the adrenal do appear to be more specific to ABV infection. Histopathology would not be a sensitive method of detecting virus-infected birds, as we identified several birds that were ABV-positive but without clinical or pathological abnormalities. IHC and RT-PCR would be more appropriate for this purpose.
The results of this study also demonstrate that RT-PCR and IHC designed to identify psittacine ABV also recognize the strain of ABV found in waterfowl. The positive percentage agreement between IHC and rtRT-PCR, performed on FFPE samples from the same block, was excellent. However, IHC did appear less sensitive than rtRT-PCR, and thus false negative results could occur with IHC if the viral load in the tissue is very low or as the result of antigen degradation. Non-specific immunohistochemical staining was observed in several cases and can be a source of false positive IHC results. In this study, we used strict criteria (the presence of strong and diffuse intranuclear staining in neurons or glial cells) to define IHC positivity (Raghav et al., Citation2010).
Both gel-based conventional RT-PCR (ABV-N gene) and rtRT-PCR (ABV-M gene) were used as a result of changes in diagnostic laboratory protocols during the study. This provided us with the opportunity to compare the results of the two testing methods. Results disagreed in only one case (of the 17 frozen brains tested), which was positive on rtRT-PCR but inconclusive on conventional RT-PCR. In parrots, assays for the M and N genes appear to have a similar high sensitivity (Gancz et al., Citation2010; Raghav et al., Citation2010); while the nucleoprotein N gene is the more highly expressed region of the genome, the matrix M gene is the more highly conserved region (Kistler et al., Citation2008).
In our laboratory, conventional RT-PCR using the N gene was not successful using FFPE tissues, whereas rtRT-PCR for the M gene was suitable for use with FFPE samples. Extraction of RNA from FFPE can result in short fragments of nucleic acids that may not be suitable for amplification in PCR assays that produce large amplicons. The use of different primers to amplify a shorter fragment in our rtRT-PCR probably explains the success of this method. The ability to perform rtRT-PCR on FFPE samples is crucial in the retrospective evaluation of disease, where frozen samples are rarely available.
IHC and PCR were carried out on brain, or spinal cord or peripheral nerve in the few cases where brain was not available. The decision to test only one tissue was a pragmatic one. Brain was selected due to the frequency of lesions in that tissue as well as the results of a study in psittacine birds that evaluated the presence of ABV in multiple tissues (Raghav et al., Citation2010). A comparison of the tissue distribution of ABV in waterfowl with that in parrots would be interesting; based on histopathologic lesions, we predict that it would be equally widespread.
The proportion of ABV-infected diseased birds was not equal in the two databases searched. The proportion of Canada geese was significantly higher at the Toronto Zoo when compared with goose cases arising elsewhere in Ontario. Recent publications about the prevalence of ABV in Canada geese and mute swans in the USA also showed that infections were often clustered within flocks and the prevalence of ABV appears to vary greatly among locations (Payne et al., Citation2011a; Guo et al., Citation2012). The Toronto Zoo goose population of approximately 150 free-flying individuals is composed of resident birds remaining on the zoo grounds year-round and migrating birds coming from the Arctic or leaving for the Great Lakes for the winter. Determining whether there is a truly higher prevalence of disease at the zoo site is made difficult by enhanced surveillance at the zoo. Numerous members of staff and the public are available to observe ill or dead birds, and a thorough necropsy is performed on every wild bird euthanized or found dead. Through the rest of Ontario, passive surveillance is carried out at a much lower rate and in a much more random manner, and more often involves the submission of birds found dead without any clinical history. The prevalence of other diseases commonly found in Ontario waterfowl, such as lead poisoning or botulism, are very rarely encountered on the zoo site but constitute a significant percentage of deaths elsewhere in Ontario, thus diluting the importance of ABV as a cause of mortality in the greater population. Finally, the Toronto Zoo environment may provide opportunities for ABV to transmit and propagate among birds, allowing virus loads to increase and permit a disease cluster to develop. Greater food availability may also allow a sick bird to survive longer than it would in the wild, increasing the opportunity for viral shedding and environmental contamination, as well as the likelihood of being observed and identified as ill. These hypotheses are supported by that fact that the proportion of infected diseased trumpeter swans was not different between the birds found at the Toronto Zoo and those found elsewhere; this species is only transient on the zoo site, thus all birds should be considered the same population. The relatively high prevalence of ABV-infected Canada geese at the zoo is of some concern for the zoo's captive collection; however, there has been no report of PDD lesions or ABV infection in any bird in the Toronto Zoo collection over the past 20 years. The possibility of transmission of ABV to other waterfowl species, to different avian families housed in outside aviaries and possibly to some mammals at the zoo remains uncertain.
As mentioned previously, ABV may be present in waterfowl that do not appear to suffer from clinical disease (Payne et al., Citation2011a; Guo et al., Citation2012). However, no necropsies were performed on any of the birds in these studies so it is possible that subclinical nervous system disease may have been present. Widespread asymptomatic infection with less common clinical disease is a consistent feature of bornavirus infections, and may be attributed to the noncytopathic effect of Bornaviridae. This pattern has been documented with BDV in horses and sheep (Lipkin & Briese, Citation2006) and is now well recognized in parrots (De Kloet & Dorrestein, Citation2009; Kistler et al., Citation2010; Lierz et al., Citation2009; Villanueva et al., Citation2010; Heffels-Redmann et al., Citation2012). The exact circumstances under which disease develops in waterfowl species is unknown but influences are probably multifactorial. The existence of frequent asymptomatic birds may also explain our difficulty in identifying negative controls in this study. A small number of birds was selected based on the absence of inclusion criteria to act as negative controls for the IHC and PCR testing. However; one Canada goose, two trumpeter swans and one mute swan within this group were positive for ABV by RT-PCR on FFPE tissues. It is of interest to note that their mean Cp (33.0) was significantly higher (i.e. lower viral load) than the mean Cp of diseased birds (24.2). Further detailed evaluation of asymptomatic birds is necessary to estimate the true prevalence of ABV infection and its relationship to the presence of clinical disease in these species.
A non-lethal method of reliably testing for ABV would assist in identifying the prevalence of infection in populations, particularly those such as the trumpeter swan that are the subject of conservation programmes. PCR testing of faeces and cloacal swabs is used in psittacine medicine, but appears to be relatively insensitive based on studies of experimentally and naturally infected parrots (Villanueva et al., Citation2010; Payne et al., Citation2011b). In our study, only one out of three cloacal swabs collected from ABV-positive geese was positive by RT-PCR, supporting the presence of intermittent faecal shedding.
The partial nucleotide sequences of the ABV-N gene recovered from seven Canada geese in this study are highly conserved among individuals and closely match recently published sequences from Canada geese and mute swans in the USA (Payne et al., Citation2011a; Guo et al., Citation2012). This unique group of ABV sequences significantly diverges from the six genotypes of ABV previously found in psittacines (Kistler et al., Citation2008; Rubbenstroth et al., Citation2012), from the one described in a canary (Weissenböck et al., Citation2009b), and from mammalian BDVs. This confirms that the waterfowl bornaviruses represent a separate, independent cluster within the family Bornaviridae, now referred as ABV-CG (Delnatte et al., Citation2011; Payne et al., Citation2011a). Payne et al. recently showed that the genome organization of ABV-CG is in fact more closely related to mammalian BDV than to other ABVs, with the presence of a regulatory open reading frame in the N/X intergenic region that is lacking in psittacine ABVs (Kistler et al., Citation2008; Rinder et al., Citation2009; Payne et al., Citation2011a; Guo et al., Citation2012). This retrospective evaluation only looked at waterfowl entered into the databases since 1992; however, we found ABV in the tissues of a bird described by Daoust and submitted to the CCWHC-Atl in 1988 (Daoust et al., Citation1991). The finding of ABV in older archival material would support the premise that this virus has been long present in North American waterfowl populations and is not a recent introduction, as the psittacine strains appear to be. As BDV sequences obtained from various species over several decades have shown remarkable sequence conservation (Dürrwald et al., Citation2006; Kistler et al., Citation2008), the recognition of an increasing number of ABVs is surprising and makes molecular characterization a critical component in the understanding of the epidemiology of ABV infection.
In conclusion, this study confirmed that ABV is present in wild Canada geese, trumpeter swans and mute swans in Canada and showed evidence that it can be associated with significant disease (Smith et al., Citation2010; Delnatte et al., Citation2011). Although our findings strongly suggest that ABV is the cause of the clinical and pathological changes observed, formal proof of a causal relationship typically requires fulfilment of Koch's postulates. This was demonstrated for ABV/PDD in several species of parrots, but has yet to be carried out in waterfowl species (Gray et al., Citation2010; Mirhosseini et al., Citation2011; Payne et al., Citation2011b; Piepenbring et al., Citation2012). ABV infection should, nonetheless, be included in the differential diagnosis for any Anseriform exhibiting upper gastrointestinal impaction or non-specific neurological signs and having non-suppurative inflammation of the central, peripheral or autonomic nervous systems. The overall impact of this disease on the health and size of populations of wild waterfowl in North America is yet to be determined.
Acknowledgements
The authors gratefully acknowledge Amy L. Kistler and Joseph L. deRisi, University of California—San Francisco for providing the rabbit polyclonal antiserum used for the IHC. The authors also thank Pierre Yves Daoust for providing the six samples from the CCWHC-Atl. The authors would like to thank Maya Kummrow and Charlene Berkvens for their key contribution early in this study, and Monika Janssen, Veronica Kay and Nicole Zaranek for their help over the summers. Carol Lee Ernst, Susan Lapos, Ana Rita Rebelo, Sarah Hoyland and Jane Coventry provided essential technical assistance. Financial support was provided by the Toronto Zoo, the Animal Health Laboratory and the CCWHC-Ont. This work was carried out in partial fulfilment of a DVSc degree by Pauline Delnatte, whose stipend was provided by the Toronto Zoo.
References
- Berhane , Y. 2004 . Studies on the etiology and pathology of proventricular dilatation disease (D.V.Sc. thesis) . University of Guelph Guelph, Ontario , , Canada , 150 .
- Berhane , Y. , Smith , D.A. , Newman , S. , Taylor , M. , Nagy , E. , Binnington , B. and Hunter , B. 2001 . Peripheral neuritis in psittacine birds with proventricular dilatation disease . Avian Pathology , 30 : 563 – 570 . doi: 10.1080/03079450120078770
- Daoust , P.Y. , Julian , R.J. , Yason , C.V. and Artsob , H. 1991 . Proventricular impaction associated with nonsuppurative encephalomyelitis and ganglioneuritis in two Canada geese . Journal of Wildlife Diseases , 27 : 513 – 517 .
- De Kloet , S.R. and Dorrestein , G.M. 2009 . Presence of avian bornavirus RNA and anti-avian bornavirus antibodies in apparently healthy macaws . Avian Diseases , 53 : 568 – 573 . doi: 10.1637/8828-040209-Reg.1
- Delnatte , P. , Berkvens , C. , Kummrow , M. , Smith , D.A. , Campbell , D. , Crawshaw , G. , Ojkic , D. and DeLay , J. 2011 . New genotype of avian bornavirus in wild geese and trumpeter swans in Canada . The Veterinary Record , 169 : 108 doi: 10.1136/vr.d4620
- Doneley , R.J.T. , Miller , R.I. and Fanning , T.E. 2007 . Proventricular dilatation disease: an emerging exotic disease of parrots in Australia . Australian Veterinary Journal , 85 : 119 – 123 . doi: 10.1111/j.1751-0813.2007.00109.x
- Dürrwald , R. , Kolodziejek , J. , Muluneh , A. , Herzog , S. and Nowotny , N. 2006 . Epidemiological pattern of classical Borna disease and regional genetic clustering of Borna disease viruses point towards the existence of to-date unknown endemic reservoir host populations . Microbes and Infection , 8 : 917 – 929 . doi: 10.1016/j.micinf.2005.08.013
- Feinstein , A.R. and Cicchetti , D.V. 1990 . High agreement but low kappa: II. Resolving the paradoxes . Journal of Clinical Epidemiology , 43 : 551 – 558 . doi: 10.1016/0895-4356(90)90159-M
- Gancz , A.Y. , Clubb , S. and Shivaprasad , H.L. 2010 . Advanced diagnostic approaches and current management of proventricular dilatation disease . Veterinary Clinics of North America: Exotic Animal Practice , 13 : 471 – 494 . doi: 10.1016/j.cvex.2010.05.004
- Gancz , A.Y. , Kistler , A.L. , Greninger , A.L. , Farnoushi , Y. , Mechani , S. , Perl , S. , Berkowitz , A. , Perez , N. , Clubb , S. and DeRisi , J.L. 2009 . Experimental induction of proventricular dilatation disease in cockatiels (Nymphicus hollandicus) inoculated with brain homogenates containing avian bornavirus 4 . Virology Journal , 6 : 100 doi: 10.1186/1743-422X-6-100
- Gray , P. , Hoppes , S. , Suchodolski , P. , Mirhosseini , N. , Payne , S. , Villanueva , I. , Shivaprasad , H.L. , Honkavuori , K.S. , Lipkin , W.I. and Briese , T. 2010 . Use of avian bornavirus isolates to induce proventricular dilatation disease in conures . Emerging Infectious Diseases , 16 : 473 – 479 . doi: 10.3201/eid1603.091257
- Gregory , C.R. , Branson , W. , Latimer , K.S. , Steffens , W. , Pesti , D. , Campagnoli , R.P. & Lukert , P. 2000 . Progress in understanding proventricular dilatation disease . In E. Bergman . Proceedings of the Annual Conference of the Association of Avian Veterinarians 269 – 275 . Weatherford , TX .
- Guo , J. , Covaleda , L. , Heatley , J. , Baroch , J. , Tizard , I. and Payne , S. 2012 . Widespread avian bornavirus infection in mute swans in the northeast United States . Veterinary Medicine , 3 : 49 – 52 .
- Heffels-Redmann , U. , Enderlein , D. , Herzog , S. , Piepenbring , A. , Bürkle , M. , Neumann , D. , Müller , H. , Capelli , S. , Müller , H. and Oberhäuser , K. 2012 . Follow-up investigations on different courses of natural avian bornavirus infections in psittacines . Avian Diseases , 56 : 153 – 159 . doi: 10.1637/9844-062811-Reg.1
- Honkavuori , K.S. , Shivaprasad , H. , Williams , B.L. , Quan , P.L. , Hornig , M. , Street , C. , Palacios , G. , Hutchison , S.K. , Franca , M. and Egholm , M. 2008 . Novel bornavirus in psittacine birds with proventricular dilatation disease . Emerging Infectious Diseases , 14 : 1883 – 1886 . doi: 10.3201/eid1412.080984
- Kistler , A.L. , Gancz , A. , Clubb , S. , Skewes-Cox , P. , Fischer , K. , Sorber , K. , Chiu , C.Y. , Lublin , A. , Mechani , S. and Farnoushi , Y. 2008 . Recovery of divergent avian bornaviruses from cases of proventricular dilatation disease: identification of a candidate etiologic agent . Virology Journal , 5 : 88 doi: 10.1186/1743-422X-5-88
- Kistler , A.L. , Smith , J.M. , Greninger , A.L. , DeRisi , J.L. and Ganem , D. 2010 . Analysis of naturally occurring avian bornavirus infection and transmission during an outbreak of proventricular dilatation disease among captive psittacine birds . Journal of Virology , 84 : 2176 – 2179 . doi: 10.1128/JVI.02191-09
- Korbel , R. & Rinder , M. 2011 . Ophtalmological findings in birds affected with PDD . In J. Samour . Proceedings of the XIth European Association of Avian Veterinarians Conference 193 . Madrid , Spain .
- Lierz , M. , Hafez , H.M. , Honkavuori , K. , Gruber , A.D. , Olias , P. , Abdelwhab , E.M. , Kohls , A. , Lipkin , I.W. , Briese , T. and Hauck , R. 2009 . Anatomic distribution of avian bornavirus in parrots, its occurrence in clinically healthy birds and ABV-antibody detection . Avian Pathology , 38 : 491 – 496 . doi: 10.1080/03079450903349238
- Lierz , M. , Herden , C. , Herzog , S. and Piepenbring , A. 2010 . Proventricular dilatation disease and avian bornavirus as a possible cause [Die neuropathische Drusenmagendilatation der Psittaziden und das aviare Bornavirus als potenzielle Ursache] . Tierärztliche Praxis Ausgabe K Kleintiere Heimtiere , 38 : 87 – 94 .
- Lipkin , W.I. & Briese , T. 2006 . Bornaviridae . In D.M. Knipe & P.M. Howley . Field's virology , 5th edn 2 , 1829 – 1851 . Philadelphia : Lippincott William & Wilkins .
- Mirhosseini , N. , Gray , P.L. , Hoppes , S. , Tizard , I. , Shivaprasad , H.L. and Payne , S. 2011 . Proventricular dilatation disease in cockatiels (Nymphicus hollandicus) after infection with a genotype 2 avian bornavirus . Journal of Avian Medicine and Surgery , 25 : 199 – 204 . doi: 10.1647/2010-030.1
- Ouyang , N. , Storts , R. , Tian , Y. , Wigle , W. , Villanueva , I. , Mirhosseini , N. , Payne , S. , Gray , P. and Tizard , I. 2009 . Histopathology and the detection of avian bornavirus in the nervous system of birds diagnosed with proventricular dilatation disease . Avian Pathology , 38 : 393 – 401 . doi: 10.1080/03079450903191036
- Payne , S. , Covaleda , L. , Jianhua , G. , Swafford , S. , Baroch , J. , Ferro , P.J. , Lupiani , B. , Heatley , J. and Tizard , I. 2011a . Detection and characterization of a distinct bornavirus lineage from healthy Canada geese (Branta canadensis) . Journal of Virology , 85 : 12053 – 12056 . doi: 10.1128/JVI.05700-11
- Payne , S. , Shivaprasad , H.L. , Mirhosseini , N. , Gray , P. , Hoppes , S. , Weissenböck , H. and Tizard , I. 2011b . Unusual and severe lesions of proventricular dilatation disease in cockatiels (Nymphicus hollandicus) acting as healthy carriers of avian bornavirus (ABV) and subsequently infected with a virulent strain of ABV . Avian Pathology , 40 : 15 – 22 . doi: 10.1080/03079457.2010.536978
- Perpiñán , D. , Fernández-Bellon , H. , López , C. and Ramis , A. 2007 . Lymphoplasmacytic myenteric, subepicardial, and pulmonary ganglioneuritis in four nonpsittacine birds . Journal of Avian Medicine and Surgery , 21 : 210 – 214 . doi: 10.1647/1082-6742(2007)21[210:LMSAPG]2.0.CO;2
- Piepenbring , A.K. , Enderlein , D. , Herzog , S. , Kaleta , E.F. , Heffels-Redmann , U. , Ressmeyer , S. , Herden , C. & Lierz , M. 2012 . Pathogenesis of avian bornavirus in experimentally infected cockatiels . Emerging Infectious Diseases , 18 , 234 – 241 . doi: 10.3201/eid1802.111525
- Raghav , R. , Taylor , M. , DeLay , J. , Ojkic , D. , Pearl , D.L. , Kistler , A.L. , DeRisi , J.L. , Ganem , D. and Smith , D.A. 2010 . Avian bornavirus is present in many tissues of psittacine birds with histopathologic evidence of proventricular dilatation disease . Journal of Veterinary Diagnostic Investigation , 22 : 495 – 508 . doi: 10.1177/104063871002200402
- Rinder , M. , Ackermann , A. , Kempf , H. , Kaspers , B. , Korbel , R. and Staeheli , P. 2009 . Broad tissue and cell tropism of avian bornavirus in parrots with proventricular dilatation disease . Journal of Virology , 83 : 5401 – 5407 . doi: 10.1128/JVI.00133-09
- Ritchie , B.W. , Gregory , C.R. , Latimer , K.S. , Steffens , W.L. , Pesti , D. , Campagnoli , R.P. & Lu Lukert , P.D. 1998 . Progress in preventing PDD, polyomavirus, and PBFD virus . In A. Romagnano . Proceedings of the Annual Conference of the Association of Avian Veterinarians 25 – 40 . Bedford , TX .
- Rubbenstroth , D. , Rinder , M. , Kaspers , B. & Staeheli , P. 2012 . Efficient isolation of avian bornaviruses (ABV) from naturally infected psittacine birds and identification of a new ABV genotype from a salmon-crested cockatoo (Cacatua moluccensis) . Veterinary Microbiology , 161 , 36 – 42 .
- Schmidt , R.E. , Reavill , D.R. and Phalen , D.N. 2003 . Pathology of Pet and Aviary Birds , Ames , IA : Iowa State Press .
- Shivaprasad , H.L. , Barr , B.C. , Woods , L.W. , Moore , J.D. , Kinde , H. , Anderson , M.L. & Droual , R. 1995 . Spectrum of lesions (pathology) of proventricular dilatation syndrome of psittacines . In M. Kornelsen . Proceedings of the Annual Conference of the Association of Avian Veterinarians 505 – 506 . Philadelphia , PA .
- Smith , D.A. , Berkvens , C. , Kummrow , M. , Campbell , D. , Ojkic , D. & DeLay , J. 2010 . Identification of an avian bornavirus in the brains of Canada geese (Branta canadensis) and trumpeter swans (Cygnus buccinator) with non-suppurative encephalitis . In C.S. Cholatal , H. Ferreyra , & A. Chirife . Proceedings of the 59th Wildlife Disease Association Annual Meeting 58 . Iguazú , Argentina .
- Steinmetz , A. , Pees , M. , Schmidt , V. , Weber , M. , Krautwald-Junghanns , M.E. and Oechtering , G. 2008 . Blindness as a sign of proventricular dilatation disease in a grey parrot (Psittacus erithacus erithacus) . Journal of Small Animal Practice , 49 : 660 – 662 . doi: 10.1111/j.1748-5827.2008.00608.x
- Vice , C.A.C. 1992 . Myocarditis as a component of psittacine proventricular dilatation syndrome in a patagonian conure . Avian Diseases , 36 : 1117 – 1119 . doi: 10.2307/1591587
- Villanueva , I. , Gray , P. , Mirhosseini , N. , Payne , S. , Hoppes , S. , Honkavuori , K.S. , Briese , T. , Turner , D. and Tizard , I. 2010 . The diagnosis of proventricular dilatation disease: use of a western blot assay to detect antibodies against avian bornavirus . Veterinary Microbiology , 143 : 196 – 201 . doi: 10.1016/j.vetmic.2009.11.041
- Weissenböck , H. , Bakonyi , T. , Sekulin , K. , Ehrensperger , F. , Doneley , R.J.T. , Dürrwald , R. , Hoop , R. , Erdélyi , K. , Gál , J. and Kolodziejek , J. 2009a . Avian bornaviruses in psittacine birds from Europe and Australia with proventricular dilatation disease . Emerging Infectious Diseases , 15 : 1453 – 1459 . doi: 10.3201/eid1509.090353
- Weissenböck , H. , Sekulin , K. , Bakonyi , T. , Hogler , S. and Nowotny , N. 2009b . Novel avian bornavirus in a nonpsittacine species (canary; Serinus canaria) with enteric ganglioneuritis and encephalitis . Journal of Virology , 83 : 11367 – 11371 . doi: 10.1128/JVI.01343-09
- Wunschmann , A. , Honkavuori , K. , Briese , T. , Lipkin , W.I. , Shivers , J. and Armien , A.G. 2011 . Antigen tissue distribution of avian bornavirus (ABV) in psittacine birds with natural spontaneous proventricular dilatation disease and ABV genotype 1 infection . Journal of Veterinary Diagnostic Investigation , 23 : 716 – 726 .